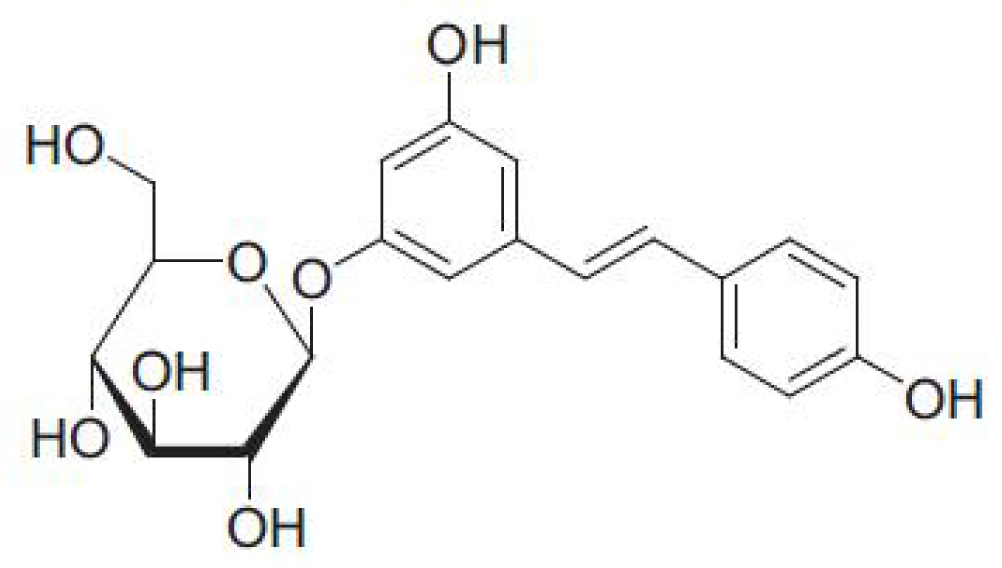
Polydatin Attenuates Hypoxic Pulmonary Hypertension and Reverses Remodeling through Protein Kinase C Mechanisms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hemodynamics during Hypopiesia and Hypoxia
2.2. Polydatin Attenuates Pulmonary Artery Remodeling and Right Ventricular Hypertrophy
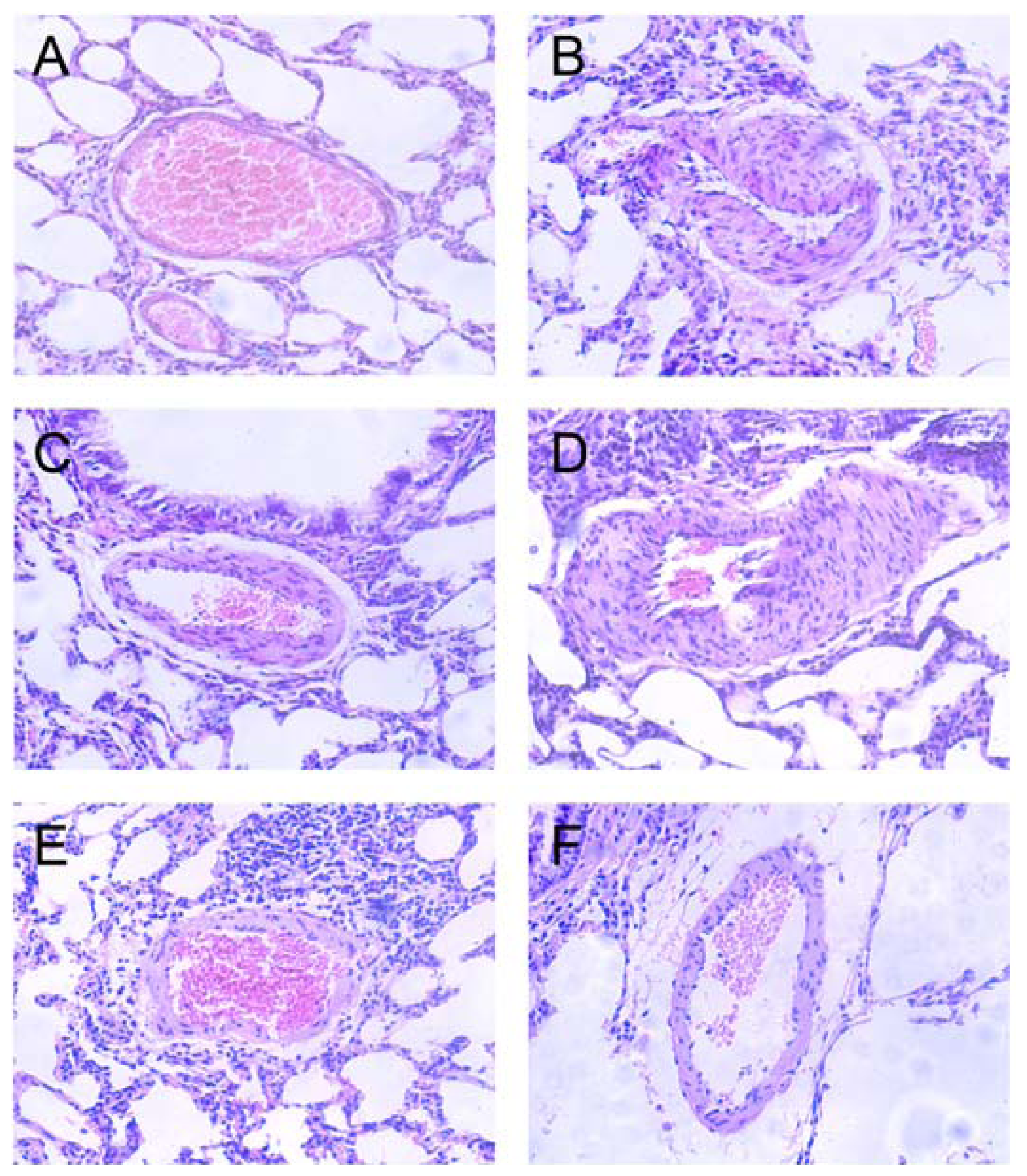
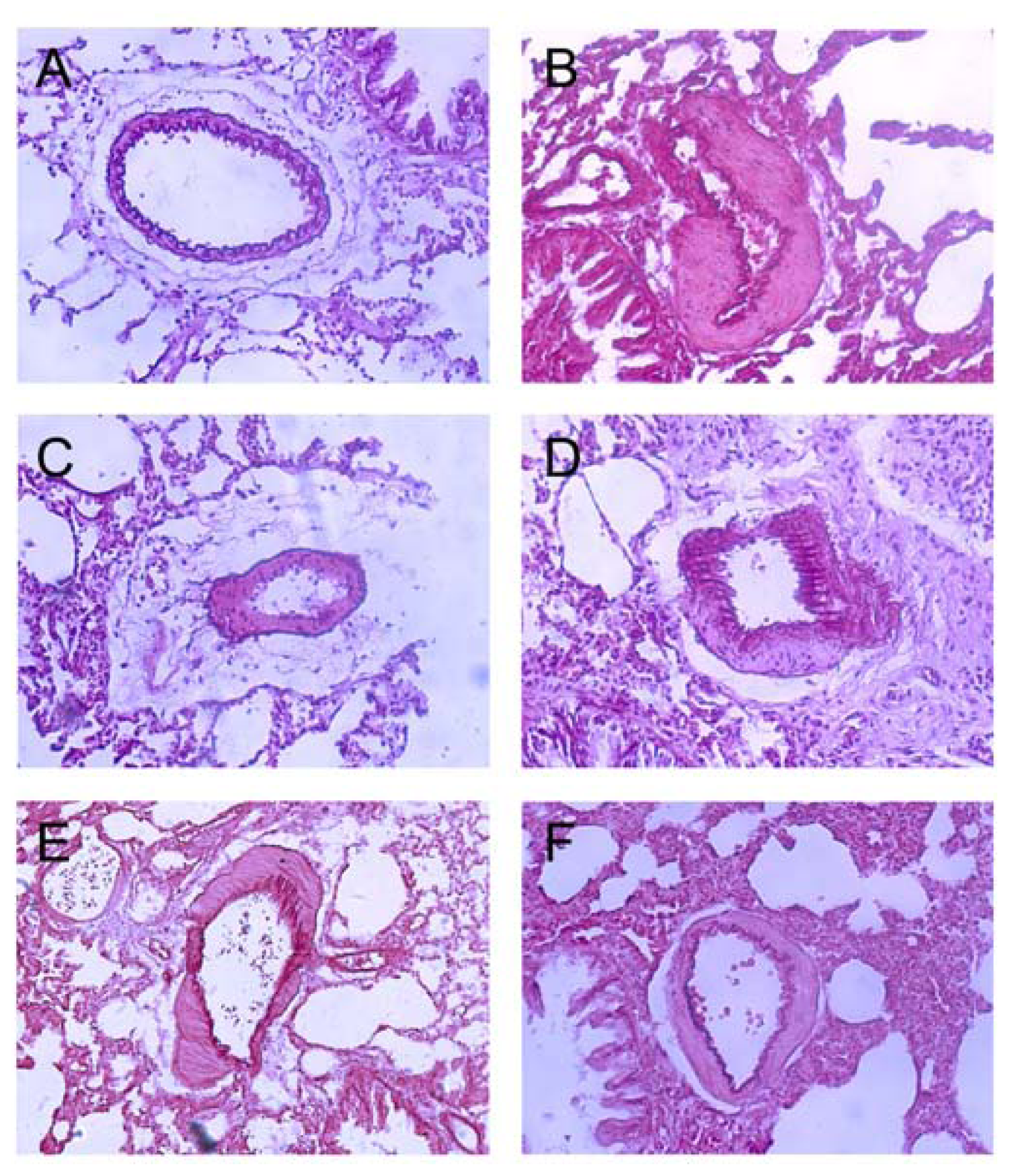
2.3. Effects of Polydatin on Pulmonary Artery Morphology
2.4. Polydatin Reverses Pulmonary Artery Remodeling
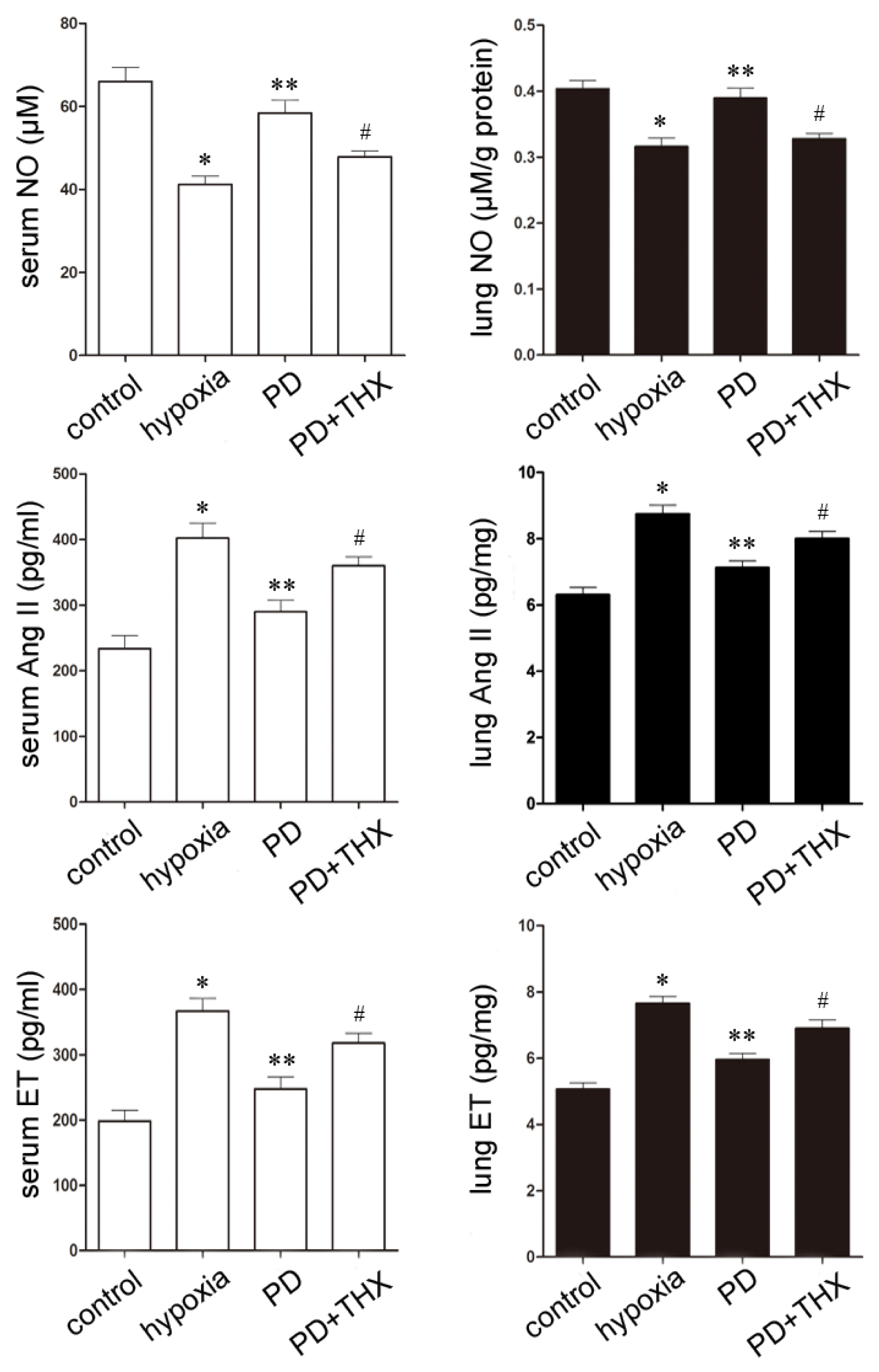
2.5. Effects of Polydatin on NO, Ang II and ET
2.6. Effect of Polydatin Is PKC-Dependent
3. Experimental Section
3.1. Reagents and Animals
3.2. Animal Groups and Models
3.3. Measurement of Hemodynamics and Right Ventricular Hypertrophy
3.4. Pulmonary Artery Histology
3.5. Forced Activation of PKC
3.6. Serum NO, ET and Ang II Assays
3.7. Lung Tissue NO, ET and Ang II Assays
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Berger, M.M.; Dehnert, C.; Bailey, D.M.; Luks, A.M.; Menold, E.; Castell, C.; Schendler, G.; Faoro, V.; Mairbäurl, H.; Bärtsch, P.; Swenson, E.R. Transpulmonary plasma ET-1 and nitrite differences in high altitude pulmonary hypertension. High Alt. Med. Biol 2009, 10, 17–24. [Google Scholar]
