Synthesis of Triptorelin Lactate Catalyzed by Lipase in Organic Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Lipase Resource
2.2. Effect of Substrate Molar Ratio
2.3. Effect of Organic Media
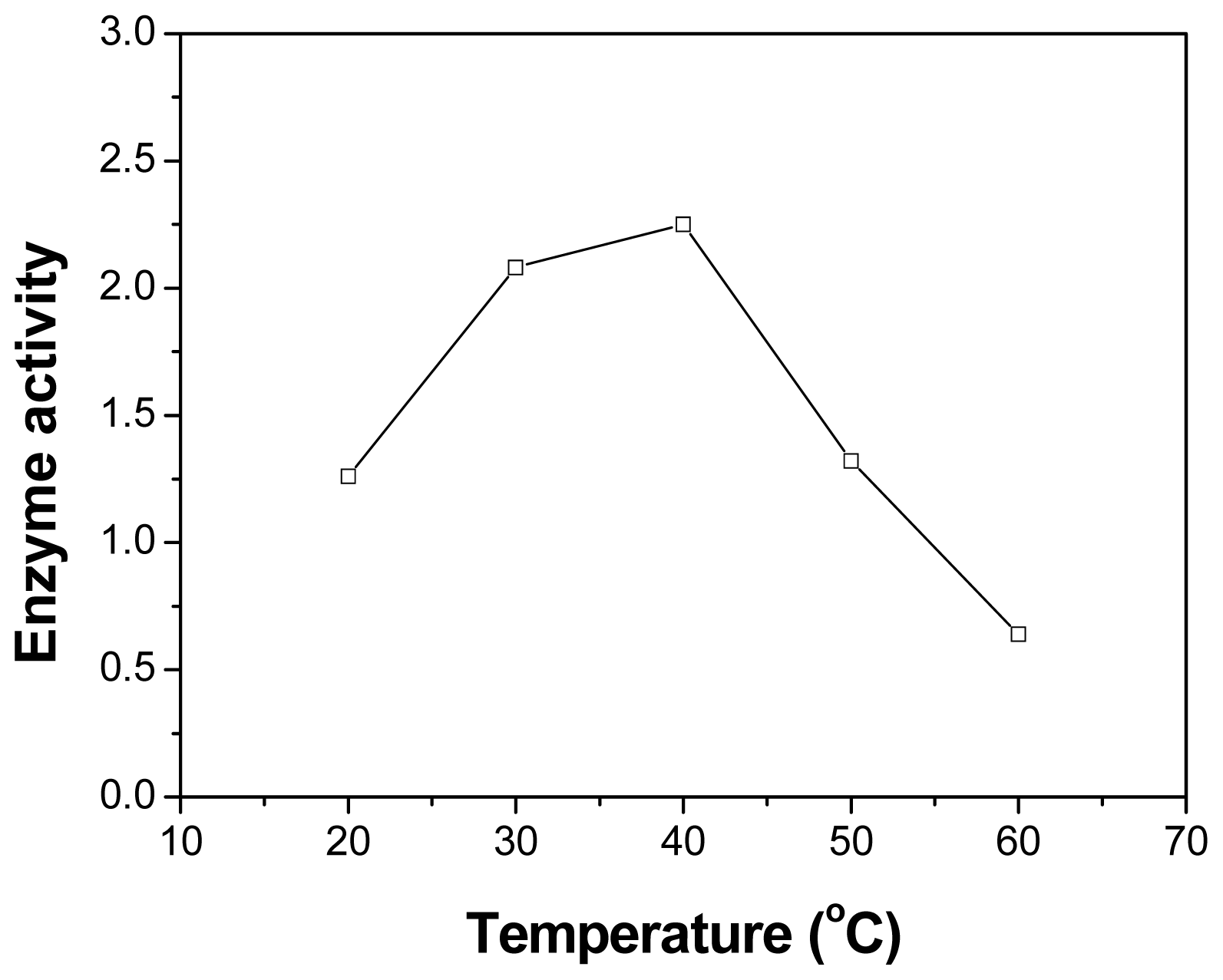
2.4. Effect of Temperature
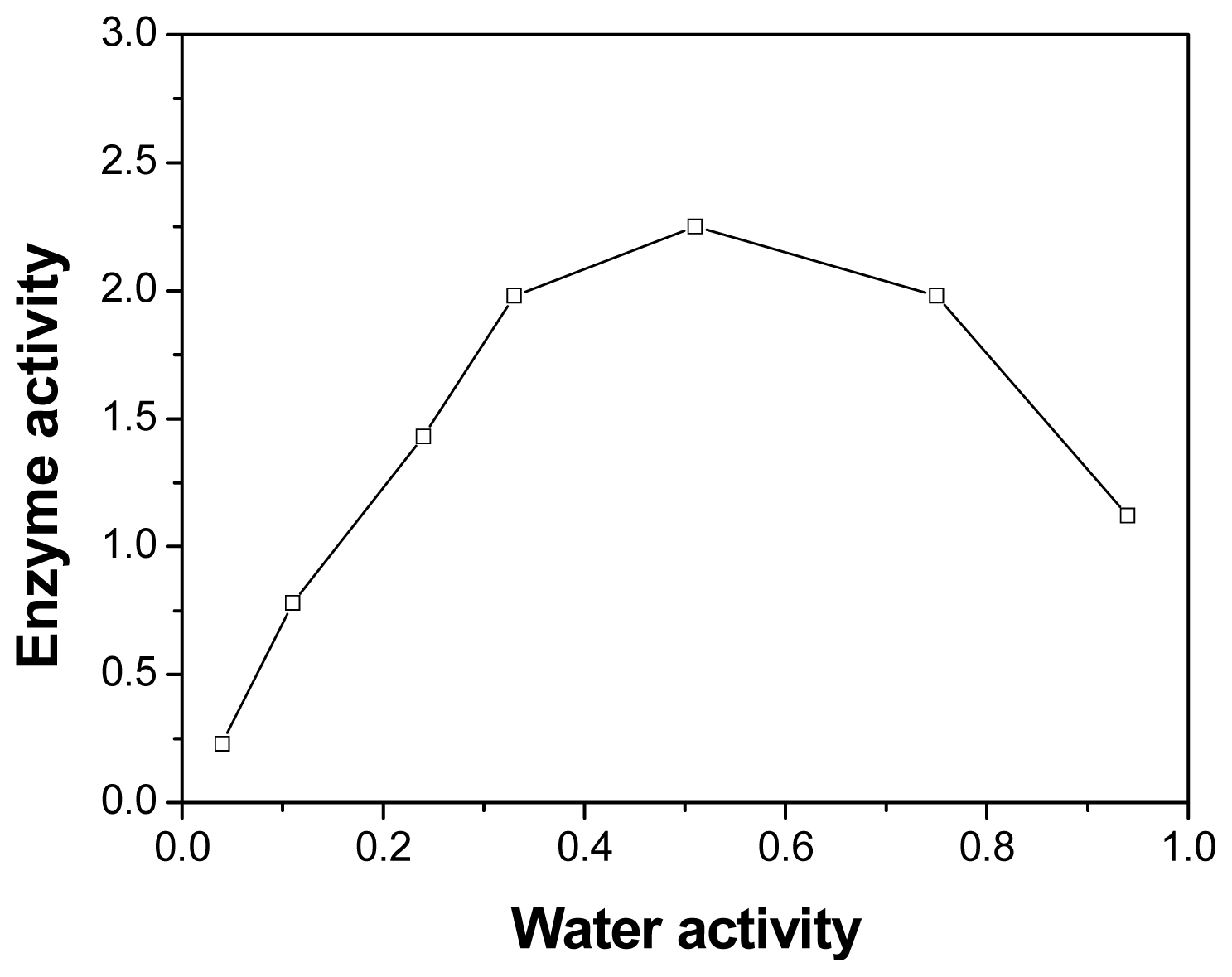
2.5. Effect of Initial Water Activity
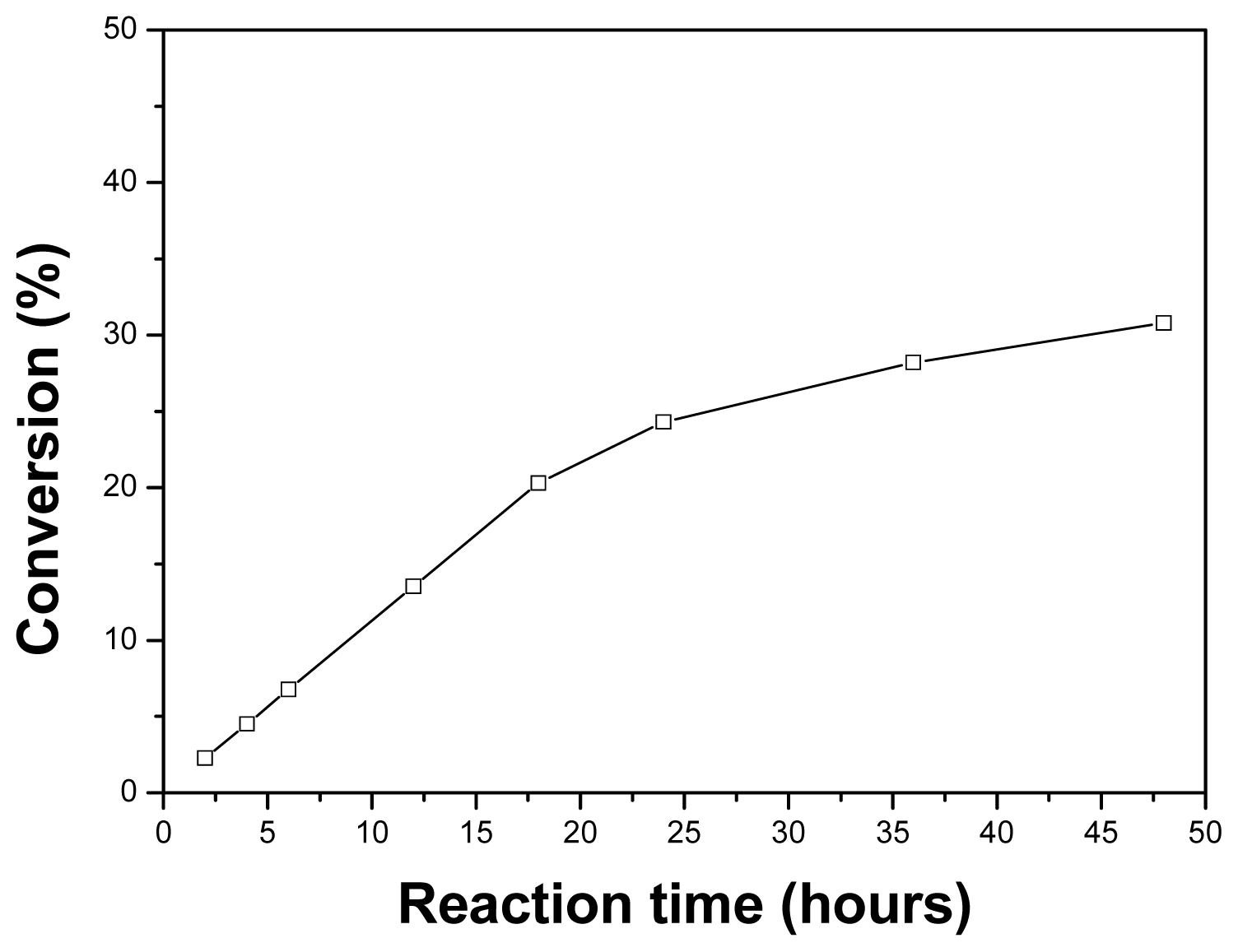
2.6. Effect of the Reaction Time
3. Experimental Section
3.1. Catalysts and Chemicals
3.2. Water Activity (aw) Control and Measurement
3.3. Esterification of Triptorelin
3.4. Analytical Methods
4. Conclusions
Acknowledgments
References
- Fuchise, T.; Kishimura, H.; Yang, Z.H.; Kojoma, M.; Toyota, E.; Sekizaki, H. Atlantic cod trypsin-catalyzed peptide synthesis with inverse substrates as acyl donor components. Chem. Pharm. Bull 2010, 58, 484–487. [Google Scholar]
- Stevenson, C. Advances in peptide pharmaceuticals. Curr. Pharm. Biotechnol 2009, 10, 122–137. [Google Scholar]
- Dahiya, R.; Mourya, R.; Agrawal, S.C. Synthesis and antimicrobial screening of peptidyl derivatives of bromocoumarins/methylimidazoles. Afr. J. Pharma. Pharmacol 2010, 4, 214–225. [Google Scholar]
- Lu, C.; Yu, X.; Huang, G.; Wang, H. Preparation and characterization of antigenic properties of gramicidin A-keyhole limpet hemocyanin and gramicidin A-ovalbumin conjugates. Afr. J. Biotechnol 2009, 8, 7051–7058. [Google Scholar]
