Pharmacokinetics of Dibutyl Phthalate (DBP) in the Rat Determined by UPLC-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
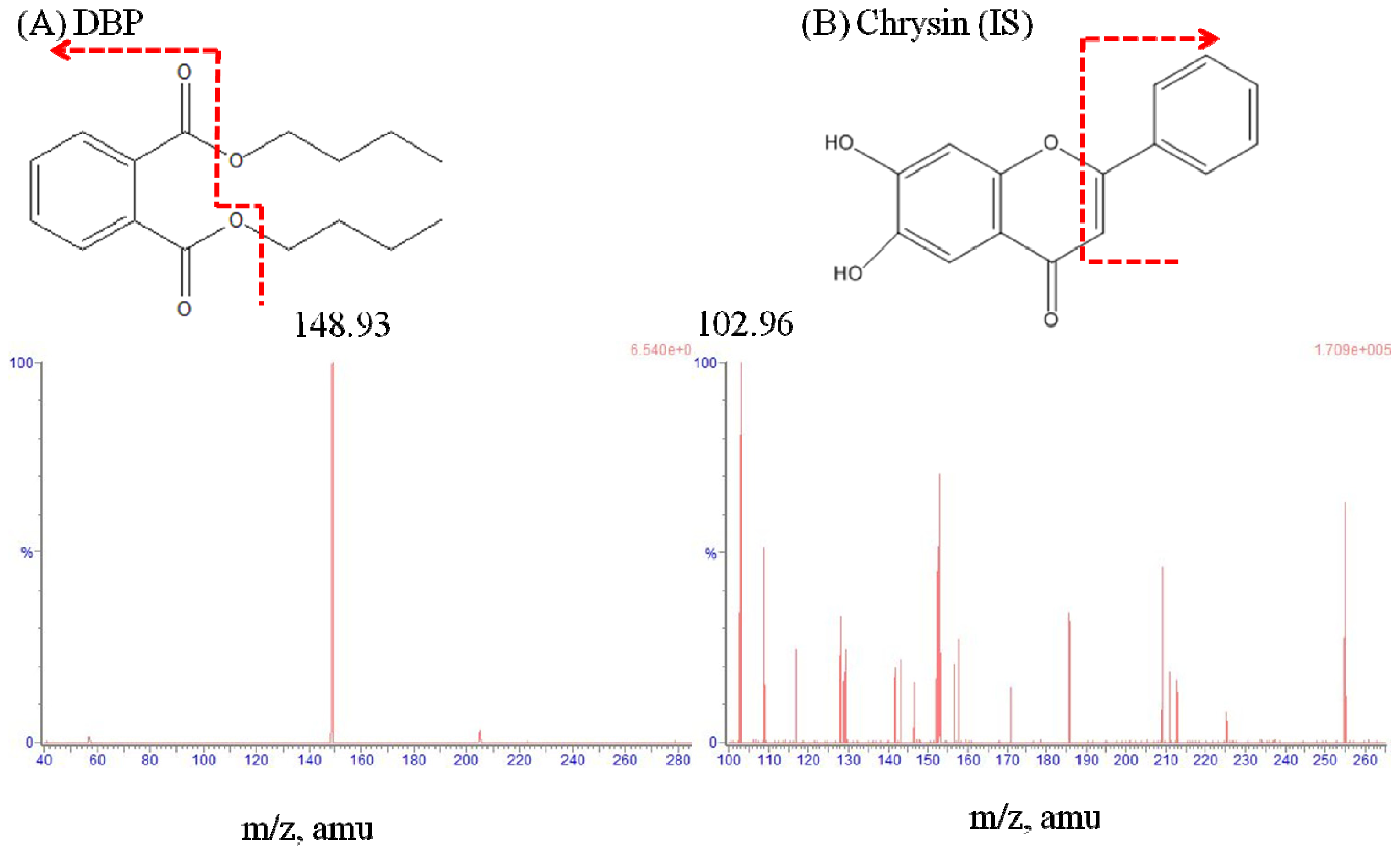
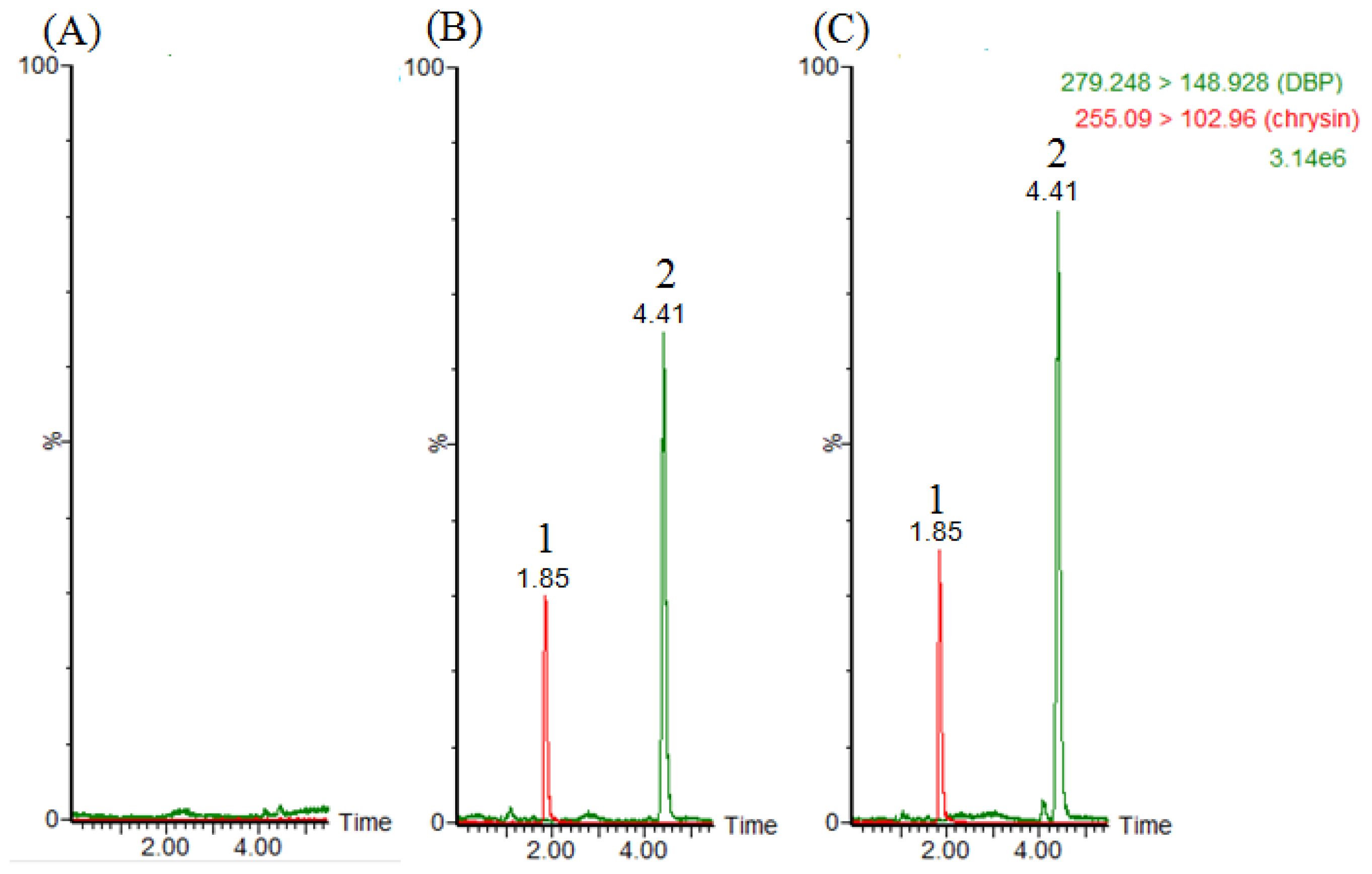
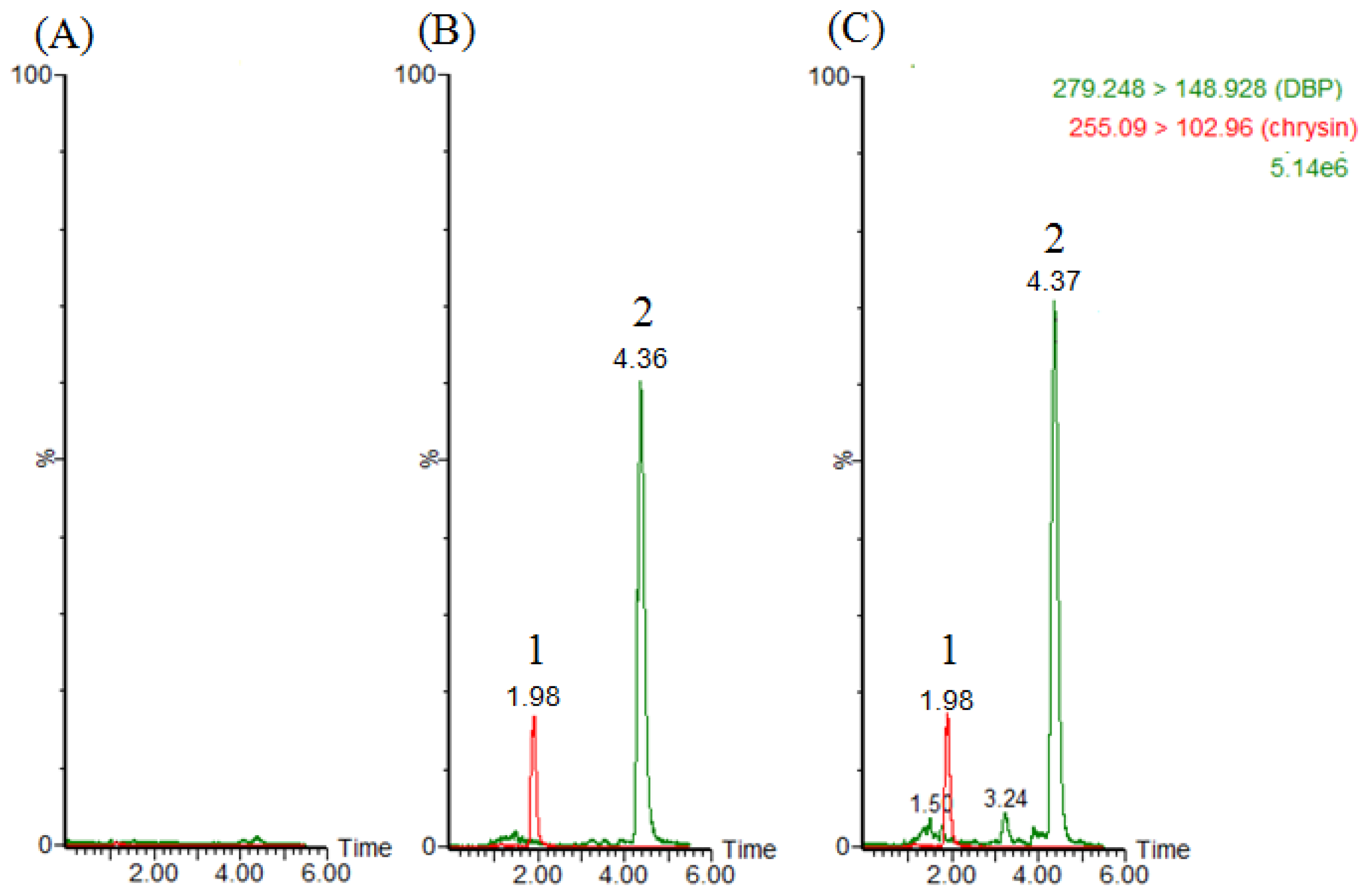
2.1. LC–MS/MS and Method Validation
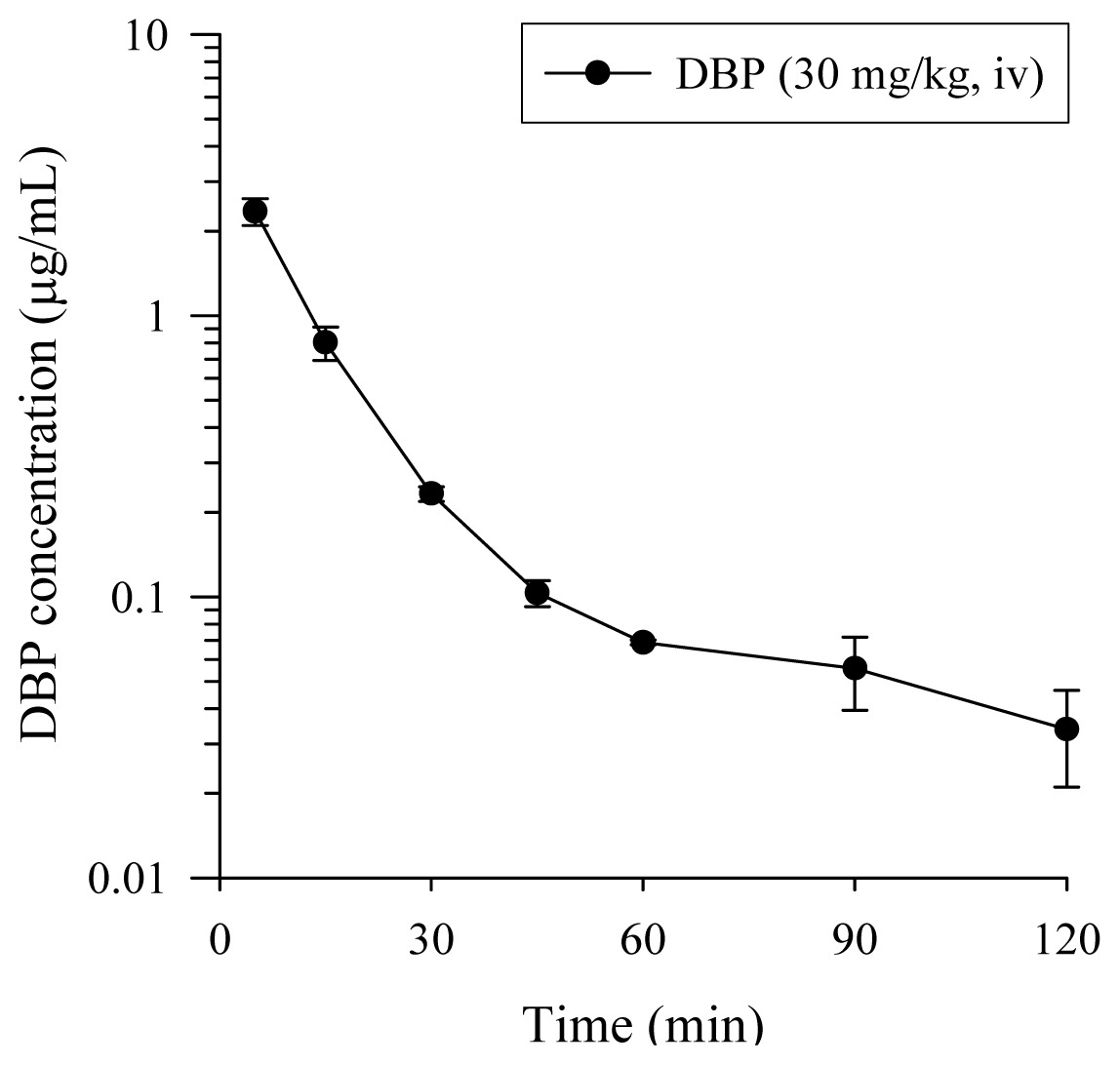
2.2. Pharmacokinetics Study of DBP
3. Experimental Section
3.1. Chemicals
3.2. Animal Experiment
3.3. LC–MS/MS and Method Validation
3.4. Blood Sampling Collection
3.5. Fecal Excretion of DBP after a Single Oral Administration
3.6. Blood and Fecal Sample Preparation
3.7. Pharmacokinetic Data Analysis and Statistics
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Blount, B.C.; Milgram, K.E.; Silva, M.J.; Malek, N.A.; Reidy, J.A.; Needham, L.L.; Brock, J.W. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal. Chem 2000, 72, 4127–4134. [Google Scholar]
- Yen, T.H.; Lin-Tan, D.T.; Lin, J.L. Food safety involving ingestion of foods and beverages prepared with phthalate-plasticizer-containing clouding agents. J. Formosan Med. Assoc 2011, 110, 671–684. [Google Scholar]
