Reflection and Transmission Coefficient of Yttrium Iron Garnet Filled Polyvinylidene Fluoride Composite Using Rectangular Waveguide at Microwave Frequencies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Powder X-ray Diffraction
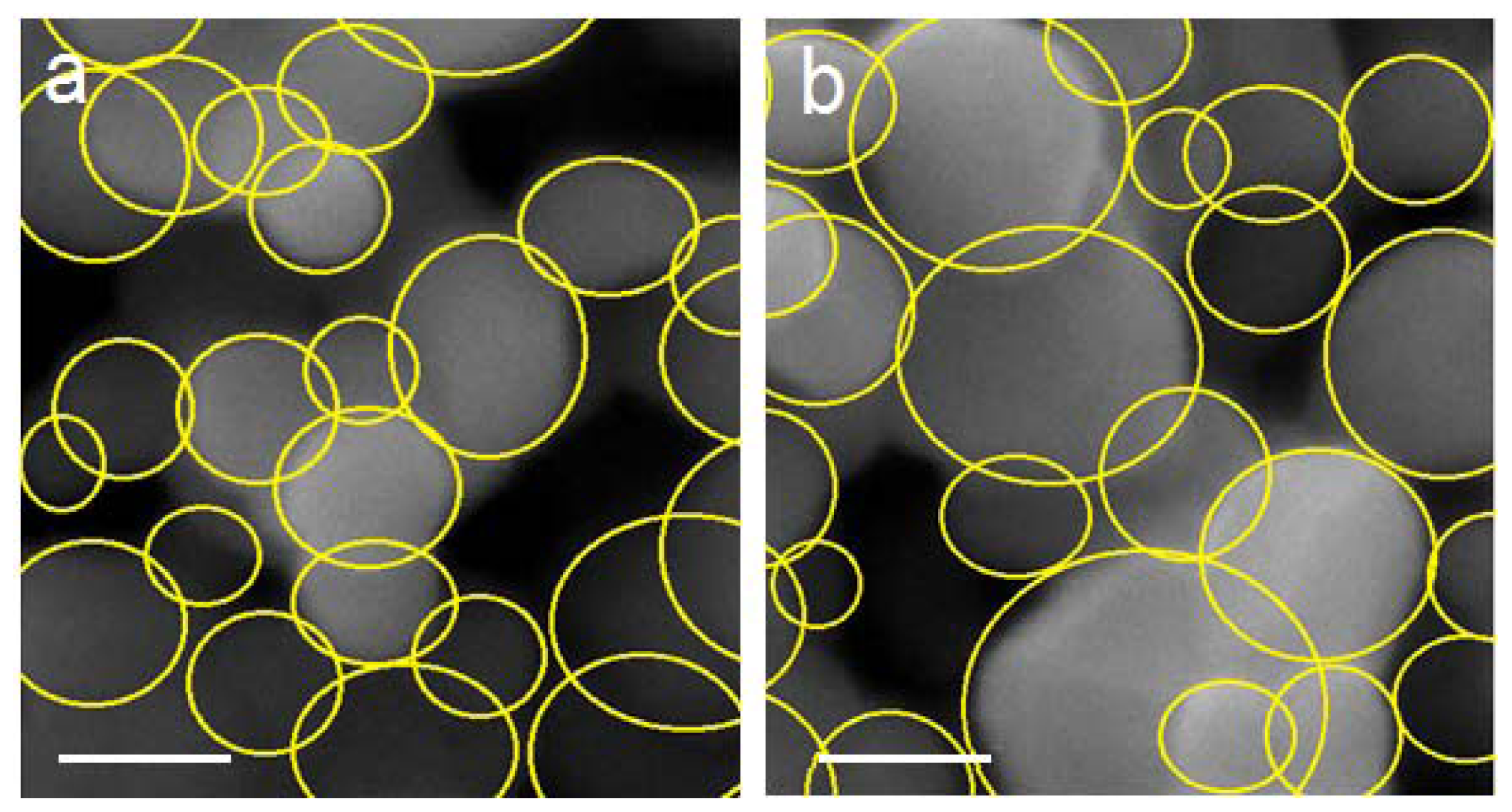
2.2. Surface Morphology Study
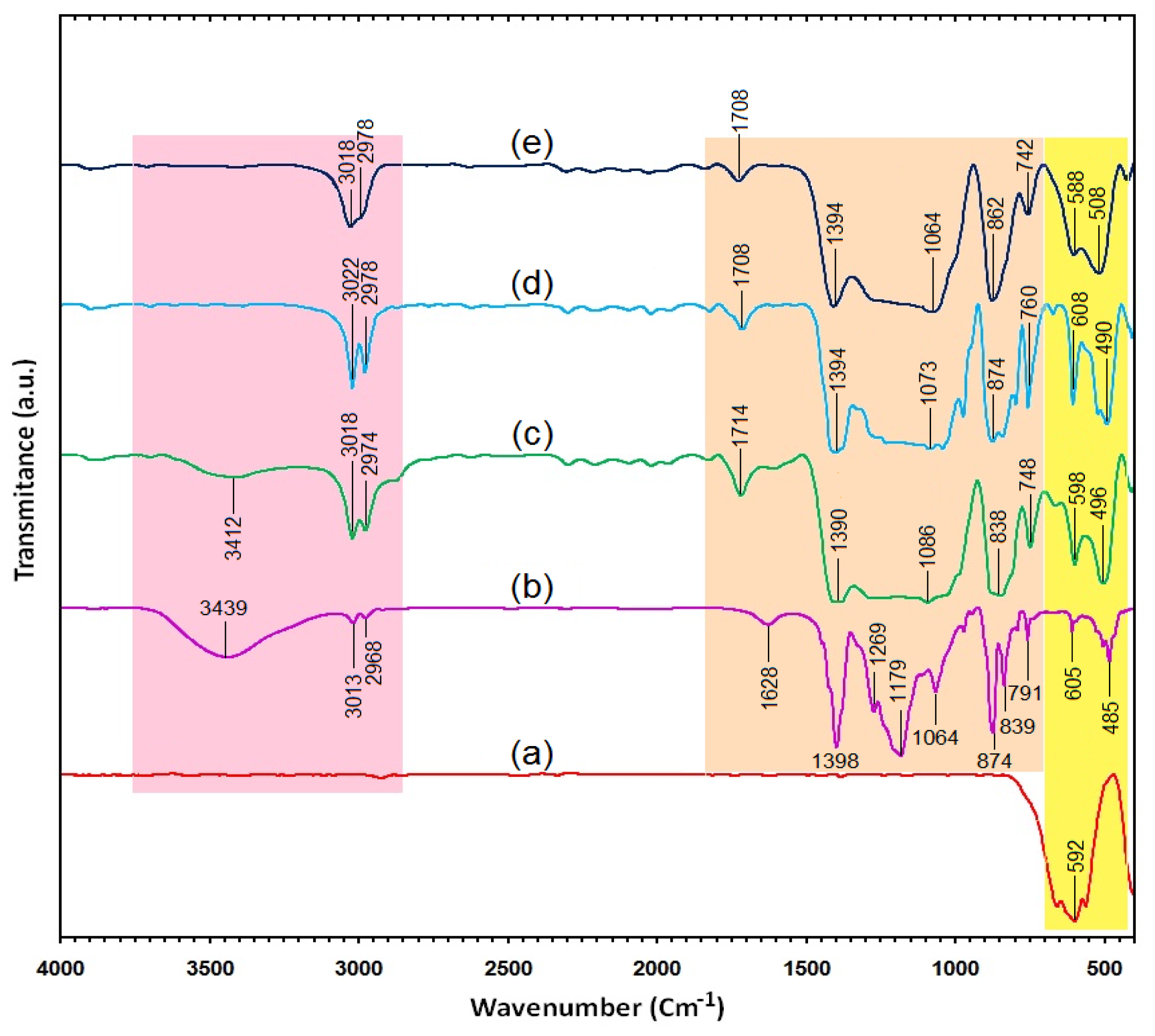
2.3. Surface Chemistry (FT-IR)
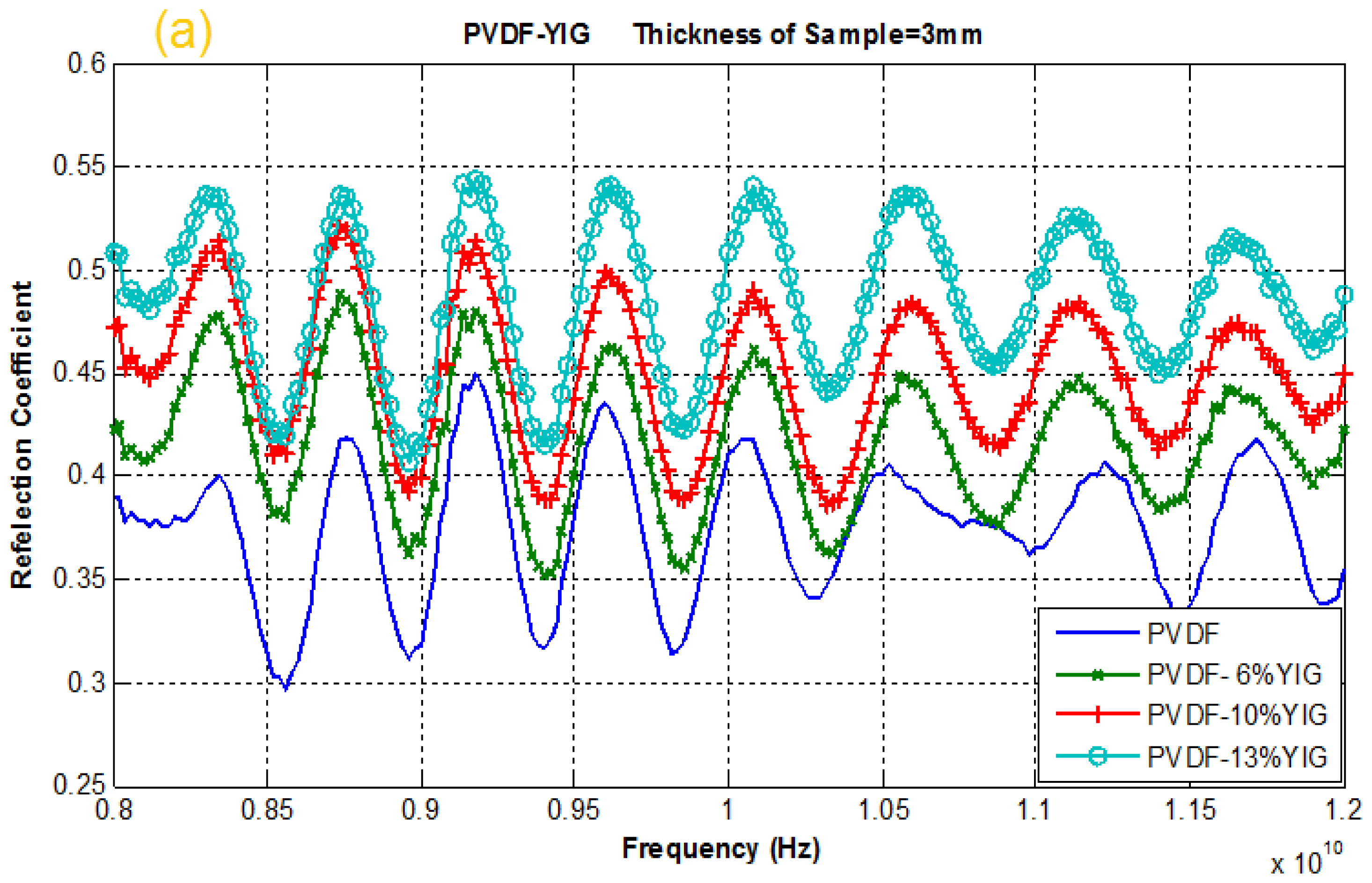
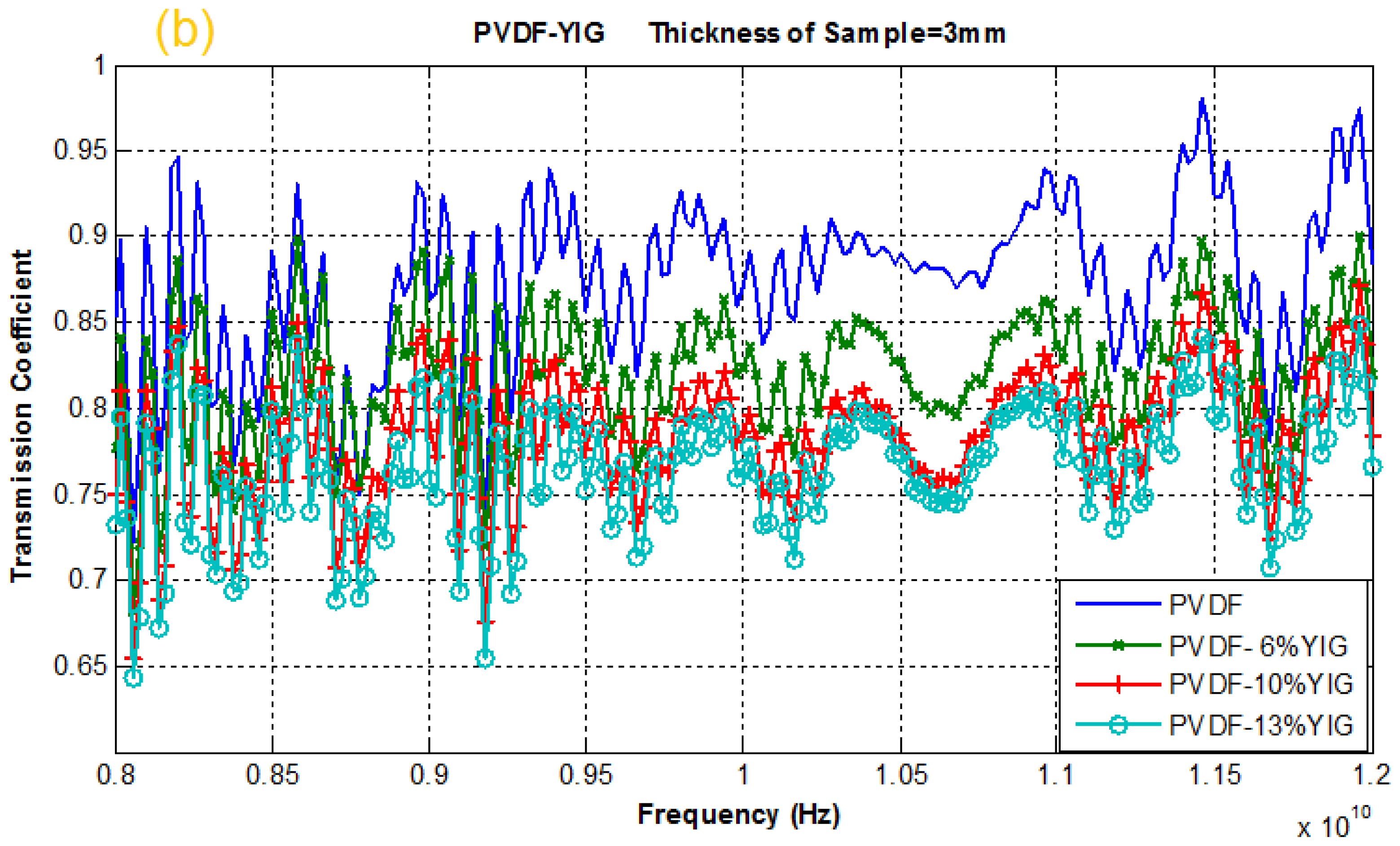
2.4. Reflection and Transmission Coefficient
3. Experimental Section
3.1. Materials and Method
3.2. Characterization
4. Conclusions
Acknowledgments
References
- Toneguzzo, P.; Acher, O.; Viau, G.; Fievet-Vincent, F.; Fievet, F. Observations of exchange resonance modes on submicrometer sized ferromagnetic particles. J. Appl. Phys 1997, 81, 5546–5548. [Google Scholar]