- Pichereau, C.; Allary, C. Therapeutic peptides under the spotlight. 2005, 88–91. [Google Scholar]
- Cheng, W.; Lim, L.-Y. Comparison of reversible and nonreversible aqueous-soluble lipidized conjugates of salmon calcitonin. Mol. Pharm 2008, 5, 610–621. [Google Scholar]
- Wang, J.; Hogenkamp, D.J.; Tran, M.; Li, W.Y.; Yoshimura, R.F.; Johnstone, T.B.; Shen, W.C.; Gee, K.W. Reversible lipidization for the oral delivery of leu-enkephalin. J. Drug Target 2006, 14, 127–136. [Google Scholar]
- Guo, Z.W.; Machiya, K.; Salamonczyc, G.M.; Sih, C.J. Total synthesis of Bastadins 2, 3, and 6. J. Org. Chem 1998, 63, 4269–4276. [Google Scholar]
- Malnar, I.; Sih, C.J. Synthesis of the bis-diaryl ether fragment of vancomycin via enzymatic oxidative phenolic coupling. Tetrahedron Lett 2000, 41, 1907–1911. [Google Scholar]
- Malnar, I.; Sih, C.J. Chloroperoxidase-catalyzed chlorination of didechloroaglucovancomycin and vancomycin. J. Mol. Catal. B Enzym 2000, 10, 545–549. [Google Scholar]
- Breddam, K.; Widmer, F.; Johansen, J.T. Carboxypeptidase Y catalyzed C-terminal modifications of peptides. Carlsberg Res. Commun 1981, 46, 121–128. [Google Scholar]
- Shah, S.; Gupta, M.N. Lipase catalyzed preparation of biodiesel from jatropha oil in a solvent free system. Process Biochem 2007, 42, 409–414. [Google Scholar]