- Jobling, S.; Reynolds, T.; White, R.; Parker, M.G.; Sumpter, J.P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ. Health Perspect 1995, 103, 582–587. [Google Scholar]
- Dobrzynska, M.M.; Tyrkiel, E.J.; Pachocki, K.A. Developmental toxicity in mice following paternal exposure to Di-N-butyl-phthalate (DBP). Biomed. Environ. Sci 2011, 24, 569–578. [Google Scholar]
- Jiang, J.T.; Sun, W.L.; Jing, Y.F.; Liu, S.B.; Ma, Z.; Hong, Y.; Ma, L.; Qin, C.; Liu, Q.; Stratton, H.J.; et al. Prenatal exposure to di-n-butyl phthalate induces anorectal malformations in male rat offspring. Toxicology 2011, 290, 322–326. [Google Scholar]
- Wine, R.N.; Li, L.H.; Barnes, L.H.; Gulati, D.K.; Chapin, R.E. Reproductive toxicity of di-n-butylphthalate in a continuous breeding protocol in Sprague-Dawley rats. Environ. Health Perspect 1997, 105, 102–107. [Google Scholar]
- Zhou, D.; Wang, H.; Zhang, J.; Gao, X.; Zhao, W.; Zheng, Y. Di-n-butyl phthalate (DBP) exposure induces oxidative damage in testes of adult rats. Syst. Biol. Reprod. Med 2010, 56, 413–419. [Google Scholar]
- Swan, S.H.; Main, K.M.; Liu, F.; Stewart, S.L.; Kruse, R.L.; Calafat, A.M.; Mao, C.S.; Redmon, J.B.; Ternand, C.L.; Sullivan, S.; et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect 2005, 113, 1056–1061. [Google Scholar]
- Alam, M.S.; Andrina, B.B.; Tay, T.W.; Tsunekawa, N.; Kanai, Y.; Kurohmaru, M. Single administration of di(n-butyl) phthalate delays spermatogenesis in prepubertal rats. Tissue Cell 2010, 42, 129–135. [Google Scholar]
- Johnson, K.J.; McCahan, S.M.; Si, X.; Campion, L.; Herrmann, R.; Barthold, J.S. The orl rat with inherited cryptorchidism has increased susceptibility to the testicular effects of in utero dibutyl phthalate exposure. Toxicol. Sci 2008, 105, 360–367. [Google Scholar]
