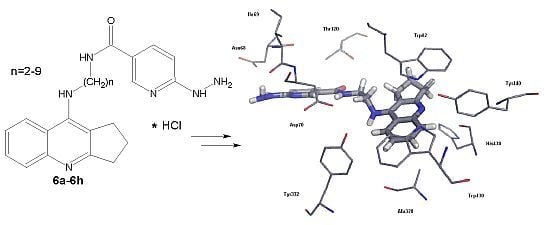
2,3-Dihydro-1H-cyclopenta[b]quinoline Derivatives as Acetylcholinesterase Inhibitors—Synthesis, Radiolabeling and Biodistribution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Pharmacological Evaluation
2.2.1. Studies of AChE/BChE Inhibition
2.2.2. Studies of Molecular Modeling
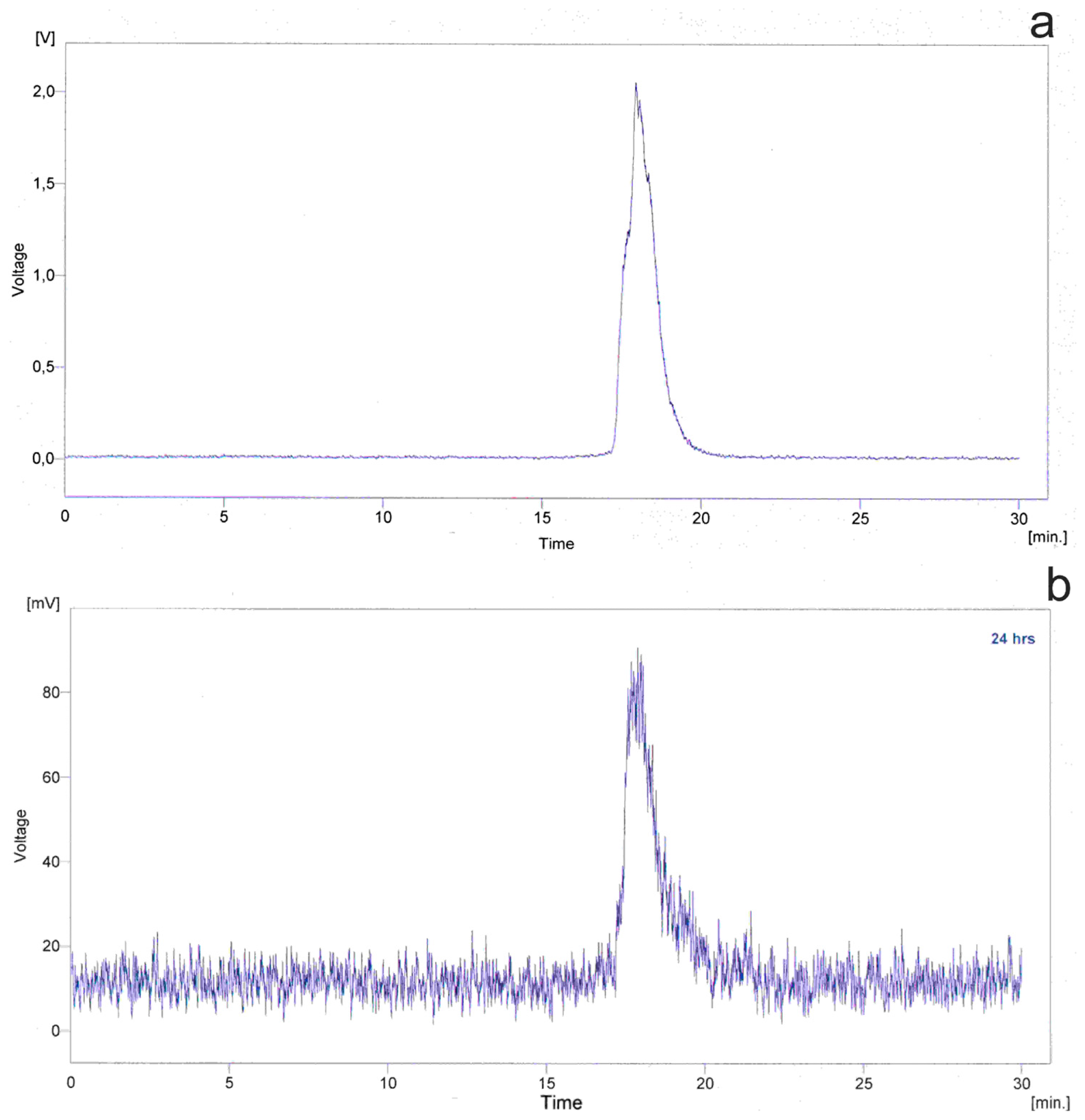
2.2.3. Radiolabeling with 99mTc and Biodistribution Studies in Rats
3. Experimental Section
3.1. Chemistry
3.1.1. 6-Hydrazinopyridine-3-carboxylic Acid (1)
3.1.2. 6-BOC-hydrazinopyridine-3-carboxylic Acid (2)
3.1.3. 9-Chloro-2,3-dihydro-1H-cyclopenta[b]quinoline (3)
3.1.4. N′-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-yl)ethane-1,2-diamine (4a)
3.1.5. N′-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-yl)propane-1,3-diamine (4b)
3.1.6. N′-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-yl)butane-1,4-diamine (4c)
3.1.7. N′-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-yl)penthane-1,5-diamine (4d)
3.1.8. N′-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-yl)hexane-1,6-diamine (4e)
3.1.9. N′-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-yl)heptane-1,7-diamine (4f)
3.1.10. N′-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-yl)octane-1,8-diamine (4g)
3.1.11. N′-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-yl)nonane-1,9-diamine (4f)
3.1.12. N-{5-[2-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-ylamino)ethylcarbamoyl]pyridin-2-yl} hydrazinecarboxylic Acid tert-Butyl Ester (5a)
3.1.13. N-{5-[3-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-ylamino)propylcarbamoyl]pyridin-2-yl} hydrazinecarboxylic Acid tert-Butyl Ester (5b)
3.1.14. N-{5-[4-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-ylamino)butylcarbamoyl]pyridin-2-yl} hydrazinecarboxylic Acid tert-Butyl Ester (5c)
3.1.15. N-{5-[5-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-ylamino)pentylcarbamoyl]pyridin-2-yl} hydrazinecarboxylic Acid tert-Butyl Ester (5d)
