1. Introduction
Methylmercury (MeHg), the more toxic form of mercury by ingestion, is a potent neurotoxin, and human beings are mainly exposed to this pollutant through fish consumption, although under certain conditions dermal contact and inhalation of mercury vapor are the main exposure routes (such as in the case of industrial workers or artisanal gold miners). Two large studies among others, contradictory in their conclusions, have been performed in recent years to assess the impact of exposure to MeHg through fish consumption on the fetal brain in the Faroe Islands [
1] and in the Seychelles [
2]. These studies focused on the possible consequences of prenatal MeHg exposure, but only a few studies aim to understand the impact of mercury-enriched fish consumption on adult populations. In the Amazonian basin, Amerindian populations are exposed to MeHg through consumption of fish containing biomagnified concentrations of this pollutant partly coming from gold mining activities. Amerindian children from the upper Maroni in French Guiana were highly contaminated with a mean of 12 μg Hg/g in hair, and were afflicted by neurological disorders, such as poor coordination of the legs, and decreased performance in the Stanford-Binet copying score [
3,
4]. However, contradictory data have been collected linking mercurial contaminations and their impact on Amazonian populations’ health. The Mundurukus Amerindians (Pará state, Brazil) present elevated mean levels of mercury in hair (14 to 16 μg/g) without overt mercury intoxication signs [
5]. The same conclusions could be drawn from the communities of Brasília Legal and São Luís do Tapajós (Pará state, Brazil) [
6]. In contrast, other authors established a correlation between mercury hair content and impact on motor performance and visual capability of a riverine population of the Tapajós river (Pará state, Brazil) [
7–
10]. Since the epidemiological studies so far available are contradictory, we found it necessary to experiment on adult animals to model the possible effects of mercurial contamination through fish consumption.
In the time-course of a study dedicated to mercurial contaminations in French Guiana, we prepared mice diets by adding mercury-containing flesh from fish caught in French Guiana. The
Hoplias aimara species, which Amerindians are fond of, was chosen because this fish is highly contaminated by MeHg (4 to 12 μg/g dw), and because this single species represents 27% of the Wayanas’ dietary mercury intake and 10.7% of the total flesh they consume [
11]. Four diets containing 0, 0.1, 1, and 7.5% aimara flesh, representing 0, 5, 62, and 520 ng MeHg per g, respectively, were given to four groups of mice for a month. The lowest fish regimen led to a mercurial contamination pressure of 1 ng mercury per day per g of body weight, which is precisely that afflicting the Wayana Amerindians. The expression of several genes was modified with mercury intoxication in the liver, kidney, and hippocampus, even at the lowest tested fish regimen. In the muscles of mice fed the lowest fish-containing diet, cytochrome
c oxidase activity decreased to 45% of that of the control muscles [
12]. Since the 0.1% fish-containing regimen proved to affect gene expression and muscle mitochondrial respiration after just one month of exposure, we decided to carry out an experiment with such a contamination pressure exposing mice for 19 months in order to check whether the adverse affects worsen with time. Two main differences can be pinpointed between the present proposed study and almost all of those dealing with MeHg toxicity published up to now in the literature: first, the form of MeHg used, naturally associated to fish in the former and added pure in the latter; second, the unprecedented low dose of MeHg with levels one hundred-times below those used in the published articles.
MeHg affects brain development and results in neuroinflammatory and neurotoxic effects, and MeHg toxicity is mediated by the generation of reactive oxygen species leading to the induction of target proteins and among others cytokines [
13]. The chemokine CCL2 is suggested to have a neuromodulatory effect and to play an essential role in various neuroinflammatory processes [
14,
15]. Using
in vitro experiments on pure rat cortical neurons in culture, we observed by blockade of the CCL2/CCR2 neurotransmission an increased neuronal cell death in response to MeHg neurotoxicity. Furthermore, CCL2 could blunt
in vitro the decrease in glutathione levels induced by MeHg [
16]. The second objective of this study was therefore to test the possibility that the chemokine CCL2 could oppose the deleterious effects of MeHg using knockout CCL2 mice, and to look at the interplay between MeHg and CCL2 deficiency at the level of gene expression.