- Bärtsch, P.; Shaw, S.; Franciolli, M.; Gnädinger, M.P.; Weidmann, P. Atrial natriuretic peptide in acute mountain sickness. J. Appl. Physiol 1988, 65, 1929–1937. [Google Scholar]
- Berger, M.M.; Hesse, C.; Dehnert, C.; Siedler, H.; Kleinbongard, P.; Bardenheuer, H.J.; Kelm, M.; Bärtsch, P.; Haefeli, W.E. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med 2005, 172, 763–767. [Google Scholar]
- Bärtsch, P.; Maggiorini, M.; Ritter, M.; Noti, C.; Vock, P.; Oelz, O. Prevention of high-altitude pulmonary edema by nifedipine. N. Engl. J. Med 1991, 325, 1284–1289. [Google Scholar]
- Swenson, E.R.; Maggiorini, M.; Mongovin, S.; Gibbs, J.S.; Greve, I.; Mairbäurl, H.; Bärtsch, P. Pathogenesis of high-altitude pulmonary edema: Inflammation is not an etiologic factor. J. Am. Med. Assoc 2002, 287, 2228–2235. [Google Scholar]
- Maggiorini, M.; Mélot, C.; Pierre, S.; Pfeiffer, F.; Greve, I.; Sartori, C.; Lepori, M.; Hauser, M.; Scherrer, U.; Naeije, R. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 2001, 103, 2078–2083. [Google Scholar]
- Maggiorini, M.; Brunner-La Rocca, H.P.; Peth, S.; Fischler, M.; Böhm, T.; Bernheim, A.; Kiencke, S.; Bloch, K.E.; Dehnert, C.; Naeije, R.; et al. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: A randomized trial. Ann. Intern. Med 2006, 145, 497–506. [Google Scholar]