3.1.16. N-{5-[6-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-ylamino)hexylcarbamoyl]pyridin-2-yl} hydrazinecarboxylic Acid tert-Butyl Ester (5e)
3.1.17. N-{5-[7-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-ylamino)heptylcarbamoyl]pyridin-2-yl} hydrazinecarboxylic Acid tert-Butyl Ester (5f)
3.1.18. N-{5-[8-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-ylamino)octylcarbamoyl]pyridin-2-yl} hydrazinecarboxylic Acid tert-Butyl Ester (5g)
3.1.19. N-{5-[9-(2,3-Dihydro-1H-cyclopenta[b]quinolin-9-ylamino)nonylcarbamoyl]pyridin-2-yl} hydrazinecarboxylic Acid tert-Butyl Ester (5h)
3.1.20. 6-Hydrazino-N-[2-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)Ethyl]nicotinamide hydrochloride (6a)
3.1.21. 6-Hydrazino-N-[3-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)propyl]nicotinamide hydrochloride (6b)
3.1.22. 6-Hydrazino-N-[4-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)butyl]nicotinamide hydrochloride (6c)
3.1.23. 6-Hydrazino-N-[5-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)pentyl]nicotinamide hydrochloride (6d)
3.1.24. 6-Hydrazino-N-[6-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)hexyl]nicotinamide hydrochloride (6e)
3.1.25. 6-Hydrazino-N-[7-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)heptyl]nicotinamide hydrochloride (6f)
3.1.26. 6-Hydrazino-N-[8-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)octyl]nicotinamide hydrochloride (6g)
3.1.27. 6-Hydrazino-N-[9-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)nonyl]nicotinamide hydrochloride (6h)
3.2. Biochemical Studies
3.3. Molecular Modeling
3.4. Spectrophotometric Experiments
3.5. Radiolabelling
- Mobile phase A: 0.9% NaCl, B: CH3CN.
- 1–25 min 50% B.
- 25–30 min 50%–100% B.
- 30–35 min 100% B.
- 35–40 min 100%–0% B.
- 0–10 min 0% B.
- 10–25 min 0%–100% B.
- 25–30 min 100% B.
- 30–35 min 100%–0% B.
