Dietary Supplementations as Neuroprotective Therapies: Focus on NT-020 Diet Benefits in a Rat Model of Stroke
Abstract
:1. Introduction
1.1. Patient Numbers and Current Methods of Care for Stroke
1.2. Dietary Supplementation and Neurogenesis
1.3. Recent Studies on Diet Supplementation Therapy in Ischemic Injury Models
1.4. Significance and Product Development
2. Preclinical Studies
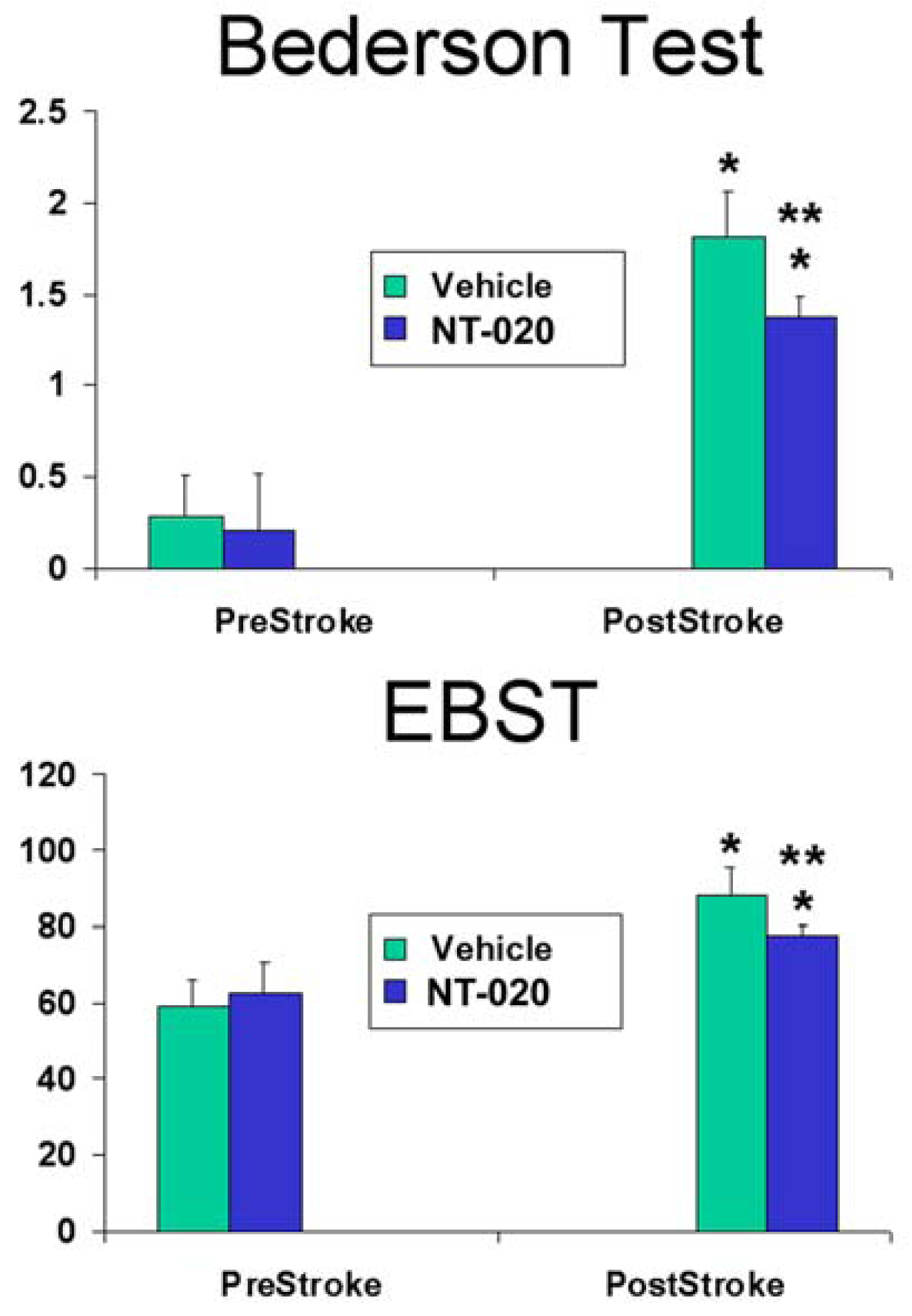
2.1. NT-020 Attenuates Stroke-Induced Behavioral Deficits
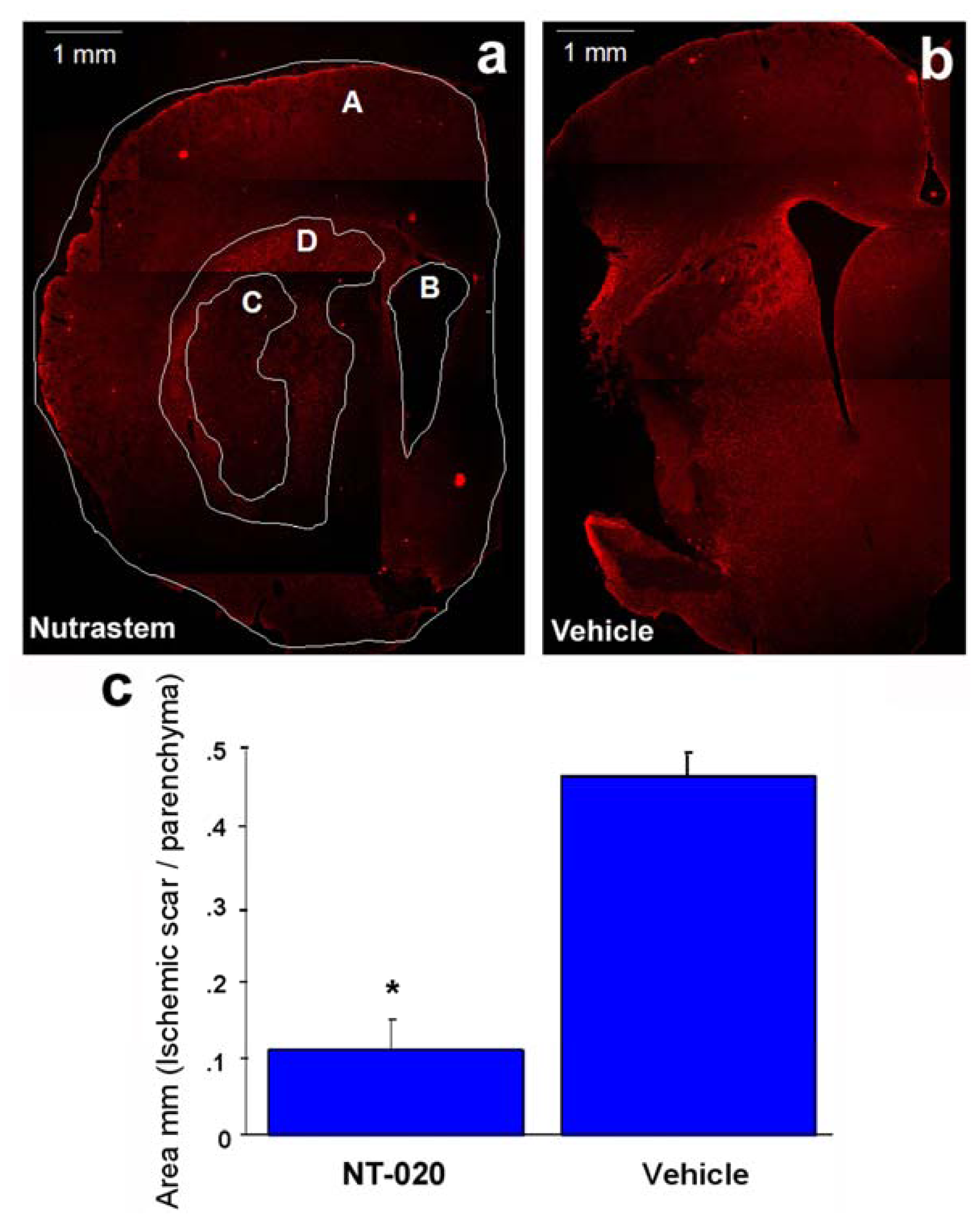
2.2. NT-020 Reduces Stroke-Induced Cerebral Infarction
2.3. NT-020 Induces Cell Proliferation in Stroke Brain