- Pardavi-Horvath, M. Characterization of nanostructured magneticmaterials. J. Magn. Magn. Mater 1999, 203, 57–59. [Google Scholar]
- Wu, Y.J.; Fu, H.P.; Hong, R.Y.; Zheng, Y.; Wei, D.G. Influence of surfactants on co-precipitation synthesis of Bi-YIG particles. J. Alloys Compd 2009, 470, 497–501. [Google Scholar]
- Vaishnava, P.P.; Senaratne, U.; Buc, E.; Naik, R.; Naik, V.M.; Tsoi, G.; Wenger, L.E.; Boolchand, P. Magnetic properties of cobalt-ferrite nanoparticles embedded in polystyrene resin. J. Appl. Phys 2006, 99, 702–703. [Google Scholar]
- Nakamura, T.; Tsutaoka, T.; Hatakeyama, K. Frequency dispersion of permeability in ferrite composite. J. Magn. Magn. Mater 1994, 138, 319–328. [Google Scholar]
- Yavuz, O.; Ram, M.K.; Aldissi, M.; Poddar, P.; Hariharan, S. Synthesis and the physical properties of MnZn ferrite and NiMnZn ferrite-polyaniline nanocomposite particles. J. Mater. Chem 2005, 15, 810–817. [Google Scholar]