3.6. Biodistribution Studies in Rats
3.6.1. Animals
3.6.2. Biodistribution in Rats
4. Conclusions
Acknowledgments
References
- Delfini, M.; di Coco, M.E.; Piccioni, F.; Porcelli, F.; Borioni, A.; Rodomonte, A.; del Giudice, M.R. Tacrine derivatives–acetylcholinesterase interaction. 1H NMR relaxation study. Bioorg. Chem 2007, 35, 243–257. [Google Scholar]
- Kurz, A.; Perneczky, R. Novel insights for the treatment of Alzheimer’s disease. Prog. Neuro-Psychopharmacol 2011, 35, 373–379. [Google Scholar]
- Sjogren, M.; Andreasen, N.; Blennow, K. Advances in the detection of Alzheimer’s disease-use of cerebrospinal fluid biomarkers. Clin. Chim. Acta 2003, 332, 1–10. [Google Scholar]
- Silmana, I.; Sussman, J.L. Acetylcholinesterase: How is structure related to function? Chem. Biol. Interact 2008, 175, 3–10. [Google Scholar]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci 2001, 2, 294–302. [Google Scholar]
- Akasofu, S.; Kimura, M.; Kosasa, T.; Sawada, K.; Ogura, H. Study of neuroprotection of donepezil, a therapy for Alzheimer’s disease. Chem. Biol. Interact 2008, 175, 222–226. [Google Scholar]
- Jia, P.; Sheng, R.; Zhang, J.; Fang, L.; He, Q.; Yang, B.; Hu, Y. Design, synthesis and evaluation of galanthamine derivatives as acetylcholinesterase inhibitors. Eur. J. Med. Chem 2009, 44, 772–784. [Google Scholar]
- Pan, L.; Tan, J.-H.; Hou, J.-Q.; Huang, S.-L.; Gu, L.-Q.; Huang, Z.-S. Design, synthesis and evaluation of isaindigotone derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. Bioorg. Med. Chem. Lett 2008, 18, 3790–3794. [Google Scholar]
- Blass, J.P. Alzheimer’s disease and Alzheimer’s dementia: Distinct but overlapping entities. Neurobiol. Aging 2002, 23, 1077–1084. [Google Scholar]
- Shah, R.S.; Lee, H.G.; Xiongwei, Z.; Perry, G.; Smith, M.A.; Castellani, R.J. Current approaches in the treatment of Alzheimer’s disease. Biomed. Pharmacother 2008, 62, 199–207. [Google Scholar]
- Salloway, S.; Mintzer, J.; Weiner, M.F.; Cummings, J.L. Disease-modifying therapies in Alzheimer’s disease. Alzheimer’s Dement 2008, 4, 65–79. [Google Scholar]
- Jin, K.; Xie, L.; Mao, X.O.; Greenberg, D.A. Alzheimer’s disease drugs promote neurogenesis. Brain Res 2006, 1085, 183–188. [Google Scholar]
- Liston, D.R.; Nielsen, J.A.; Villalobos, A.; Chapin, D.; Jones, S.B.; Hubbard, S.T.; Shalaby, I.A.; Ramirez, A.; Nason, D.; White, W.F. Pharmacology of selective acetylcholinesterase inhibitors: Implications for use in Alzheimer’s disease. Eur. J. Pharmacol 2004, 486, 9–17. [Google Scholar]