After 19 months feeding with a diet containing lyophilized aimara fish flesh at a final concentration of 5 ng MeHg/g of food giving a contamination pressure of 1 ng MeHg/day/g of body weight, we analyzed the mercury accumulation in tissues, the MeHg demethylation, the metallothionein content, the gene expression perturbation, and the contribution of the Ccl2 gene to the genetic response of tissues to MeHg contamination. We also performed a histological analysis of MeHg impact on the liver.
3. Results
3.1. Mercury Quantification and Demethylation Patterns
When considering the Experiment 1, as soon as three months of exposure, a significant difference of mercury accumulation between control and aimara-fed mice could be observed (
Table 3).
After 7 months of exposure the body parts and tissues displaying the highest mercury concentration were hair (180 ng/g) and kidney (300 ng/g, dw) followed by the liver (32 ng/g, dw). Surprisingly, despite the fact that MeHg is a neurotoxic compound, the brain accumulated low levels of mercury (12–15 ng/g, dw). After 19 months of exposure, the mercury concentrations increased slightly in several organs, except the kidney and stomach. Between 7 and 19 months of exposure, we quantified increased mercury concentrations in hair and brain (1.7-fold), in muscles (1.5-fold), and in the liver (1.3-fold).
MeHg demethylation was observed after three months of exposure in the kidney (40%) and the liver (30%) (
Table 4). At this time, no demethylation was evidenced in muscles and the brain. After 7 months of exposure, demethylation had increased in the kidney and liver, reaching 70% in both organs, and was apparent in muscles (10%). The brain remained refractory to such a process.
In Experiment 2, a control fish group was added in which mice were fed a salmon flesh-containing diet. After three or 14 months of exposure, the salmon-fed mice accumulated the same concentrations of mercury than the control group (
Table 5) with only two exceptions: eyes and kidney after 14 months of exposure. The aimara-fed mice accumulated much higher levels of mercury than the two other groups. As observed in Experiment 1, hair, the kidney and the liver were the main accumulators of mercury, and a net increase in mercury concentrations was noticed between three and 14 months of exposure. After 14 months of exposure the ratios of mercury concentrations in tissues from aimara-fed mice over that of salmon-fed mice were 24, 24 and 10 in hair, the kidney and liver, respectively.
The same conclusions can be drawn from the results of Experiment 3: after three months of exposure, no differences between the wild-type control and salmon-fed groups, and much higher mercury concentrations in tissues of mice fed the aimara-containing diet (
Table 6).
The ratio of mercury concentrations in tissues from aimara-fed mice over that of salmon-fed mice were 19, 21 and 15 in hair, the kidney and liver, respectively. During the time-course of Experiment 3, we carefully measured the food pellet consumption and the production of feces. Since the urinary excretion of mercury is very low in rodents (below 1%) [
25] we neglected it in our assessment. On a daily basis, we observed that mice were consuming 4.2 ± 0.18 g of food pellets/animal, and excreting 1.7 ± 0.1 g of feces/mouse. Feces contained after three months of exposure 19 ng Hg/g dry weight (
Table 6) or 5.7 ng Hg/g “fresh” weight. Thus, the daily level of mercury excretion was 9.7 ng Hg/mouse. Since the aimara food contained 5.4 ng Hg/g of food pellets, this made a daily intake of 22.7 ng Hg/mouse. Therefore, feces retained 43% of the daily ingested mercury (9.7 ng of excreted Hg over 22.7 ng of ingested Hg), giving a trophic transfer rate of mercury equal to 57%.
3.3. MeHg-Induced Gene Expression Perturbations
The expression of genes involved in the mitochondrial metabolism (
Atp5a1,
Atp6,
Cox1,
Cox4,
Cytb,
Mt-Nd4,
Ndusf8,
Sdhb,
Uqcrc2), the response towards oxidative stress (
Hsp25,
Sod1,
Sod2), the detoxification process (
Gsta4,
Mdr1a,
Mt2), the apoptotic signaling (
Bax), and brain microglial markers (
Cd11b,
Gfap,
Mcp1) were assessed. Mice coming from the Experiment 3 were sampled for the gene expression study after three months of exposure to MeHg through fish consumption. In the liver, the respiratory genes
Sdhb,
Cytb and
Cox1, representing the electron transfer chain complexes II, III, and IV, respectively, were up-regulated in the salmon-fed group compared to control group (2.5-, 4-, and 4.8-times, respectively) but repressed in the aimara-fed group compared to the salmon-fed group (3.8-, 5-, and 6.7-times, respectively) (
Table 8). The
Sod1,
Sod2,
Mt2,
Mdr1a, and
Bax genes also were repressed in the aimara-fed group compared to the salmon-fed group.