- Du, J.; Sun, L.N.; Xing, W.W.; Huang, B.K.; Jia, M.; Wu, J.Z.; Zhang, H.; Qin, L.P. Lipid-lowering effects of polydatin from Polygonum cuspidatum in hyperlipidemic hamsters. Phytomedicine 2009, 16, 652–658. [Google Scholar]
- Kerem, Z.; Bilkis, I.; Flaishman, M.A.; Sivan, L. Antioxidant activity and inhibition of alpha-glucosidase by trans-resveratrol, piceid, and a novel trans-stilbene from the roots of Israili Rumex bucephalophorus L. J. Agric. Food Chem 2006, 544, 1243–1247. [Google Scholar]
- Sheng, C.; Yu, Y.H.; Zhao, K.S.; Qin, W.; Wang, C.H. Hypotensive resuscitation combined with polydatin improve microcirculation and survival in a rabbit model of uncontrolled hemorrhagic shock in pregnancy. J. Surg. Res 2011, 168, 103–110. [Google Scholar]
- Lee, S.H.; Ryu, S.Y.; Kim, H.B.; Kim, M.Y.; Chun, Y.J. Induction of apoptosis by 3,4′-dimethoxy-5-hydroxystilbene in human promyeloid leukemic HL-60 cells. Planta Med 2002, 68, 123–127. [Google Scholar]
- Miao, Q.; Wang, S.; Miao, S.; Wang, J.; Xie, Y.; Yang, Q. Cardioprotective effect of polydatin against ischemia/reperfusion injury: Roles of protein kinase C and mito KATP activation. Phytomedicine 2011, 19, 8–12. [Google Scholar]
- Cheng, Y.; Zhang, H.T.; Sun, L.; Guo, S.; Ouyang, S.; Zhang, Y.; Xu, J. Involvement of cell adhesion molecules in polydatin protection of brain tissues from ischemia-reperfusion injury. Brain Res 2006, 1110, 193–200. [Google Scholar]