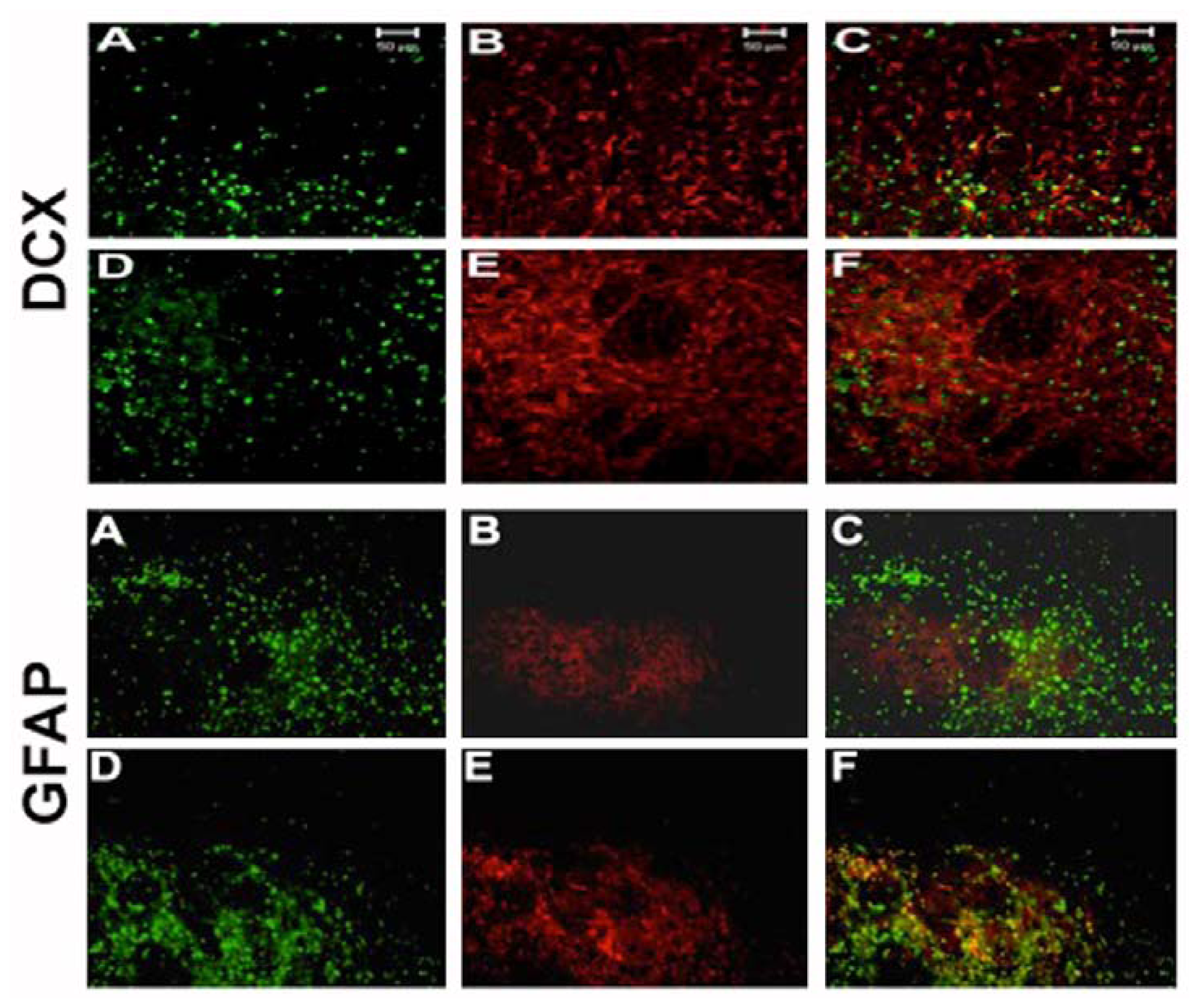
2.4. NT-020 Promotes Neurogenesis in Stroke Brain
3. Caveats to Consider for Translational Research of NT-020
4. Conclusions
- Disclosure StatementP.C. Bickford is a co-founder and C.V. Borlongan is a consultant of Natura Therapeutics.