In the kidney, there were no differences between gene expression from mice fed the control and those fed salmon-containing diets. However, in the kidney from mice fed the aimara diet, a significant decrease in gene expression was noticed for Mt-Nd4, Cox4, and Atp6 genes as compared to control mice. This down-regulated pattern of gene expression indicates that the mitochondrial metabolism was impacted by the aimara-containing diet.
In muscles, both fish-containing diets resulted in the down-regulation of the respiratory genes Ndusf8 and Sdhb, and that of Sod1, Sod2, and Bax genes compared to the control mice. This indicated that MeHg was not involved but rather that some fish nutriments common to both salmon and aimara flesh were responsible for such a gene expression pattern.
In the brain, both fish-containing diets resulted in the up-regulation of the pro-inflammatory genes Cd11b, Mcp1, and Gfap, compared to control mice, suggesting that the fish-containing diets increased the inflammatory status in this organ. Nevertheless, in the brains of mice fed the aimara-containing diet, several genes were found up-regulated as compared to control mice. These genes were Bax, Mdr1a, Sod1, Sod2, Cox1 and Cytb. In addition, Cytb and Ndusf8 genes were up-regulated in the brains of mice fed the aimara flesh compared to those fed salmon.
Mice coming from the Experiment 2 were sampled for the gene expression study after 14 months of exposure to MeHg through fish consumption. In the liver, all of the 15 scrutinized genes were repressed in the aimara-fed group compared to the salmon-fed group (between 5 and 14-times) (
Table 9), confirming the observations made after three months of exposure (Experiment 3).
In the kidney, both fish-containing diets resulted in the down-regulation of several genes involved in the mitochondrial respiration (Ndusf8, Sdhb), the response to oxidative stress (Gsta4, Sod2), and the general stress response (Mt2, Hsp25, Mdr1a). The influence of MeHg that was observed after 3 months of exposure in this organ had vanished after 14 months.
In muscles, contrary to the three months exposure, during which no influence of MeHg could be pinpointed, after 14 months of exposure, 13 out of 15 tested genes showed a down-regulation pattern of expression in the aimara-fed group compared to salmon-fed and control groups. These genes comprised all the 9 tested respiratory genes, the oxidative stress responsive genes Gst4a, Sod1 and Sod2, and the stress responsive gene Mdr1a.
In the brain, the influence of MeHg that was observed after three months of exposure in this organ had disappeared after 14 months. Salmon flesh triggered the down-regulation of Atp5a1, Hsp25 and Mdr1a genes.
The differential expression of genes in tissues of mice fed the aimara diet was recorded (
Table 10) after having considered as non significant that of genes observed for both fish-containing diets (
i.e., genes for which expression varied after exposure to both salmon and aimara flesh), taking the control diet as a reference.
It appeared that except for brain of mice fed aimara flesh after a three month-long exposure, the differential expressions indicated a repression of the responsive genes. In addition, the time-course of gene response was different between tissues: the response observed after three months in the kidney and the brain vanished after 14 months, whereas a response was significant only after 14 months in muscles and conserved in the liver between three and 14 months.
3.4. Influence of the CCL2 Chemokine on MeHg-Induced Gene Expression Modifications
After three months of exposure, in mice knockout for
Ccl2 gene, much higher mercury concentrations were found in tissues of mice fed the aimara-containing diet compared to mice fed the basic diet (
Table 6). The ratios of mercury concentrations in tissues from aimara-fed KO mice over that of KO mice fed the basic regimen were 17, 20 and 17 in hair, the kidney and liver, respectively, very close to those observed in
Ccl2+/+ mice. Therefore, the lack of
Ccl2 gene did not influence the kinetic and pattern of MeHg accumulation within the mice bodies.
When looking at the interplay between MeHg and
Ccl2 knockout, no significant influence of aimara-containing diet was observed on gene expression in the liver, kidney and muscles of KO mice (
Table 11).