- Pain, T.; Yang, X.M.; Critz, S.D.; Yue, Y.; Nakano, A.; Liu, G.S.; Heusch, G.; Cohen, M.V.; Downey, J.M. Opening of mitochondrial K (ATP) channels triggers the preconditioned state by generating free radicals. Circ. Res 2000, 87, 460–466. [Google Scholar]
- Murata, M.; Akao, M.; O’Rourke, B.; Marbán, E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca2+ overload during simulated ischemia and reperfusion: Possible mechanism of cardioprotection. Circ. Res 2001, 89, 891–898. [Google Scholar]
- Yellon, D.M.; Downey, J.M. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol. Rev 2003, 83, 1113–1151. [Google Scholar]
- Parra, V.M.; Macho, P.; Domenech, R.J. Late cardiac preconditioning by exercise in dogs is mediated by mitochondrial potassium channels. J. Cardiovasc. Pharmacol 2010, 56, 268–274. [Google Scholar]
- Das, M.; Dempsey, E.C.; Bouchey, D.; Reyland, M.E.; Stenmark, K.R. Chronic hypoxia induces exaggerated growth responses in pulmonary artery adventitial fibroblasts: Potential contribution of specific protein kinase C isozymes. Am. J. Respir. Cell. Mol. Biol 2000, 22, 15–25. [Google Scholar]
- Barman, S.A.; Zhu, S.; White, R.E. Protein kinase C inhibits BKCa channel activity in pulmonary arterial smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol 2004, 286, L149–L155. [Google Scholar]