References
- Wolf, P.A.; Cobb, J.L.; D’Agostino, R.B. Epidemiology of Stroke. In Stroke: Pathophysiology, Diagnosis, and Management; Barnett, H.J., Stein, B.M., Mohr, J.P., Yatsu, F.M., Eds.; Churchill Livingstone: New York, NY, USA, 1992; pp. 3–27. [Google Scholar]
- Moskowitz, M.A.; Lo, E.H.; Ladecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar]
- Acosta, S.; Jernberg, J.; Sanberg, C.D.; Sanberg, P.R.; Small, B.J.; Gemma, C.; Bickford, P.C. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res 2010, 13, 581–588. [Google Scholar]
- Wang, X.; Qin, X.; Demirtas, H. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 2007, 369, 1876–1882. [Google Scholar]
- Arab, L.; Liebeskind, D.S. Tea, flavonoids and stroke in man and mouse. Arch. Biochem. Biophys 2010, 501, 31–36. [Google Scholar]
- Bachstetter, A.D.; Jernberg, J.; Schlunk, A.; Vila, J.L.; Hudson, C. Spirulina promotes stem cell genesis and protects against LPS induced declines in neural stem cell proliferation. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Zavala-Alarcon, E.; Cecena, F.; Ashar, R.; Patel, R.; van Poppel, S.; Carlson, R. Safety of elective—Including “high risk”—Percutaneous coronary interventions without on-site cardiac surgery. Am. Heart J 2004, 148, 676–683. [Google Scholar]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar]
- Duan, W.; Guo, Z.; Jiang, H.; Ware, M.; Mattson, M.P. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 2003, 144, 2446–2453. [Google Scholar]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci 1999, 19, 8114–8121. [Google Scholar]
- Dupret, D.; Revest, J.-M.; Koehl, M.; Ichas, F.; de Giorgi, F. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Vukovic, J.; Blackmore, D.G.; Jhaveri, D.; Bartlett, P.F. Activation of neural precursors in the adult neurogenic niches. Neurochem. Int 2011, 59, 341–346. [Google Scholar]
- Borlongan, C.V.; Glover, L.E.; Tajiri, N.; Kaneko, Y.; Freeman, T.B. The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog. Neurobiol 2011, 95, 213–228. [Google Scholar]
- Borlongan, C.V. Bone marrow stem cell mobilization in stroke: A “bonehead” may be good after all! Leukemia 2011, 25, 1674–1686. [Google Scholar]
- Borlongan, C.V.; Sanberg, P.R.; Freeman, T.B. Neural transplantation for neurodegenerative disorders. Lancet 1999, 353, SI29–SI30. [Google Scholar]
- Haas, S.; Weidner, N.; Winkler, J. Adult stem cell therapy in stroke. Curr. Opin. Neurol 2005, 18, 59–64. [Google Scholar]
- Picard-Riera, N.; Nait-Oumesmar, B.; Baron-van Evercooren, A. Endogenous adult neural stem cells: Limits and potential to repair the injured central nervous system. J. Neurosci. Res 2004, 15, 76, 223–231. [Google Scholar]
- Bachstetter, A.D.; Pabon, M.M.; Cole, M.J.; Hudson, C.E.; Sanberg, P.R.; Willing, A.E.; Bickford, P.C.; Gemma, C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci 2008, 9. [Google Scholar] [CrossRef]
- Bickford, P.C.; Tan, J.; Shytle, R.D.; Sanberg, C.D.; El-Badri, N.; Sanberg, P.R. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev 2006, 15, 118–123. [Google Scholar]