In the brain from KO mice, only a down-regulation of the respiratory gene Ndusf8 was noticed. Therefore, the loss of Ccl2 gene resulted in a great upheaval of gene expression patterns, especially in the liver and brain, compared to wild-type Ccl2+/+ mice. Most spectacular was the absence, in the liver of KO mice, of the repression pattern that had been observed in Ccl2+/+ mice when animals were fed the aimara-containing diet. In the brain, the up-regulation of the pro-inflammatory genes Cd11b and Gfap that had been observed in Ccl2+/+ mice fed with both the aimara- and salmon-containing diets was seen no more in KO mice fed the aimara diet, meaning that somehow the Ccl2 gene monitors the expression of these genes in response to fish consumption. In the brain of Ccl2+/+ mice fed the aimara-containing diet, seven genes out of 13 were found up-regulated, whereas six of them presented a basal expression, and the Ndusf8 gene was even down-regulated in KO mice fed the aimara diet.
3.5. MeHg Causes Histological Alterations in Liver
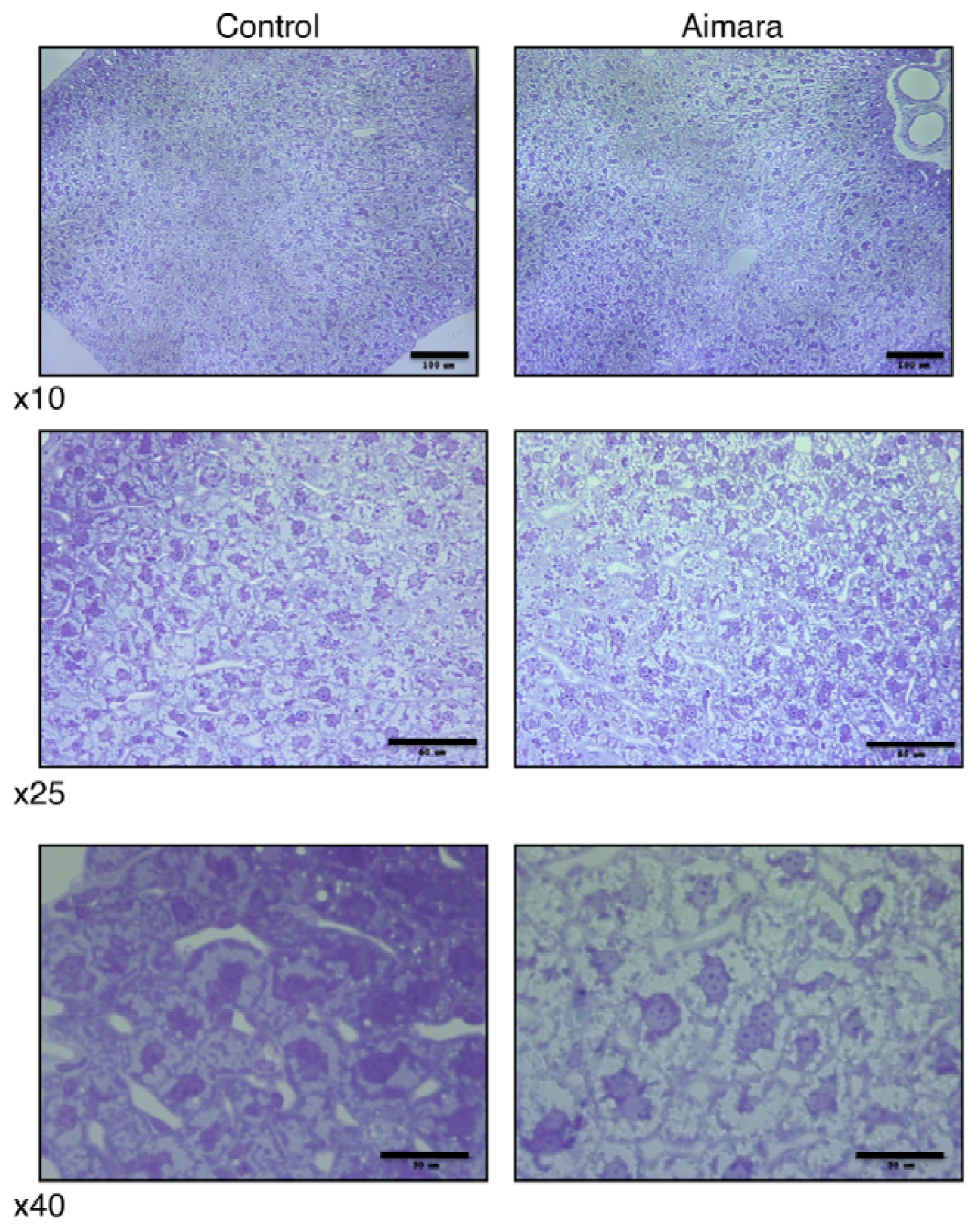
Since the organ accumulating the highest MeHg concentration was the kidney, we thought that it might be possible to observe damage at the histological level. In fact, we could see no differences between the kidneys of mice fed the control and the aimara-containing diet, either under optical microscopy or transmission electron microscopy. Surprisingly, when we looked at the liver after 7 months of exposure, we found that the area of hepatic cells tended to be larger in the livers of mice fed the aimara-containing diet (
Figure 1).