- Kazantseva, N.E.; Bespyatykh, Y.I.; Sapurina, I.; Stejskal, J.; Vilcáková, J.; Sáha, P. Magnetic materials based on manganese-zinc ferrite with surface-organized polyaniline coating. J. Magn. Magn. Mater 2006, 301, 155–165. [Google Scholar]
- Suwanboon, S. Structural and optical properties of nanocrystalline ZnO powder from sol-gel method. Sci. Asia 2008, 34, 31–34. [Google Scholar]
- Lee, J.; Easteal, A.J.; Pal, U.; Bhattacharyya, D. Evaluation of ZnO nanostructures in sol-gel synthesis. Curr. Appl. Phys 2009, 9, 792–796. [Google Scholar]
- Vafaee, M.; Ghamsari, M.S. Preparation and characterization of ZnO nanoparticles by a novel sol-gel route. Mater. Lett 2007, 61, 3265–3268. [Google Scholar]
- Ligthart, L.P. A fast computational technique for accurate permittivity determination using transmission line methods. IEEE Trans. Microw. Theory Tech 1983, 31, 249–254. [Google Scholar]
- Dang, Z.M.; Nan, C.W. Dielectric properties of LTNO ceramics and LTNO/PVDF composites. Ceram. Int 2005, 31, 349–351. [Google Scholar]
- Judovits, L. Thermal analysis of poly(vinylidene fluoride) film. Thermochim. Acta 2006, 442, 92–94. [Google Scholar]
- Dang, Z.M.; Wu, J.B.; Fan, L.Z.; Nan, C.W. Dielectric behavior of Li and Ti co-doped NiO/PVDF composite. Chem. Phys. Lett 2003, 376, 389–394. [Google Scholar]
- Zak, A.K.; Gan, W.C.; Majid, W.H.A.; Velayutham, T.S. Experimental and theoretical dielectric studies of PVDF/PZT nanocomposite thin films. Ceram. Int 2011, 37, 1653–1660. [Google Scholar]
- Vaqueiro, P.; Crosnier-Lopez, M.P.; López-Quintela, M.A. Synthesis and characterization of Yttrium iron garnet nanoparticles. J. Solid State Chem 1996, 126, 161–168. [Google Scholar]