- Won, K.; Hong, J.K.; Kim, K.J.; Moon, S.J. Lipase catalyzed enantioselective esterification of racemic ibuprofen coupled with pervaporation. Process Biochem 2006, 41, 264–269. [Google Scholar]
- Wu, J.Y.; Liu, S.W. Influence of alcohol concentration on lipase catalyzed enantioselective esterification of racemic naproxen in isooctane: Under controlled water activity. Enzyme Microb. Technol 2000, 26, 124–130. [Google Scholar]
- Carrea, G.; Otto, L.G.; Riva, S. Role of solvents in the control of enzyme selectivity in organic media. Trends Biotechnol 1995, 13, 63–70. [Google Scholar]
- Gorman, L.A.S.; Dordick, J.S. Organic solvents strip water off enzymes. Biotechnol. Bioeng 1992, 39, 392–397. [Google Scholar]
- Serdakowski, A.L.; Dordick, J.S. Enzyme activation for organic solvents made easy. Trends Biotechnol 2008, 26, 48–54. [Google Scholar]
- Wehtje, E.; Costes, D.; Adlercreutz, P. Enantioselectivity of lipases: Effects of water activity. J. Mol. Catal. B Enzymatic 1997, 3, 221–230. [Google Scholar]
- Halling, P.J. Organic liquids and biocatalysts: Theory and practice. Trends Biotechnol 1989, 7, 50–52. [Google Scholar]
- Ghamgui, H.; Karra-Chaabouni, M.; Bezzine, S.; Miled, N.; Gargouri, Y. Production of isoamyl acetate with immobilized Staphylococcus simulans lipase in a solvent-free system. Enzyme Microb. Technol 2006, 38, 788–794. [Google Scholar]