- Struve, M.F.; Gaido, K.W.; Hensley, J.B.; Lehmann, K.P.; Ross, S.M.; Sochaski, M.A.; Willson, G.A.; Dorman, D.C. Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats. Birth Defects Res. B 2009, 86, 345–354. [Google Scholar]
- Fennell, T.R.; Krol, W.L.; Sumner, S.C.; Snyder, R.W. Pharmacokinetics of dibutylphthalate in pregnant rats. Toxicol. Sci 2004, 82, 407–418. [Google Scholar]
- Clewell, R.A.; Kremer, J.J.; Williams, C.C.; Campbell, J.L.; Sochaski, M.A.; Andersen, M.E.; Borghoff, S.J. Kinetics of selected di-n-butyl phthalate metabolites and fetal testosterone following repeated and single administration in pregnant rats. Toxicology 2009, 255, 80–90. [Google Scholar]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. Lond. B 2009, 364, 2063–2078. [Google Scholar]
- Guo, Z.; Wei, D.; Wang, M.; Wang, S. Determination of six phthalic acid esters in orange juice packaged by PVC bottle using SPE and HPLC-UV: Application to the migration study. J. Chromatogr. Sci 2010, 48, 760–765. [Google Scholar]
- Yan, H.; Cheng, X.; Liu, B. Simultaneous determination of six phthalate esters in bottled milks using ultrasound-assisted dispersive liquid-liquid microextraction coupled with gas chromatography. J. Chromatogr. B 2011, 879, 2507–2512. [Google Scholar]
- Holadova, K.; Prokupkova, G.; Hajslova, J.; Poustka, J. Headspace solid-phase microextraction of phthalic acid esters from vegetable oil employing solvent based matrix modification. Anal. Chim. Acta 2007, 582, 24–33. [Google Scholar]
- Kondo, F.; Ikai, Y.; Hayashi, R.; Okumura, M.; Takatori, S.; Nakazawa, H.; Izumi, S.; Makino, T. Determination of five phthalate monoesters in human urine using gas chromatography-mass spectrometry. Bull. Environ. Contam. Toxicol 2010, 85, 92–96. [Google Scholar]
- Blount, B.C.; Silva, M.J.; Caudill, S.P.; Needham, L.L.; Pirkle, J.L.; Sampson, E.J.; Lucier, G.W.; Jackson, R.J.; Brock, J.W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect 2000, 108, 979–982. [Google Scholar]
- Guidance for Industry: Bioanalytical Method Validation; US Food and Drug Administration: Rockville, MD, USA, 2001.