- Tuder, R.M.; Cool, C.D.; Yeager, M.; Taraseviciene-Stewart, L.; Bull, T.M.; Voelkel, N.F. The pathobiology of pulmonary hypertension. Endothelium. Clin. Chest Med 2001, 22, 405–418. [Google Scholar]
- Cacoub, P.; Dorent, R.; Nataf, P.; Carayon, A.; Riquet, M.; Noe, E.; Piette, J.C.; Godeau, P.; Gandjbakhch, I. Endothelin-1 in the lungs of patients with pulmonary hypertension. Cardiovasc. Res 1997, 33, 196–200. [Google Scholar]
- Giaid, A.; Saleh, D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med 1995, 333, 214–221. [Google Scholar]
- Jeffery, T.K.; Wanstall, J.C. Pulmonary vascular remodeling: A target for therapeutic intervention in pulmonary hypertension. Pharmacol. Ther 2001, 92, 1–20. [Google Scholar]
- Orte, C.; Polak, J.M.; Haworth, S.G.; Yacoub, M.H.; Morrell, N.W. Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary plexiform pulmonary hypertension. J. Pathol 2000, 192, 379–384. [Google Scholar]
- Underwood, D.C.; Bochnowicz, S.; Osborn, R.R.; Luttmann, M.A.; Hay, D.W. Nonpeptide endothelin receptor antagonists. X. Inhibition of endothelin-1- and hypoxia-induced pulmonary pressor responses in the guinea pig by the endothelin receptor antagonist, SB 217242. J. Pharmacol. Exp. Ther 1997, 283, 1130–1137. [Google Scholar]