- Vendrame, M.; Gemma, C.; de Mesquita, D.; Collier, L.; Bickford, P.C.; Sanberg, C.D.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev 2005, 14, 595–604. [Google Scholar]
- Yasuhara, T.; Hara, K.; Maki, M.; Masuda, T.; Sanberg, C.D.; Sanberg, P.R.; Bickford, P.C.; Borlongan, C.V. Dietary supplementation exerts neuroprotective effects in ischemic stroke model. Rejuvenation Res 2008, 11, 201–214. [Google Scholar]
- Jones, N.E.; Heyland, D.K. Pharmaconutrition: A new emerging paradigm. Curr. Opin. Gastroenterol 2008, 24, 215–222. [Google Scholar]
- Joseph, J.A.; Shukitt-Hale, B.; Casadesus, G. Reversing the deleterious effects of aging on neuronal communication and behavior: Beneficial properties of fruit polyphenolic compounds. Am. J. Clin. Nutr 2005, 81, 313S–316S. [Google Scholar]
- Valente, T.; Hidalgo, J.; Bolea, I.; Ramirez, B.; Angles, N.; Reguant, J.; Morello, J.R.; Gutierrez, C.; Boada, M.; Unzeta, M. A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neurogenesis in the subventricular zone and hippocampus of adult mouse brain. J. Alzheimers Dis. 2009, 18, 849–865. [Google Scholar]
- Venna, V.R.; Deplanque, D.; Allet, C.; Belarbi, K.; Hamdane, M.; Bordet, R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology 2009, 34, 199–211. [Google Scholar]
- Ahmet, I.; Wan, R.; Mattson, M.P.; Lakatta, E.G.; Talan, M. Cardioprotection by intermittent fasting in rats. Circulation 2005, 112, 3115–3121. [Google Scholar]
- Anson, R.M.; Guo, Z.; de Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar]
- Mattson, M.P.; Duan, W.; Guo, Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: Cellular and molecular mechanisms. J. Neurochem 2003, 84, 417–431. [Google Scholar]
- Mattson, M.P.; Duan, W.; Wan, R.; Guo, Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx 2004, 1, 111–116. [Google Scholar]
- Yu, Z.F.; Mattson, M.P. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: Evidence for a preconditioning mechanism. J. Neurosci. Res 1999, 57, 830–839. [Google Scholar]
- Wang, Y.; Chang, C.F.; Chou, J.; Chen, H.L.; Deng, X.; Harvey, B.K.; Cadet, J.L.; Bickford, P.C. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp. Neurol 2005, 193, 75–84. [Google Scholar]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.G.; Smith, M.A.; Joseph, J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci 2004, 7, 309–316. [Google Scholar]
- Bazan, H.A.; Lu, Y.; Thoppil, D.; Fitzgerald, T.N.; Hong, S.; Dardik, A. Diminished omega-3 fatty acids are associated with carotid plaques from neurologically symptomatic patients: Implications for carotid interventions. Vasc. Pharmacol 2009, 51, 331–336. [Google Scholar]
- Blondeau, N.; Nguemeni, C.; Debruyne, D.N.; Piens, M.; Wu, X.; Pan, H.; Hu, X.; Gandin, C.; Lipsky, R.H.; Plumier, J.C.; Marini, A.M.; Heurteaux, C. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: A versatile potential therapy for stroke. Neuropsychopharmacology 2009, 34, 2548–2559. [Google Scholar]
- Zheng, G.Q.; Cheng, W.; Wang, Y.; Wang, X.M.; Zhao, S.Z.; Zhou, Y. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J. Ethnopharmacol 2011, 133, 724–728. [Google Scholar]