- Sorensen, L.K. Determination of phthalates in milk and milk products by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom 2006, 20, 1135–1143. [Google Scholar]
- Niino, T.I.T.; Ishiwata, H.; Takeda, K.; Onodera, S. Characterization of Human Salivary Esterase in Enzymatic Hydrolysis of Phthalate Esters. J. Health Sci 2003, 49, 76–81. [Google Scholar]
- Matheus, M.; Meirelles, R.S. BAME-Esterase activity in plasmas from blood of human placental interveillous space and umbilical cord vessels (author’s transl). Reproduccion 1976, 3, 253–263. [Google Scholar]
- Chambers, E.; Wagrowski-Diehl, D.M.; Lu, Z.; Mazzeo, J.R. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatogr. B 2007, 852, 22–34. [Google Scholar]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm 1978, 6, 165–175. [Google Scholar]
- Kremer, J.J.; Williams, C.C.; Parkinson, H.D.; Borghoff, S.J. Pharmacokinetics of monobutylphthalate, the active metabolite of di-n-butylphthalate, in pregnant rats. Toxicol. Lett 2005, 159, 144–153. [Google Scholar]
- Tanaka, A.; Matsumoto, A.; Yamaha, T. Biochemical studies on phthalic esters. III. Metabolism of dibutyl phthalate (DBP) in animals. Toxicology 1978, 9, 109–123. [Google Scholar]
- Rowland, I.R.; Cottrell, R.C.; Phillips, J.C. Hydrolysis of phthalate esters by the gastro-intestinal contents of the rat. Food Cosmetics Toxicol 1977, 15, 17–21. [Google Scholar]
- Brunner, L.J.; Bai, S. Effect of dietary oil intake on hepatic cytochrome P450 activity in the rat. J. Pharm. Sci 2000, 89, 1022–1027. [Google Scholar]
- Saillenfait, A.M.; Sabate, J.P.; Gallissot, F. Diisobutyl phthalate impairs the androgen-dependent reproductive development of the male rat. Reprod. Toxicol 2008, 26, 107–115. [Google Scholar]

| Nominal concentration (μg/mL) | Precision (R.S.D.%) | Accuracy (Bias%) |
|---|---|---|
| Inter-day | ||
| 0.05 | 15.97 | −1.17 |
| 0.1 | 11.87 | −7.27 |
| 0.25 | 8.23 | −2.57 |
| 0.5 | 4.70 | 6.07 |
| 1 | 1.33 | −1.67 |
| 2.5 | 0.02 | 0.02 |
| Intra-day | ||
| 0.05 | 15.27 | 10.15 |
| 0.1 | 6.36 | −7.16 |
| 0.25 | 4.58 | −0.79 |
| 0.5 | 8.10 | 5.10 |
| 1 | 2.59 | −1.69 |
| 2.5 | 0.07 | 0.04 |
| Nominal concentration (μg/g) | Precision (R.S.D. %) | Accuracy (Bias %) |
|---|---|---|
| Inter-day | ||
| 0.125 | 4.89 | 11.93 |
| 0.25 | 11.31 | −1.38 |
| 0.5 | 11.86 | −3.58 |
| 1.25 | 8.03 | −3.71 |
| 2.5 | 1.05 | 0.11 |
| Intra-day | ||
| 0.125 | 17.50 | 14.23 |
| 0.25 | 9.88 | 4.17 |
| 0.5 | 7.43 | 3.48 |