- Goirand, F.; Bardou, M.; Guerard, P.; Dumas, J.P.; Rochette, L.; Dumas, M. ETA, mixed ETA/ETB receptor antagonists, and protein kinase C inhibitor prevent acute hypoxic pulmonary vasoconstriction: Influence of potassium channels. J. Cardiovasc. Pharmacol 2003, 41, 117–125. [Google Scholar]
- Jin, N.; Packer, C.S.; Rhoades, R.A. Pulmonary arterial hypoxic contraction: Signal transduction. Am. J. Physiol. Lung Cell. Mol. Physiol 1992, 263, L73–L78. [Google Scholar]
- Weissmann, N.; Voswinckel, R.; Hardebusch, T.; Rosseau, S.; Ghofrani, H.A.; Schermuly, R.; Seeger, W.; Grimminger, F. Evidence for a role of protein kinase C in hypoxic pulmonary vasoconstriction. Am. J. Physiol. Lung Cell. Mol. Physiol 1999, 276, L90–L95. [Google Scholar]
- Tsai, B.M.; Wang, M.; Pitcher, J.M.; Meldrum, K.K.; Meldrum, D.R. Hypoxic pulmonary vasoconstriction and pulmonary artery tissue cytokine expression are mediated by protein kinase C. Am. J. Physiol. Lung Cell. Mol. Physiol 2004, 287, L1215–L1219. [Google Scholar]
- Krotova, K.Y.; Zharikov, S.I.; Block, E.R. Classical isoforms of PKC as regulators of CAT-1 transporter activity in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol 2003, 284, L1037–L1044. [Google Scholar]