- Tian, R.; Yang, C.-H.; Wei, X.-F.; Xun, E.-N.; Wang, R.; Cao, S.-G.; Wang, Z.; Wang, L. Optimization of APE1547-catalyzed Enantioselective Esterification of (R/S)-2-methyl-1-butanol in an Ionic Liquid. Biotechnol. Bioprocess Eng 2011, 16, 337–342. [Google Scholar]


| Lipase | Porcine pancreatic lipase (PPL) | Pseudomonas sp. (PSL) | Pseudomonas fluorescens lipase (Lipase AK, AKL) | Candida cylindracea A.Y. lipase (AYL) | Candida rugosa lipase (CRL) | Novozyme 435 |
|---|---|---|---|---|---|---|
| Activity (μmol/h/g) | 2.25 | 0.67 | 1.32 | 0.54 | 0.27 | 1.47 |
| Organic solvents | Log P | Enzyme activity (μmol/h/g) |
|---|---|---|
| Dichloromethane | 1.25 | 1.75 |
| Benzene | 2.0 | 2.25 |
| Toluene | 2.5 | 1.95 |
| Cyclohexane | 3.2 | 1.87 |
| n-hexane | 3.5 | 1.45 |
| n-Heptane | 4.0 | 0.88 |
| Isooctane | 4.5 | 0.33 |
| Fragment ions | Structure | Calculated m/z | Measured m/z |
|---|---|---|---|
| b1 | Pyr | 112.04 | - |
| b2 | Pyr-His | 249.10 | 249.1 |
| b3 | Pyr-His-Trp | 435.18 | 435.1 |
| b4 | Pyr-His-Trp-Ser(lactate) | 594.23 | 594.2 |
| b5 | Pyr-His-Trp-Ser(lactate)-Tyr | 757.29 | - |
| b6 | Pyr-His-Trp-Ser(lactate)-Tyr-d-Trp | 943.37 | 943.4 |
| b7 | Pyr-His-Trp-Ser(lactate)-Tyr-d-Trp-Leu | 1056.46 | - |
| b8 | Pyr-His-Trp-Ser(lactate)-Tyr-d-Trp-Leu-Arg | 1212.56 | 1212.6 |
| b9 | Pyr-His-Trp-Ser(lactate)-Tyr-d-Trp-Leu-Arg-Pro | 1309.61 | - |
| (M+H)+ | Pyr-His-Trp-Ser(lactate)-Tyr-d-Trp-Leu-Arg-Pro-Gly-NH2 | 1383.66 | 1383.6 |