- Morse, D.; Choi, A.M. Heme oxygenase-1: From bench to bedside. Am. J. Respir. Crit. Care Med 2005, 172, 660–670. [Google Scholar]
- Saleem, S.; Zhuang, H.; Biswal, S.; Christen, Y.; Dore, S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke 2008, 39, 3389–3396. [Google Scholar]
- Maswood, N.; Young, J.; Tilmont, E.; Zhang, Z.; Gash, D.M.; Gerhardt, G.A.; Grondin, R.; Roth, G.S.; Mattison, J.; Lane, M.A.; et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 18171–18176. [Google Scholar]
- Mattson, M.P. The need for controlled studies of the effects of meal frequency on health. Lancet 2005, 365, 1978–1980. [Google Scholar]
- Borlongan, C.V.; Hadman, M.; Sanberg, C.D.; Sanberg, P.R. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004, 35, 2385–2389. [Google Scholar]
- Borlongan, C.V.; Lind, J.G.; Dillon-Carter, O.; Yu, G.; Hadman, M.; Cheng, C.; Carroll, J.; Hess, D.C. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res 2004, 1010, 108–116. [Google Scholar]
- Langdon, K.D.; Clarke, J.; Corbett, D. Long-term exposure to high fat diet is bad for your brain: Exacerbation of focal ischemic brain injury. Neuroscience 2011, 182, 82–87. [Google Scholar]
- Puig, K.L.; Floden, A.M.; Adhikari, R.; Golovko, M.Y.; Combs, C.K. Amyloid precursor protein and proinflamatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Stromberg, I.; Gemma, C.; Vila, J.; Bickford, P.C. Blueberry- and spirulina-enriched diets enhance striatal dopamine recovery and induce a rapid, transient microglia activation after injury of the rat nigrostriatal dopamine system. Exp. Neurol 2005, 196, 298–307. [Google Scholar]
- Borlongan, C.V.; Lind, J.G.; Dillon-Carter, O.; Yu, G.; Hadman, M.; Cheng, C.; Carroll, J.; Hess, D.C. Intracerebral xenografts of mouse bone marrow cells in adult rats facilitate restoration of cerebral blood flow and blood-brain barrier. Brain Res 2004, 1009, 26–33. [Google Scholar]
- Borlongan, C.V.; Lind, J.G.; Dillon-Carter, O.; Yu, G.; Hadman, M.; Cheng, C.; Carroll, J.; Hess, D.C. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res 2004, 1010, 108–116. [Google Scholar]
- Borlongan, C.V.; Yamamoto, M.; Takei, N.; Kumazaki, M.; Ungsuparkorn, C.; Hida, H.; Sanberg, P.R.; Nishino, H. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J 2000, 14, 1307–1317. [Google Scholar]
- Beech, J.S.; Reckless, J.; Mosedale, D.E.; Grainger, D.J.; Williams, S.C.; Menon, D.K. Neuroprotection in ischemia-reperfusion injury: An antiinflammatory approach using a novel broad-spectrum chemokine inhibitor. J. Cereb. Blood Flow Metab 2001, 21, 683–689. [Google Scholar]
- Ito, D.; Tanaka, K.; Suzuki, S.; Dembo, T.; Fukuuchi, Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 2001, 32, 1208–1215. [Google Scholar]
- Marks, L.; Carswell, H.V.; Peters, E.E.; Graham, D.I.; Patterson, J.; Dominiczak, A.F.; Macrae, I.M. Characterization of the microglial response to cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension 2001, 38, 116–122. [Google Scholar]
- Wang, X.; Qin, X.; Demirtas, H. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 2007, 369, 1876–1882. [Google Scholar]