- Davis, K.L.; Pochwik, P. Tacrine. Lancet 1995, 11, 625–630. [Google Scholar]
- Musiał, A.; Bajda, M.; Malawska, B. Development of acetylcholinesterase inhibitors for Alzheimer’s disease treatment. Curr. Med. Chem 2007, 14, 2654–2679. [Google Scholar]
- Tumiatti, V.; Minarini, A.; Bolognesi, M.L.; Milelli, A.; Rosini, M.; Melchiorre, C. Tacrine derivatives and Alzheimer’s disease. Curr. Med. Chem 2010, 17, 1825–1838. [Google Scholar]
- Hu, M.-K.; Wu, L.-J.; Hsiao, G.; Yen, M.-H. Homodimeric tacrine congeners as acetylcholinesterase inhibitors. J. Med. Chem 2002, 45, 2277–2282. [Google Scholar]
- Rydberg, E.H.; Brumshtein, B.; Greenblatt, H.M.; Wong, D.M.; Shaya, D.; Williams, L.D.; Carlier, P.R.; Pang, Y.-P.; Silman, I.; Sussman, J.L. Complexes of alkylene-linked tacrine dimers with Torpedo californica acetylcholinesterase: Binding of bis(5)-tacrine produces a dramatic rearrangement in the active-site gorge. J. Med. Chem 2006, 49, 5491–5500. [Google Scholar]
- Li, W.; Mak, M.; Jiang, H.; Wang, Q.; Pang, Y.; Chen, K.; Han, Y. Novel anti-Alzheimer’s dimer Bis(7)-cognitin: Cellular and molecular mechanisms of neuroprotection through multiple targets. Neurotherapeutics 2009, 6, 187–201. [Google Scholar]
- Carlier, P.R.; Han, Y.F.; Chow, E.S.; Li, C.P.; Wang, H.; Lieu, T.X.; Wong, H.S.; Pang, Y.P. Evaluation of short-tether Bis-THA AChE inhibitors. A further test of the dual binding site hypothesis. Bioorg. Med. Chem 1999, 7, 351–357. [Google Scholar]
- Shen, Y.; Yu, Y.; Lv, H.; Feng, L.; Zhang, G. Design, synthesis and evaluation of tacrine based acetylcholinesterase inhibitors. Lett. Drug Des. Discov 2010, 7, 341–345. [Google Scholar]
- Zhou, J.; Hu, X.; Zhang, H.; Qian, H.; Huang, W.; Qi, F.; Zhang, Y. Synthesis and biological evaluation of 5,6-dihydro-benzo[c]acridin-7-ol derivatives as anti-Alzheimer’s disease drugs. Lett. Drug Des. Discov 2009, 6, 623–628. [Google Scholar]
- Belluti, F.; Piazzi, L.; Bisi, A.; Gobbi, S.; Bartolini, M.; Cavalli, A.; Valenti, P.; Rampa, A. Design, synthesis, and evaluation of benzophenone derivatives as novel acetylcholinesterase inhibitors. Eur. J. Med. Chem 2009, 44, 1341–1348. [Google Scholar]
- Tomassoli, I.; Ismaili, L.; Pudlo, M.; de los Ríos, C.; Soriano, E.; Colmena, I.; Gandía, L.; Rivas, L.; Samadi, A.; Marco-Contelles, J.; et al. Synthesis, biological assessment and molecular modeling of new dihydroquinoline-3-carboxamides and dihydroquinoline-3-carbohydrazide derivatives as cholinesterase inhibitors, and Ca channel antagonists. Eur. J. Med. Chem 2011, 46, 1–10. [Google Scholar]