| y9 | His-Trp-Ser(lactate)-Tyr-d-Trp-Leu-Arg-Pro-Gly-NH2 | 1272.63 | - |
| y8 | Trp-Ser(lactate)-Tyr-d-Trp-Leu-Arg-Pro-Gly-NH2 | 1135.57 | 1135.4 |
| y7 | Ser(lactate)-Tyr-d-Trp-Leu-Arg-Pro-Gly-NH2 | 949.49 | 949.3 |
| y6 | Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2 | 790.44 | 790.3 |
| y5 | D-Trp-Leu-Arg-Pro-Gly-NH2 | 627.37 | - |
| y4 | Leu-Arg-Pro-Gly-NH2 | 441.29 | 441.2 |
| y3 | Arg-Pro-Gly-NH2 | 328.21 | 328.1 |
| y2 | Pro- Gly-NH2 | 172.11 | 172.1 |
| y1 | Gly-NH2 | 75.03 | - |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhuang, H.; Wang, Z.; Wang, J.; Zhang, H.; Xun, E.; Chen, G.; Yue, H.; Tang, N.; Wang, L. Synthesis of Triptorelin Lactate Catalyzed by Lipase in Organic Media. Int. J. Mol. Sci. 2012, 13, 9971-9979. https://doi.org/10.3390/ijms13089971
Zhuang H, Wang Z, Wang J, Zhang H, Xun E, Chen G, Yue H, Tang N, Wang L. Synthesis of Triptorelin Lactate Catalyzed by Lipase in Organic Media. International Journal of Molecular Sciences. 2012; 13(8):9971-9979. https://doi.org/10.3390/ijms13089971
Chicago/Turabian StyleZhuang, Hong, Zhi Wang, Jiaxin Wang, Hong Zhang, Erna Xun, Ge Chen, Hong Yue, Ning Tang, and Lei Wang. 2012. "Synthesis of Triptorelin Lactate Catalyzed by Lipase in Organic Media" International Journal of Molecular Sciences 13, no. 8: 9971-9979. https://doi.org/10.3390/ijms13089971