When analyzing the distribution of the cell areas, we were able to highlight a shift toward the greater values, with means equal to 384 ± 13 and 461 ± 15 μm
2 for control and aimara-fed mice, respectively (
Table 12), the difference being statistically significant as assessed by the Mann-Whitney
U-test (
p < 0.05). 62% of the control cells had an area comprising between 100 and 400 μm
2, against 37% for the aimara-fed mice.
Autometallographic detection allowed us to detect metal deposits within the cytoplasm of hepatic cells. These metal clusters appeared as globular bodies containing little stains denser to electrons than the surrounding part of the globule (
Figure 2). These metal corpuscles could only be observed in hepatic cells from mice fed the aimara-containing diet (panels D–H) and were absent in liver cells from those fed the control diet (panels A–C).
4. Discussion
The aimara-containing diet selected in this study is aimed to mimic the mercurial contamination of the Wayana population through fish consumption. The aimara diet is enriched in MeHg, whereas the salmon and control diets contain only trace amounts of MeHg. Therefore, as a rule of thumb, any effects specifically observed in mice fed the aimara diet should be attributed to MeHg. Effects observed in mice fed on both the aimara and salmon diets should be attributed to nutrients contained in fish flesh and common to salmon and aimara species.
In a previous article [
12], with the same aimara-containing diet and at the same dose, a four-fold up-regulation of
Cox1 and
Mt2 genes in the liver was observed after a one-month exposure. In the present article, and after three and 14 months of exposure, we observed a four- and 14-fold down-regulation of
Cox1 and
Mt2 genes, respectively. This apparent discrepancy might well be due to the levels of accumulated total mercury in the liver which were much higher at three and 14 months, as compared to one month of exposure, with concentrations equal to 67, 77 and 6.7 ng Hg/g, respectively. Thus, the same type of regulation pattern with 10- and 11-times more accumulated mercury cannot be expected.
After three months of exposure, the aimara diet specifically resulted in the brain in the up-regulation of several genes encoding respiratory subunit proteins (Cox1 and Cytb), and superoxide dismutase isoforms (Sod1 and Sod2). This impact vanished after 14 months, suggesting that this organ could adapt or tolerate MeHg with time. The same holds true in the kidney in which the aimara diet-specific effect on the down-regulation of three respiratory genes was obvious after three months, but disappeared after 14 months. In muscles, the reverse was observed since a specific effect of the aimara diet could be observed after 14 months but not after three. The effects of aimara flesh were constant with time in the liver in which all the 15 tested genes were still down-regulated after 14 months. Correspondingly, we observed a decreased respiratory activity in isolated mitochondria from the brain, the kidney and liver after three months of exposure (data not shown). Finally, the aimara-associated MeHg effects were most prominent in the liver, despite the fact that this organ accumulated 13 and six-times less mercury than the kidney after three and 14 months, respectively. Therefore, there is not an obligate relationship between mercury accumulation and effect intensity. This fact was confirmed by the histological impact observed on hepatic cells which area distribution shifted towards the greater values.