- Hussein, M.Z.B.; Ghotbi, M.Y.; Yahaya, A.H.; Abd Rahman, M.Z. Synthesis and characterization of (zinc-layered-gallate) nanohybrid using structural memory effect. Mater. Chem. Phys 2009, 113, 491–496. [Google Scholar]
- Wang, P.; Wang, Z.; Wu, Z.; Zhou, Q.; Yang, D. Effect of hypochlorite cleaning on the physiochemical characteristics of polyvinylidene fluoride membranes. Chem. Eng. J 2010, 162, 1050–1056. [Google Scholar]
- Aravindan, V.; Vickraman, P.; Prem Kumar, T. Polyvinylidene fluoride-hexafluoropropylene (PVdF-HFP)-based composite polymer electrolyte containing LiPF3(CF3CF2)3. J. Non Cryst. Solids 2008, 354, 3451–3457. [Google Scholar]
- Zhao, X.; Song, L.; Fu, J.; Tang, P.; Liu, F. Experimental and DFT investigation of surface degradation of polyvinylidene fluoride membrane in alkaline solution. Surf. Sci 2011, 605, 1005–1015. [Google Scholar]
- Solaimani, H.; Abbas, Z.; Yahya, N.; Soleimani, H.; Yeganeh-Gotbi, M. Determination of complex permittivity and permeability of lanthanum iron garnet filled PVDF-polymer composite using rectangular waveguide and Nicholson-Ross-Weir (NRW) method at X-band frequencies. Measurement 2012, 45, 1621–1625. [Google Scholar]
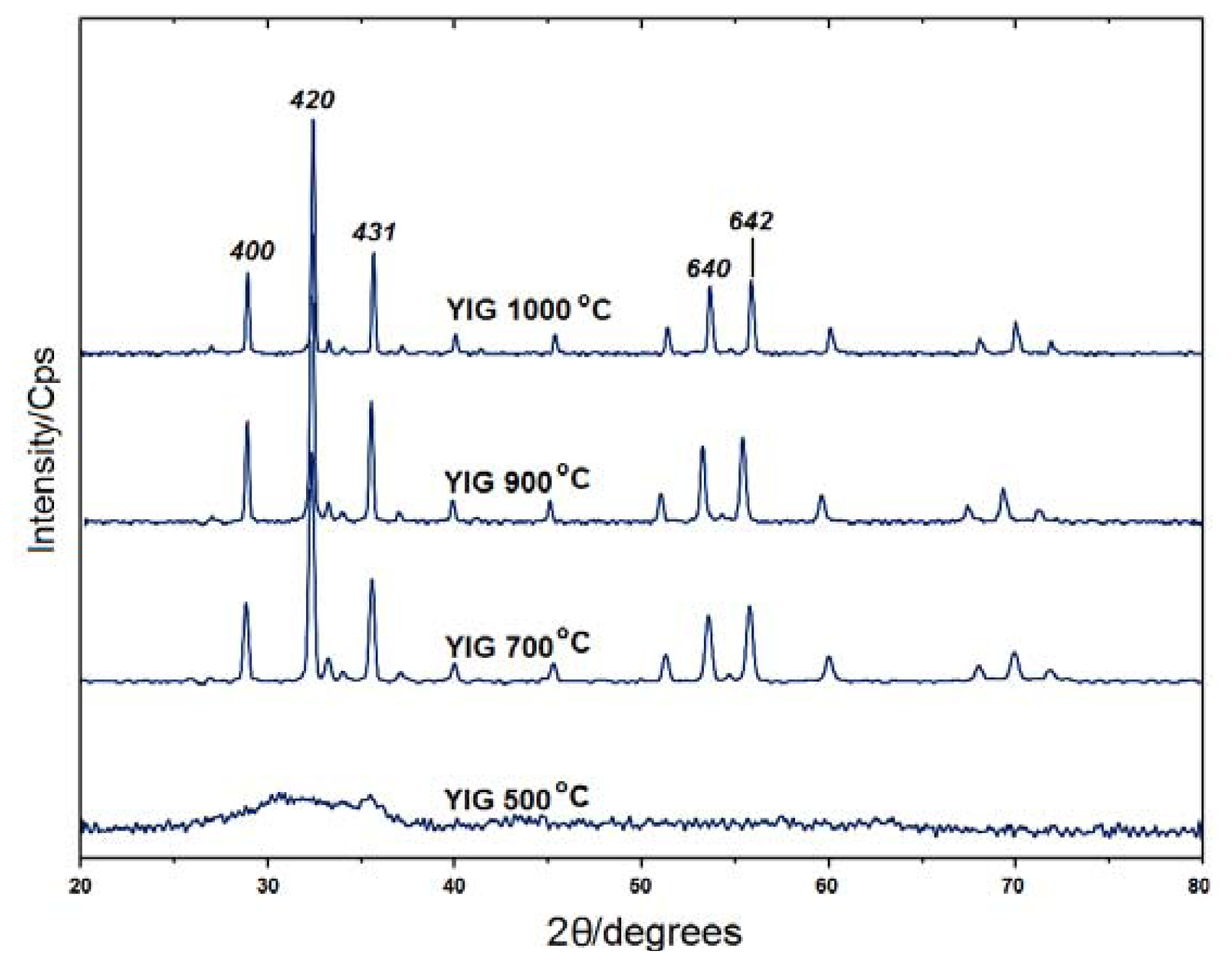
| Temperature | 2θ | d-Spacing (A) | FWHM | Crystallite Size D (nm)a |
|---|---|---|---|---|
| 500 °C | * | * | * | * |
| 700 °C | 32.3280 | 2.76930 | 0.1624 | 53.24 |
| 900 °C | 32.3592 | 2.76670 | 0.1624 | 53.35 |
| 1000 °C | 32.3441 | 2.76795 | 0.1299 | 66.56 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Soleimani, H.; Abbas, Z.; Yahya, N.; Shameli, K.; Soleimani, H.; Shabanzadeh, P. Reflection and Transmission Coefficient of Yttrium Iron Garnet Filled Polyvinylidene Fluoride Composite Using Rectangular Waveguide at Microwave Frequencies. Int. J. Mol. Sci. 2012, 13, 8540-8548. https://doi.org/10.3390/ijms13078540
Soleimani H, Abbas Z, Yahya N, Shameli K, Soleimani H, Shabanzadeh P. Reflection and Transmission Coefficient of Yttrium Iron Garnet Filled Polyvinylidene Fluoride Composite Using Rectangular Waveguide at Microwave Frequencies. International Journal of Molecular Sciences. 2012; 13(7):8540-8548. https://doi.org/10.3390/ijms13078540
Chicago/Turabian StyleSoleimani, Hassan, Zulkifly Abbas, Noorhana Yahya, Kamyar Shameli, Hojjatollah Soleimani, and Parvaneh Shabanzadeh. 2012. "Reflection and Transmission Coefficient of Yttrium Iron Garnet Filled Polyvinylidene Fluoride Composite Using Rectangular Waveguide at Microwave Frequencies" International Journal of Molecular Sciences 13, no. 7: 8540-8548. https://doi.org/10.3390/ijms13078540