- Samadi, A.; Valderas, C.; de los Ríos, C.; Bastida, A.; Chioua, M.; González-Lafuente, L.; Colmena, I.; Gandía, L.; Romero, A.; del Barrio, L.; et al. Cholinergic and neuroprotective drugs for the treatment of Alzheimer and neuronal vascular diseases. II. Synthesis, biological assessment, and molecular modelling of new tacrine analogues from highly substituted 2-aminopyridine-3-carbonitriles. Bioorg. Med. Chem 2011, 19, 122–133. [Google Scholar]
- Abrams, M.J.; Juweid, M.; TenKate, C.I.; Schwartz, D.A.; Hauser, M.M.; Gaul, F.E. Technetium-99-m-Human Polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J. Nucl. Med 1990, 31, 2022–2028. [Google Scholar]
- Dorronsoro, I.; Alonso, D.; Castro, A. Synthesis and biological evaluation of tacrine-thiadiazolidinone hybrids as dual acetylcholinesterase inhibitors. Arch. Pharm. Chem. Life Sci 2005, 338, 18–23. [Google Scholar]
- Rosini, M.; Andrisano, V.; Bartolini, M.; Bolognesi, M.L.; Hrelia, P.; Minarini, A.; Tarozzi, A.; Melchiorre, C. Rational approach to discover multipotent anti-Alzheimer drugs. J. Med. Chem 2005, 48, 360–363. [Google Scholar]
- Fang, L.; Kraus, B.; Lehman, J. Tacrine–Ferulic acid hybrids as multi-potent anti-Alzheimer drug candidates. Bioorg. Med. Chem 2008, 18, 2905–2909. [Google Scholar]
- Carlier, P.R.; Chow, E.S.-W.; Han, Y.F.; Liu, J.; El Yazak, J.; Pang, Y.-P. Heterodimeric tacrine-based acetylcholinesterase inhibitors: Investigating ligand-peripheral site interactions. J. Med. Chem 1999, 42, 4225–4231. [Google Scholar]
- Fang, L.; Appenroth, D.; Decker, M.; Kiehntopf, M.; Lupp, A.; Peng, S.; Fleck, C.; Zhang, Y.; Lehmann, J. Tacrine hybrid compounds improve scopolamine-induced cognition impairment and show less hepatotoxicity. J. Med. Chem 2008, 51, 7666–7669. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm 1961, 7, 88–95. [Google Scholar]
- Cheng, Y.C.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharm 1973, 22, 3099–3108. [Google Scholar]
- Tipton, K.F. Commentary: Enzyme kinetics in relation to enzyme inhibitors. Biochem. Pharm 1973, 22, 2933–2941. [Google Scholar]
- Neşe Çokuğraş, A. Butyrylcholinesterase: Structure and physiological importance. Turk. J. Biochem 2003, 28, 54–61. [Google Scholar]
- Liu, S.; Edwards, D.S. 99mTc-Labeled small peptides as diagnostic radiopharmaceuticals. Chem. Rev 1999, 99, 2235–2268. [Google Scholar]