| 1.25 | 4.50 | −6.43 |
| 2.5 | 0.82 | 1.39 |
| Nominal concentration | Set 1 | Set 2 | Set 3 | ME (%) | RE (%) |
|---|---|---|---|---|---|
| DBP in plasma (μg/mL) | |||||
| 0.125 | 18030 ± 469 | 17659 ± 951 | 17041 ± 533 | 97.89 | 96.58 |
| 1.25 | 149945 ± 4210 | 147095 ± 3886 | 148575 ± 11081 | 98.94 | 100.92 |
| 2.5 | 285965 ± 2679 | 282224 ± 1116 | 280990 ± 6802 | 98.69 | 99.57 |
| Average | - | - | - | 98.50 ± 0.54 | 99.02 ± 2.22 |
| IS (1 μg/mL) in Plasma | 146906 ± 6353 | 156220 ± 5833 | 158258 ± 6815 | 106.38 ± 2.03 | 107.73 ± 1.20 |
| DBP in feces (μg/g) | |||||
| 0.125 | 17662 ± 554 | 17791 ± 722 | 17535 ± 187 | 100.7 | 98.63 |
| 1.25 | 154574 ± 3608 | 152536 ± 1775 | 152825 ± 2346 | 100.65 | 100.18 |
| 2.5 | 309409 ± 4973 | 311060 ± 2234 | 309193 ± 5762 | 100.54 | 99.39 |
| Average | - | - | - | 100.63 ± 0.08 | 99.4 ± 0.77 |
| IS (1 μg/mL) in feces | 113790 ± 15520 | 113189 ± 3439 | 105573 ± 1652 | 100.70 ± 11.05 | 93.31 ± 1.7 |
| Pharmacokinetic parameters | DBP (30 mg/kg, i.v.) |
|---|---|
| AIC of one-compartment | (−23.57) ± 3.66 |
| AIC of two-compartment | (−37.09) ± 6.30 |
| A (μg/mL) | 6.04 ± 1.31 |
| B (μg/mL) | 0.18 ± 0.06 |
| α (1/min) | 0.15 ± 0.03 |
| β (1/min) | 0.02 ± 0.01 |
| AUC (min μg/mL) | 57.8 ± 5.93 |
| t1/2,α (min) | 5.77 ± 1.14 |
| t1/2,β (min) | 217 ± 131 |
| CL(mL/min/kg) | 551 ± 64 |
| MRT (min) | 123 ± 78 |
| Parameters | DBP | Chrysin (IS) |
|---|---|---|
| Ionization mode | ESI (+) | ESI (+) |
| Capillary voltage (kV) | 3.11 | 3.11 |
| Cone voltage (V) | 22 | 66 |
| Collision energy (eV) | 14 | 32 |
| Desolvation temperature (°C) | 400 | 400 |
| Desolvation gas flow (L/h) | 800 | 800 |
| Multiple reaction monitoring | m/z 279.25 > 148.93 | m/z 255.09 > 102.96 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, L.-W.; Hou, M.-L.; Tsai, T.-H. Pharmacokinetics of Dibutyl Phthalate (DBP) in the Rat Determined by UPLC-MS/MS. Int. J. Mol. Sci. 2013, 14, 836-849. https://doi.org/10.3390/ijms14010836
Chang L-W, Hou M-L, Tsai T-H. Pharmacokinetics of Dibutyl Phthalate (DBP) in the Rat Determined by UPLC-MS/MS. International Journal of Molecular Sciences. 2013; 14(1):836-849. https://doi.org/10.3390/ijms14010836
Chicago/Turabian StyleChang, Li-Wen, Mei-Ling Hou, and Tung-Hu Tsai. 2013. "Pharmacokinetics of Dibutyl Phthalate (DBP) in the Rat Determined by UPLC-MS/MS" International Journal of Molecular Sciences 14, no. 1: 836-849. https://doi.org/10.3390/ijms14010836