- Michelakis, E.D.; McMurtry, M.S.; Wu, X.C.; Dyck, J.R.; Moudgil, R.; Hopkins, T.A.; Lopaschuk, G.D.; Puttagunta, L.; Waite, R.; Archer, S.L. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: Role of increased expression and activity of voltage-gated potassium channels. Circulation 2002, 105, 244–250. [Google Scholar]
- Li, J.; Zhang, P.; Zhang, Q.Y.; Zhang, S.M.; Guo, H.T.; Bi, H.; Wang, Y.M.; Sun, X.; Liu, J.C.; Cheng, L.; et al. Effects of U50,488H on hypoxia pulmonary hypertension and its underlying mechanism. Vascul. Pharmacol 2009, 51, 72–77. [Google Scholar]
- Peng, P.; Huang, L.Y.; Li, J.; Fan, R.; Zhang, S.M.; Wang, Y.M.; Hu, Y.Z.; Sun, X.; Kaye, A.D.; Pei, J.M. Distribution of kappa-opioid receptor in the pulmonary artery and its changes during hypoxia. Anat. Rec 2009, 292, 1062–1067. [Google Scholar]
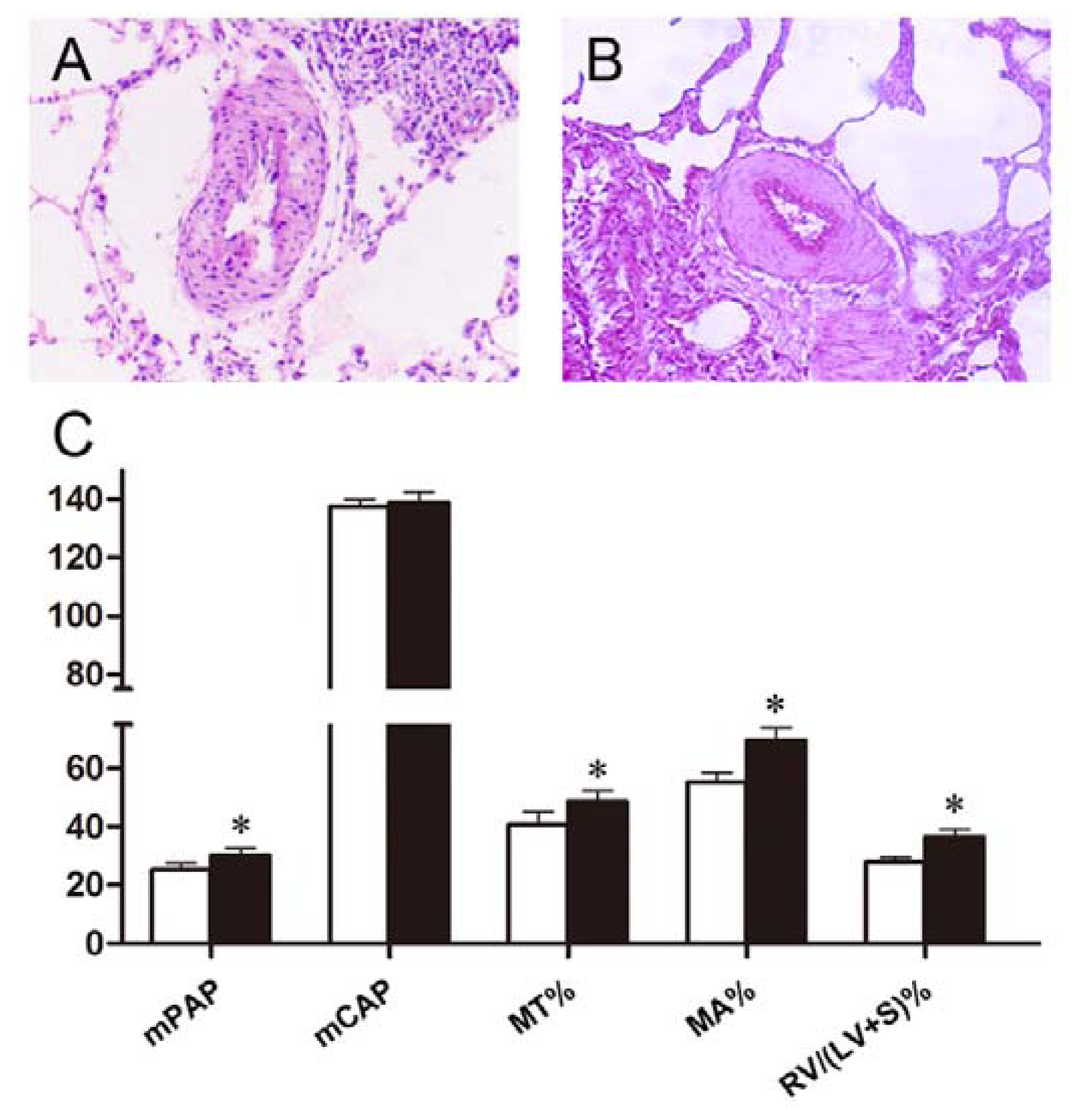
| mPAP (mmHg) | mCAP (mmHg) | |
|---|---|---|
| control | 18.74 ± 1.74 | 138.05 ± 3.55 |
| hypoxia | 32.93 ± 3.08 * | 140.15 ± 5.81 |
| silaenafil | 26.08 ± 3.93 ** | 138.20 ± 2.77 |
| 5 mg/kg PD | 30.34 ± 2.19 | 139.48 ± 4.27 |
| 10 mg/kg PD | 27.71 ± 2.61 ** | 138.72 ± 3.22 |
| 20 mg/kg PD | 25.21 ± 2.40 ** | 137.67 ± 4.53 |
| MT% | MA% | RV/(LV + S)% | RV/BW (mg/g) | |
|---|---|---|---|---|
| control | 31.63 ± 2.66 | 43.54 ± 3.17 | 22.20 ± 1.21 | 0.56 ± 0.08 |
| hypoxia | 50.72 ± 4.50 * | 72.99 ± 4.47 * | 37.67 ± 2.57 * | 0.92 ± 0.14 * |
| silaenafil | 39.28 ± 5.26 ** | 49.84 ± 6.34 ** | 25.57 ± 2.57 ** | 0.63 ± 0.13 ** |