- Kahles, T.; Luedike, P.; Endres, M.; Galla, H.J.; Steinmetz, H.; Busse, R.; Neumann-Haefelin, T.; Brandes, R.P. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 2007, 38, 3000–3006. [Google Scholar]
- Lenzsér, G.; Kis, B.; Snipes, J.A.; Gáspár, T.; Sándor, P.; Komjáti, K.; Szabó, C.; Busija, D.W. Contribution of poly(ADP-ribose) polymerase to postischemic blood-brain barrier damage in rats. J. Cereb. Blood Flow Metab 2007, 27, 1318–1326. [Google Scholar]
- Hamby, A.M.; Suh, S.W.; Kauppinen, T.M.; Swanson, R.A. Use of a poly(ADP-ribose) polymerase inhibitor to suppress inflammation and neuronal death after cerebral ischemia-reperfusion. Stroke 2007, 38, 632–636. [Google Scholar]
- Kauppinen, T.M.; Suh, S.W.; Berman, A.E.; Hamby, A.M.; Swanson, R.A. Inhibition of poly(ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J. Cereb. Blood Flow Metab 2009, 29, 820–829. [Google Scholar]
- Garbuzova-Davis, S.; Haller, E.; Saporta, S.; Kolomey, I.; Nicosia, S.V.; Sanberg, P.R. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res 2007, 1157, 126–137. [Google Scholar]
- Garbuzova-Davis, S.; Sanberg, C.D.; Kuzmin-Nichols, N.; Willing, A.E.; Gemma, C.; Bickford, P.C.; Miller, C.; Rossi, R.; Sanberg, P.R. Human umbilical cord blood treatment in a mouse model of ALS: Optimization of cell dose. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Saporta, S.; Haller, E.; Kolomey, I.; Bennett, S.P.; Potter, H.; Sanberg, P.R. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS One 2007, 2. [Google Scholar] [CrossRef]
- Zhu, Y.; Bickford, P.C.; Sanberg, P.; Giunta, B.; Tan, J. Blueberry opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activation protein kinase. Rejuvenation Res 2008, 11, 891–901. [Google Scholar]
- Borlongan, C.V.; Tajima, Y.; Trojanowski, J.Q.; Lee, V.M.; Sanberg, P.R. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp. Neurol 1998, 149, 310–321. [Google Scholar]
- Borlongan, C.V.; Chopp, M.; Steinberg, G.K.; Bliss, T.M.; Li, Y.; Lu, M.; Hess, D.C.; Kondziolka, D. Potential of stem/progenitor cells in treating stroke: The missing steps in translating cell therapy from laboratory to clinic. Regen. Med 2008, 3, 249–250. [Google Scholar]
- Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 2009, 40, 510–515.



© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaneko, Y.; Cortes, L.; Sanberg, C.; Acosta, S.; Bickford, P.C.; Borlongan, C.V. Dietary Supplementations as Neuroprotective Therapies: Focus on NT-020 Diet Benefits in a Rat Model of Stroke. Int. J. Mol. Sci. 2012, 13, 7424-7444. https://doi.org/10.3390/ijms13067424
Kaneko Y, Cortes L, Sanberg C, Acosta S, Bickford PC, Borlongan CV. Dietary Supplementations as Neuroprotective Therapies: Focus on NT-020 Diet Benefits in a Rat Model of Stroke. International Journal of Molecular Sciences. 2012; 13(6):7424-7444. https://doi.org/10.3390/ijms13067424
Chicago/Turabian StyleKaneko, Yuji, Lourdes Cortes, Cyndy Sanberg, Sandra Acosta, Paula C. Bickford, and Cesar V. Borlongan. 2012. "Dietary Supplementations as Neuroprotective Therapies: Focus on NT-020 Diet Benefits in a Rat Model of Stroke" International Journal of Molecular Sciences 13, no. 6: 7424-7444. https://doi.org/10.3390/ijms13067424