The metallothionein protein (MT) quantification shows that in those tissues in which MT concentration increased, MeHg demethylation occurred. This holds true for the kidney after three and seven months of exposure, and in muscles at seven months, in which tissues 40, 100, and 120% increases in MT concentrations were noted, respectively, paralleled by a 40, 70, and 10% of MeHg demethylation. This can be taken as a molecular sign of divalent mercurial contamination, since only Hg2+ has been reported yet to induce MT accumulation in contaminated tissues. However, the reciprocal situation does not stand since MeHg demethylation is not necessarily accompanied by a MT concentration increase. This is the case in the liver in which a 30 and 70% MeHg demethylation was noticed after three and seven months of exposure, respectively, with a level of MT similar to control at three months and significantly decreased (36%) at seven months. This peculiar situation can be explained by the 127-times down-regulation of mt2 gene in liver at three months, a repression still maintained at 14 months. The reason why the mt2 gene is repressed in a mercury demethylating tissue is unknown, but this illustrates that at low doses of metal contaminant, far below those used in classical studies, unexpected gene response can arise. In addition to this genetic parameter, the decrease of MT level in contaminated liver after seven months of exposure is also probably linked to the formation of insoluble corpuscles of this metal in liver cells (as seen in microscopy), which decreases the proportion of bioavailable inorganic mercury for MTs.
The suppression of the synthesis of the chemokine CCL2 in the corresponding KO mice resulted in important changes in gene expression patterns in the liver and brain. After three months of exposure to aimara-containing diet, eight genes over 10 selected (
Sdhb,
Cytb,
Cox1,
Sod1,
Sod2,
Mt2,
Mdr1a and
Bax) were repressed in wild-type mice liver whereas none presented a differential expression in KO
Ccl2−/− mice. In wild-type mice brain, six genes over 12 selected (
Cytb,
Cox1,
Sod1,
Sod2,
Mdr1a and
Bax) presented a stimulated expression, whereas all of them remained at the basal level of expression in KO
Ccl2−/− mice. Chemokines were first identified as being responsible for the maturation and trafficking of leukocytes, in particular in inflammatory diseases [
26]. Therefore, the results obtained in the liver suggest that the aimara diet-induced repression of genes involved in the mitochondrial respiration (
Sdhb,
Cytb, and
Cox1), in the oxidative stress neutralization (
Sod1 and
Sod2), and in xenobiotic detoxification (
Mdr1a), is triggered by an inflammatory process in which intervenes the CCL2 action. In keeping with this, the involvement of CCL2 in molecular mechanisms of hepatic damage has been assessed in mice lacking CCL2 after a carbon tetrachloride (CCl
4) challenge, since this toxic determines liver injury, inflammation and oxidative stress. In KO
Ccl2−/− mice, the lack of CCL2 afforded protection from CCl
4-induced damages and the development of oxidative stress [
27]. Several data indicate that many hepatic pathologies are linked to CCL2. For instance, (1) liver fibrosis depends on recruitment of monocytes into the liver and precisely the chemokine receptor CCR2 and its ligand CCL2 participate in regulating monocyte subset infiltration [
28,
29]; (2) CCL2 induces human hepatoma cell migration and invasion [
30]; (3) Obesity activates hepatocyte expression of CCL2 leading to hepatic recruitment of CCR2(+) myeloid cells that promote hepatosteatosis. Reciprocally, reduced hepatic steatosis in obese mice deficient in the CCR2 receptor was observed [
31]; (4) inhibition of CCR2 could improve diet-induced obesity and related metabolic disorders, such as insulin resistance and hepatic steatosis, by suppressing inflammation in adipose tissue [
32]; (5) hyperhomocysteinemia is a metabolic disorder associated with liver injury and chronic inflammation through induction of CCL2 production in the liver [
33]. With such a scheme in mind, one may propose that the CCL2-mediated repression of genes in the liver impedes this organ to mount a proper response against MeHg contamination, and constitutes one of the molecular bases accounting for MeHg toxicity in the liver. The situation in the brain is reversed: instead of a CCL2-based gene repression we observed a CCL2-based gene stimulation. In such a case, in the brain, the lack of CCL2 production would impede the brain in adapting to MeHg toxic effects. In agreement with such a view, we have reported that on the same groups of mice than used in the present study, the aimara-containing diet in the mice cortex provoked a decrease in CCL2 concentrations, a neuronal cell death and a microglial activation.
Ccl2 KO mice that were fed a vegetal control food already presented a decrease in cortical neuronal cell density in comparison with wild-type animals under similar diet conditions showing that the presence of CCL2 is required for normal neuronal survival. Moreover,
Ccl2 KO mice showed a more pronounced cortical neuronal cell death than wild-type mice in response to the aimara diet [
16]. Thus, CCL2 would contribute to MeHg toxicity in the liver, and protect against it in the brain.