| 5 mg/kg PD | 46.27 ± 3.88 | 68.85 ± 3.26 | 34.23 ± 1.92 ** | 0.80 ± 0.17 |
| 10 mg/kg PD | 45.01 ± 4.25 ** | 58.85 ± 4.74 ** | 30.63 ± 1.44 ** | 0.75 ± 0.13 ** |
| 20 mg/kg PD | 40.75 ± 4.38 ** | 55.27 ± 3.41 ** | 27.87 ± 1.48 ** | 0.73 ± 0.12 ** |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miao, Q.; Shi, X.-P.; Ye, M.-X.; Zhang, J.; Miao, S.; Wang, S.-W.; Li, B.; Jiang, X.-X.; Zhang, S.; Hu, N.; et al. Polydatin Attenuates Hypoxic Pulmonary Hypertension and Reverses Remodeling through Protein Kinase C Mechanisms. Int. J. Mol. Sci. 2012, 13, 7776-7787. https://doi.org/10.3390/ijms13067776
Miao Q, Shi X-P, Ye M-X, Zhang J, Miao S, Wang S-W, Li B, Jiang X-X, Zhang S, Hu N, et al. Polydatin Attenuates Hypoxic Pulmonary Hypertension and Reverses Remodeling through Protein Kinase C Mechanisms. International Journal of Molecular Sciences. 2012; 13(6):7776-7787. https://doi.org/10.3390/ijms13067776
Chicago/Turabian StyleMiao, Qing, Xiao-Peng Shi, Ming-Xiang Ye, Jin Zhang, Shan Miao, Si-Wang Wang, Bo Li, Xiu-Xiu Jiang, Song Zhang, Nan Hu, and et al. 2012. "Polydatin Attenuates Hypoxic Pulmonary Hypertension and Reverses Remodeling through Protein Kinase C Mechanisms" International Journal of Molecular Sciences 13, no. 6: 7776-7787. https://doi.org/10.3390/ijms13067776