- Minarini, A.; Milelli, A.; Tumiatti, V.; Rosini, M.; Simoni, E.; Bolognesi, M.L.; Andrisano, V.; Bartolini, M.; Motori, E.; Angeloni, C.; et al. Cystamine-Tacrine dimer: A new multi-target-directed ligand as potential therapeutic agent for Alzheimer’s disease treatment. Neuropharmacology 2012, 62, 997–1003. [Google Scholar]
- Binder, J.; Paar, M.; Jun, D.; Pohanka, M.; Hrabinova, M.; Opletalova, V.; Kuca, K. New bisquaternary isoquinolinium inhibitors of brain cholinesterases-synthesis and anticholinesterase. Lett. Drug Des. Discov 2010, 7, 1–4. [Google Scholar]
- Martins, C.; Carmo Carreiras, M.; León, R.; de los Ríos, C.; Bartolini, M.; Andrisano, V.; Iriepa, I.; Moraleda, I.; Gálvez, E.; García, M.; et al. Synthesis and biological assessment of diversely substituted furo[2,3-b]quinolin-4-amine and pyrrolo[2,3-b]quinolin-4-amine derivatives, as novel tacrine analogues. Eur. J. Med. Chem 2011, 46, 6119–6130. [Google Scholar]
- Antequera, D.; Bolos, M.; Spuch, C.; Pascual, C.; Ferrer, I.; Fernandez-Bachiller, M.I.; Rodríguez-Franco, M.I.; Carro, E. Effects of a tacrine-8-hydroxyquinoline hybrid (IQM-622) on Aβ accumulation and cell death: Involvement in hippocampal neuronal loss in Alzheimer’s disease. Neurobiol. Dis 2012, 46, 682–691. [Google Scholar]
- Akula, M.R.; Kabalka, G.W. Synthesis of 7-[123I]iodotacrine: A potential SPECT agent to map acetylcholinesterase. J. Labelled Compd. Radiopharm 1999, 42, 959–964. [Google Scholar]
- Nishioka, K.; Kamada, T.; Kanamaru, H. 14C-Labeling of a tetrahydroacridine, a novel CNS-selective cholinesterase inhibitor. J. Labelled Compd. Radiopharm 1992, 31, 553–560. [Google Scholar]
- Leman, L.; Kitson, S.L.; Brown, R.T.; Cairns, J.; Watters, W.; McMordie, A.; Murrell, V.; Marfurt, J. Synthesis of isotopically labelled [14C]ZT-1 (Debio-9902), [d3]ZT-1 and (−)-[d3]huperzine A, a new generation of acetylcholinesterase inhibitors. J. Labelled Compd. Radiopharm 2011, 54, 720–730. [Google Scholar]
- De Vos, F.; Santens, P.; Vermeirsch, H.; Dewolf, I.; Dumont, F.; Slegers, G.; Dierckx, R.A.; de Reuck, J. Pharmacological evaluation of [11C]donepezil as tracer for visualization of acetylcholinesterase by PET. Nucl. Med. Biol 2000, 27, 745–747. [Google Scholar]
- Szymański, P.; Żurek, E.; Mikiciuk-Olasik, E. New tacrine-hydrazinonicotinamide hybrids as acetylcholinesterase inhibitors of potential interest for the early diagnostics of Alzheimer’s disease. Pharmazie 2006, 61, 269–273. [Google Scholar]