One may ask about the relevance of MeHg liver effects to clinical or population-based adverse effects. Indeed, most of studies dealing with MeHg focus on neurotoxic outcomes making believe that the liver is not a target for MeHg toxicity. First, it has been reported that deaths from liver cancer, chronic liver disease, and liver cirrhosis were significantly more frequent among registered Minamata disease patients than among the general population of Minamata City [
34]. And in rodents, numerous observations showed a MeHg-induced hepatic toxicity. Rats, exposed daily for 4 weeks to 1.0 mg Hg/kg of body weight, were described as having decreased activities in glucose 6-phosphatase, alkaline phosphatase, ATPase and succinic dehydrogenase in the liver [
35], along with hepatic mitochondria swelling [
36]. In kittens fed daily for 11 months with tuna containing 0.3–0.5 mg Hg/kg, ultrastructural changes were observed in the liver with proliferation of the smooth endoplasmic reticulum and abnormal mitochondria morphology [
37]. In rats exposed to 140 mg MeHg/kg/day for 100 days, leukocyte infiltration was observed in the liver [
38]. MeHg triggers an oxidative stress in the liver since it has been shown in mice after MeHg exposure, hepatic lipid peroxidation [
39], increase in thiobarbituric acid reactive species, decrease in ascorbic acid content [
40], and inhibition of the hepatic thioredoxin reductase [
41]. In rat liver, after MeHg exposure, a decrease in glutathione level [
42], an increased superoxide generation in mitochondria, and increased glutathione peroxidase and decreased superoxide dismutase activities [
43] were observed. Oxidative DNA damages in the liver after MeHg exposure have been demonstrated [
42,
44], and also MeHg-induced hepatic DNA methylation [
45]. These published data fit well with our data showing ultrastructural modifications and repression of respiratory genes and superoxide dismutase genes in the liver.
When addressing the question as to whether the mouse is an appropriate model for the mercurial intoxication of the Wayana Amerindians, a good criterion consists in a comparison of the trophic transfer rate of mercury. Dietary MeHg is readily and efficiently absorbed by the human gastrointestinal tract, to a reported level of 95% to 100% [
46]. Here, we could calculate a trophic transfer rate of 57% for wild-type mouse, meaning that the impact of aimara diet could be greater in the case of Wayanas Amerindians. This trophic transfer rate is close to that recorded in rats for which the fecal excretion of mercury was about 68% [
25].
In the present study, mice were fed an aimara diet representing a daily contamination pressure of 1 μg/kg body weight. This is far below the doses of MeHg given to mice found in the literature. A literature survey of the recent articles dealing with MeHg contamination in mice through diet or drinking water these last 2 years, identifies the commonly-used daily doses which were in decreasing order of concentration: 5.4 [
47], 2 [
40], 1.4 [
48], 1 [
49,
50], and 0.5 mg MeHg/kg body weight [
51]. These concentrations are 5400- to 500-times greater than the contamination pressure we have used in the present study. Finally, it has been found that MeHg could impair motor and cognitive functions in mice daily gavaged or fed with 50 [
52] and 10 μg MeHg/kg body weight [
53], respectively, equivalent to doses 50 and 10-times higher than in the present study. In the latter work, the mercury content in the brains of adult mice exposed as adults was 63 ng/g, fresh weight (fw), and that in the 3-month old mice exposed prenatally was 28 ng/g, fw [
53]. This is three times higher than the value of 10 ng/g, fw (31 ng/g, dw) found in the brains of mice fed the aimara diet in the third experiment of the present study. The reported 90-percentile value of mercury concentration in the cortex of individual Norwegian people without occupational exposure to mercury was found to be 28 ng/g, fw [
54], a value 3-times higher than that in the brain of aimara-fed mice in the present study. Therefore, after just three months of feeding with a diet containing 0.15% aimara flesh, mouse brain mercury levels were three-fold below the highest values found in human brains from heavy fish consumers. Swedish people are modest fish consumers and the mercury mean concentrations in the human kidney from the Swedish population are 0.7 μg/g, dw, for women and 0.4 μg/g, dw, for men with an overall distribution range of 0.04–2.1 μg/g, dw [
55]. In the kidneys of mice fed 0.15% aimara flesh over three months (experiment 3), the tissue mercury concentration reached a value of 0.91 μg/g, dw, equivalent to mid-range values found in the human kidney from modest fish consumers.