- Szymański, P.; Markowicz, M.; Mikiciuk-Olasik, E. Synthesis and biological activity of derivatives of tetrahydroacridine as acetylcholinesterase inhibitors. Bioorg. Chem 2011, 39, 138–142. [Google Scholar]
- Alvarez, A.; Opazo, C.; Alarcon, R.; Garrido, J.; Inestrosa, N.C. Acetylcholinesterase promotes the aggregation of amyloid-β-peptide fragments by forming a complex with the growing fibrils. J. Mol. Biol 1997, 272, 348–361. [Google Scholar]
- Alvarez, A.; Alarcon, R.; Opazo, C.; Campos, E.O.; Munoz, F.J.; Calderon, F.H.; Dajas, F.; Gentry, M.K.; Doctor, B.P.; de Mello, F.G.; et al. Stable complexes involving acetylcholinesterase and amyloid-β peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer’s fibrils. J. Neurosci 1998, 18, 3213–3223. [Google Scholar]
- Inagaki, K.; Morita, T.; Fujii, K.; Kosaka, N.; Hara, T. Mediastinal lymph node staging in non-small cell lung cancer using PET with C-11 choline and F-18 FDG. J. Nucl. Med 1999, 40, 59–60. [Google Scholar]
- Hara, T.; Kosaka, N.; Kondo, T.; Kishi, H.; Kobori, O. Imaging of brain tumor, lung cancer, esophagus cancer, colon cancer, prostate cancer, and bladder cancer with [C-11]choline. J. Nucl. Med 1997, 38, 250. [Google Scholar]
- Reske, S.N.; Blumstein, N.M.; Neumaier, B.; Gottfried, H.W.; Finsterbusch, F.; Kocot, D.; Möller, P.; Glatting, G.; Perner, S. Imaging prostate cancer with 11C-choline PET/CT. J. Nucl. Med 2006, 47, 1249–1254. [Google Scholar]
- Giovacchini, G.; Picchio, M.; Coradeschi, E.; Scattoni, V.; Bettinardi, V.; Cozzarini, C.; Freschi, M.; Fazio, F.; Messa, C. [11C]Choline uptake with PET/CT for the initial diagnosis of prostate cancer: Relation to PSA levels, tumour stage and anti-androgenic therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1065–1073. [Google Scholar]
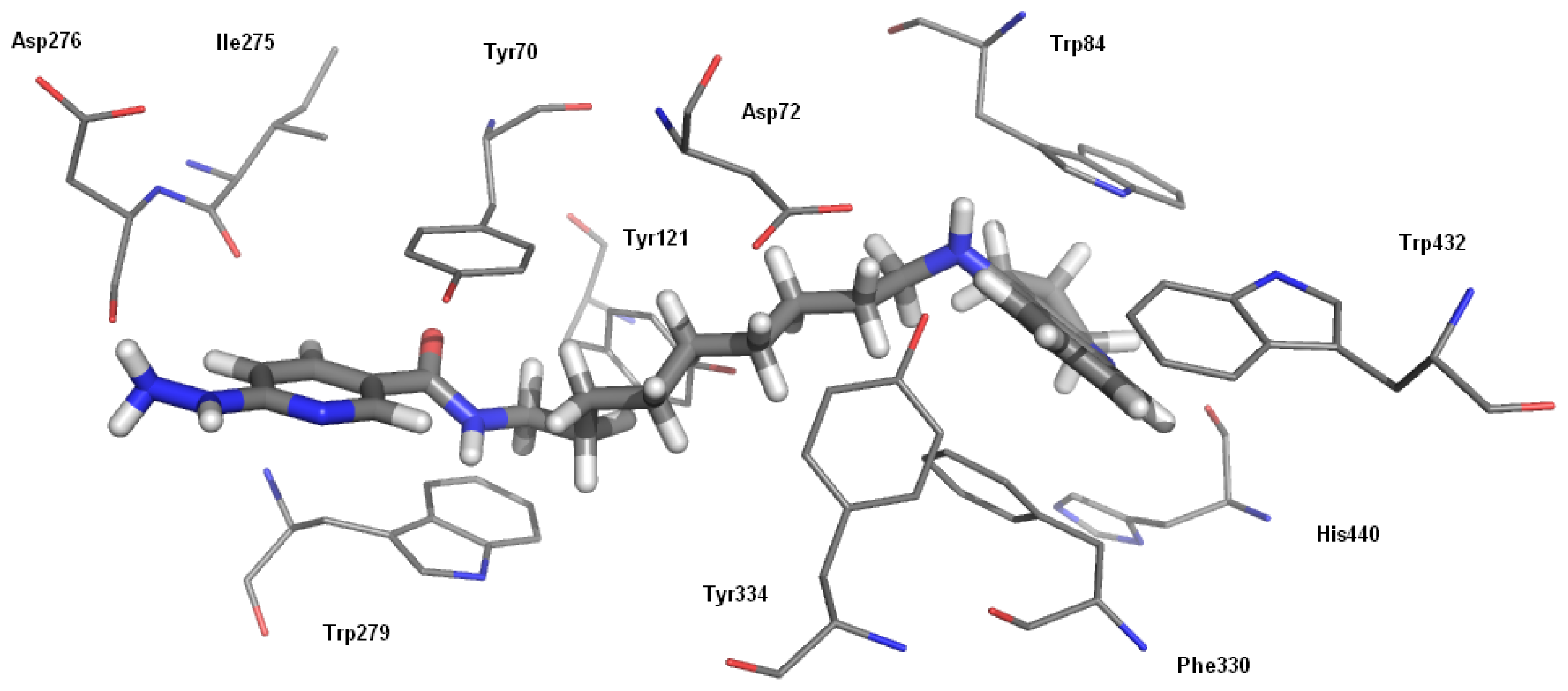
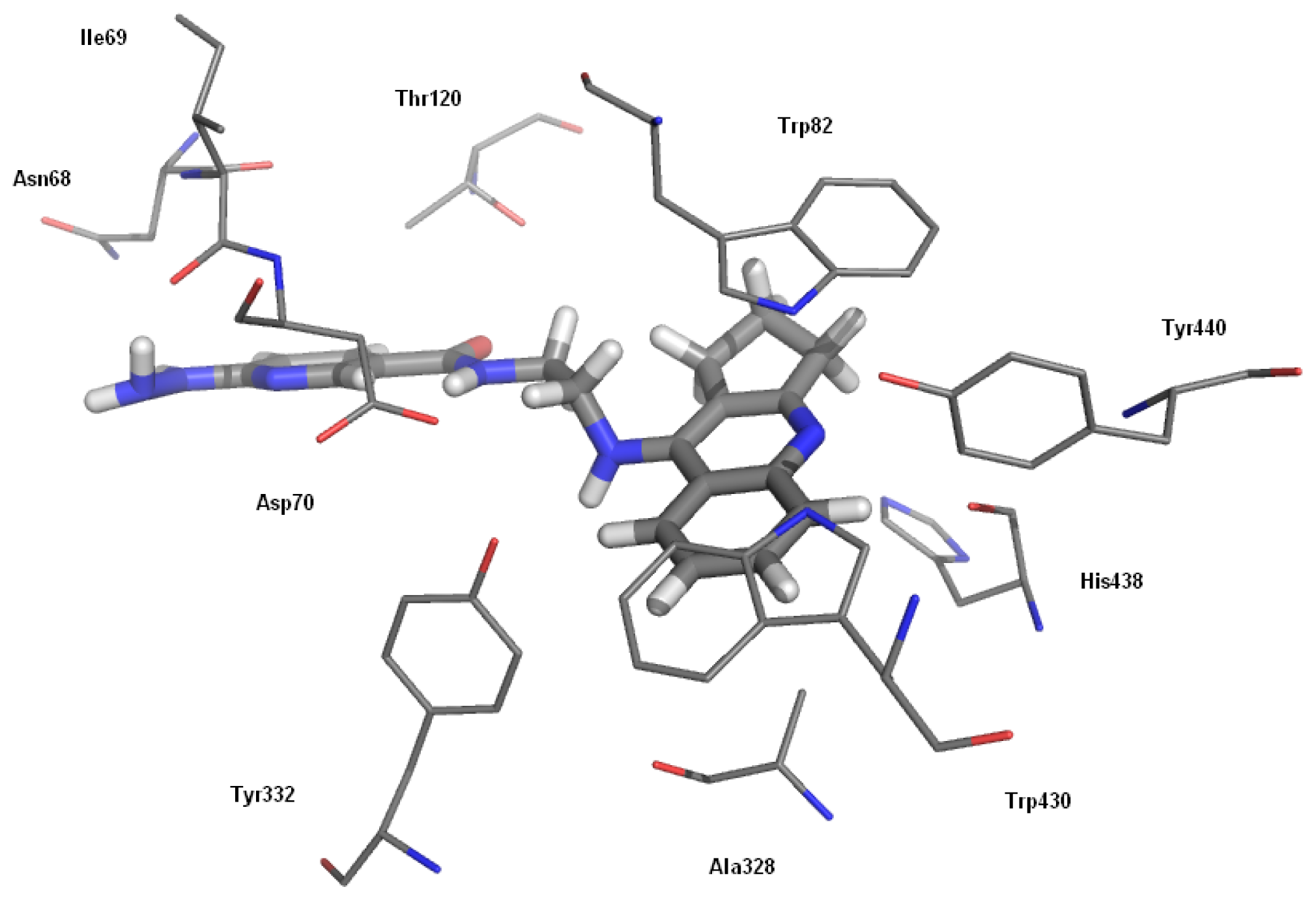




| Compound | AChE IC50, (nM) ± SEM a | BChE IC50, (nM) ± SEM b | Selectivity for AChE c | Selectivity for BChE d |
|---|---|---|---|---|
| 6a | 31.50 ± 1.9 | 164.00 ± 1.6 | 5.21 | 0.19 |
| 6b | 19.30 ± 1.1 | 197.00 ± 3.4 | 10.21 | 0.10 |
| 6c | 22.40 ± 1.7 | 2650.00 ± 9.5 | 118.30 | 0.01 |
| 6d | 7.84 ± 2.2 | 4240.00 ± 2.2 | 540.82 | 0.00 |
| 6e | 41.60 ± 1.0 | 16600.00 ± 17.5 | 399.04 | 0.00 |
| 6f | 17.60 ± 0.8 | 22700.00 ± 20.6 | 1289.77 | 0.00 |
| 6g | 5.17 ± 1.4 | 19600.00 ± 16.4 | 3791.10 | 0.00 |
| 6h | 3.65 ± 0.5 | 17100.00 ± 21.3 | 4684.93 | 0.00 |
| tacrine | 5.46 ± 1.0 | 2.44 ± 0.6 | 0.45 | 2.24 |
| 99mTc-6a (% Dose/g) | ||||
|---|---|---|---|---|
| Organs | 5 min | 60 min | 120 min | 24 h |
| Blood | 0.850 ± 0.108 | 0.114 ± 0.015 | 0.091 ± 0.017 | 0.033 ± 0.004 |
| Plasma | 0.921 ± 0.124 | 0.183 ± 0.024 | 0.150 ± 0.027 | 0.052 ± 0.003 |
| Pancreas | 0.551 ± 0.109 | 0.117 ± 0.019 | 0.092 ± 0.015 | 0.067 ± 0.006 |
| Liver | 5.752 ± 0.742 | 5.160 ± 0.586 | 5.630 ± 0.508 | 5.095 ± 0.478 |
| Adrenals | 1.186 ± 0.231 | 0.900 ± 0.102 | 0.890 ± 0.040 | 0.793 ± 0.137 |
| Kidney | 3.838 ± 0.264 | 1.556 ± 0.148 | 1.332 ± 0.209 | 1.026 ± 0.130 |
| Lung | 3.497 ± 0.239 | 2.010 ± 0.323 | 1.824 ± 0.438 | 1.354 ± 0.066 |
| Heart | 0.779 ± 0.078 | 0.241 ± 0.013 | 0.204 ± 0.025 | 0.147 ± 0.009 |
| Spleen | 0.999 ± 0.218 | 1.840 ± 0.618 | 2.172 ± 0.391 | 2.631 ± 0.199 |
| Stomach | 0.206 ± 0.044 | 0.469 ± 0.438 | 0.311 ± 0.153 | 0.571 ± 0.389 |
| Intestine | 0.977 ± 0.313 | 4.242 ± 0.113 | 3.661 ± 1.491 | 0.196 ± 0.055 |
| Colon | 0.108 ± 0.031 | 0.044 ± 0.003 | 1.566 ± 1.539 | 1.809 ± 0.976 |
| Testes | 0.054 ± 0.004 | 0.033 ± 0.001 | 0.030 ± 0.005 | 0.022 ± 0.003 |
| Skin | 0.290 ± 0.039 | 0.196 ± 0.017 | 0.169 ± 0.029 | 0.100 ± 0.010 |
| Muscle | 0.157 ± 0.004 | 0.056 ± 0.007 | 0.048 ± 0.006 | 0.037 ± 0.005 |
| Thyroid | 0.551 ± 0.040 | 0.225 ± 0.021 | 0.189 ± 0.009 | 0.124 ± 0.037 |
| Brain | 0.049 ± 0.003 | 0.013 ± 0.001 | 0.014 ± 0.003 | 0.007 ± 0.001 |
| Fat | 0.305 ± 0.060 | 0.120 ± 0.019 | 0.105 ± 0.017 | 0.070 ± 0.017 |
| Femur | 0.330 ± 0.036 | 0.202 ± 0.015 | 0.218 ± 0.031 | 0.232 ± 0.037 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Szymański, P.; Lázničková, A.; Lázniček, M.; Bajda, M.; Malawska, B.; Markowicz, M.; Mikiciuk-Olasik, E. 2,3-Dihydro-1H-cyclopenta[b]quinoline Derivatives as Acetylcholinesterase Inhibitors—Synthesis, Radiolabeling and Biodistribution. Int. J. Mol. Sci. 2012, 13, 10067-10090. https://doi.org/10.3390/ijms130810067
Szymański P, Lázničková A, Lázniček M, Bajda M, Malawska B, Markowicz M, Mikiciuk-Olasik E. 2,3-Dihydro-1H-cyclopenta[b]quinoline Derivatives as Acetylcholinesterase Inhibitors—Synthesis, Radiolabeling and Biodistribution. International Journal of Molecular Sciences. 2012; 13(8):10067-10090. https://doi.org/10.3390/ijms130810067
Chicago/Turabian StyleSzymański, Paweł, Alice Lázničková, Milan Lázniček, Marek Bajda, Barbara Malawska, Magdalena Markowicz, and Elżbieta Mikiciuk-Olasik. 2012. "2,3-Dihydro-1H-cyclopenta[b]quinoline Derivatives as Acetylcholinesterase Inhibitors—Synthesis, Radiolabeling and Biodistribution" International Journal of Molecular Sciences 13, no. 8: 10067-10090. https://doi.org/10.3390/ijms130810067