Biosysthesis of Corn Starch Palmitate by Lipase Novozym 435
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Enzyme
2.2. Methods
2.2.1. Starch Pretreatment
2.2.2. General Procedure for Lipase Esterification
2.2.3. Determination of the Degree of Substitution by Methanolysis and GC Analysis
2.2.4. Methanolysis of the Starch Palmitate and GC Analysis of the Methyl Palmitate
2.2.5. Calculation DS of the Starch Palmitate
2.2.6. Preparation of Emulsions
2.2.7. Analytical Methods
3. Results and Discussion
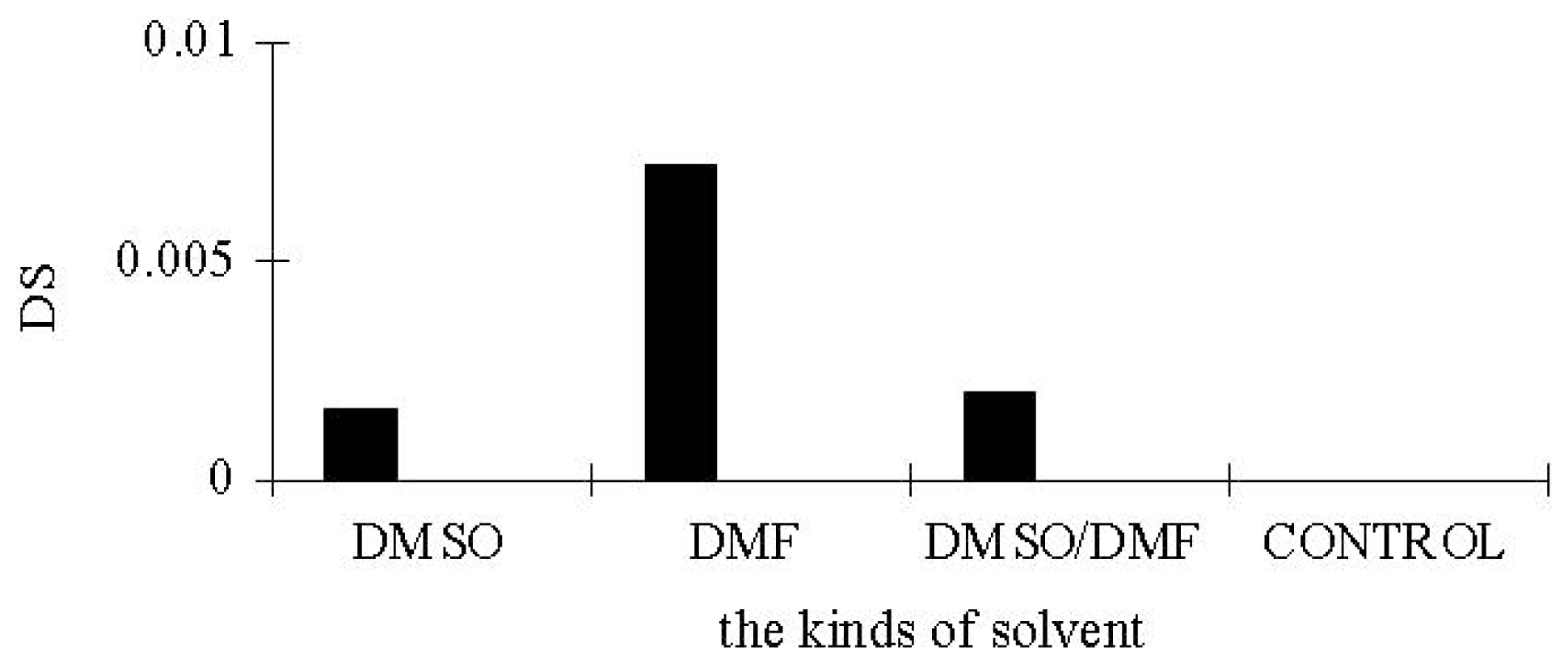
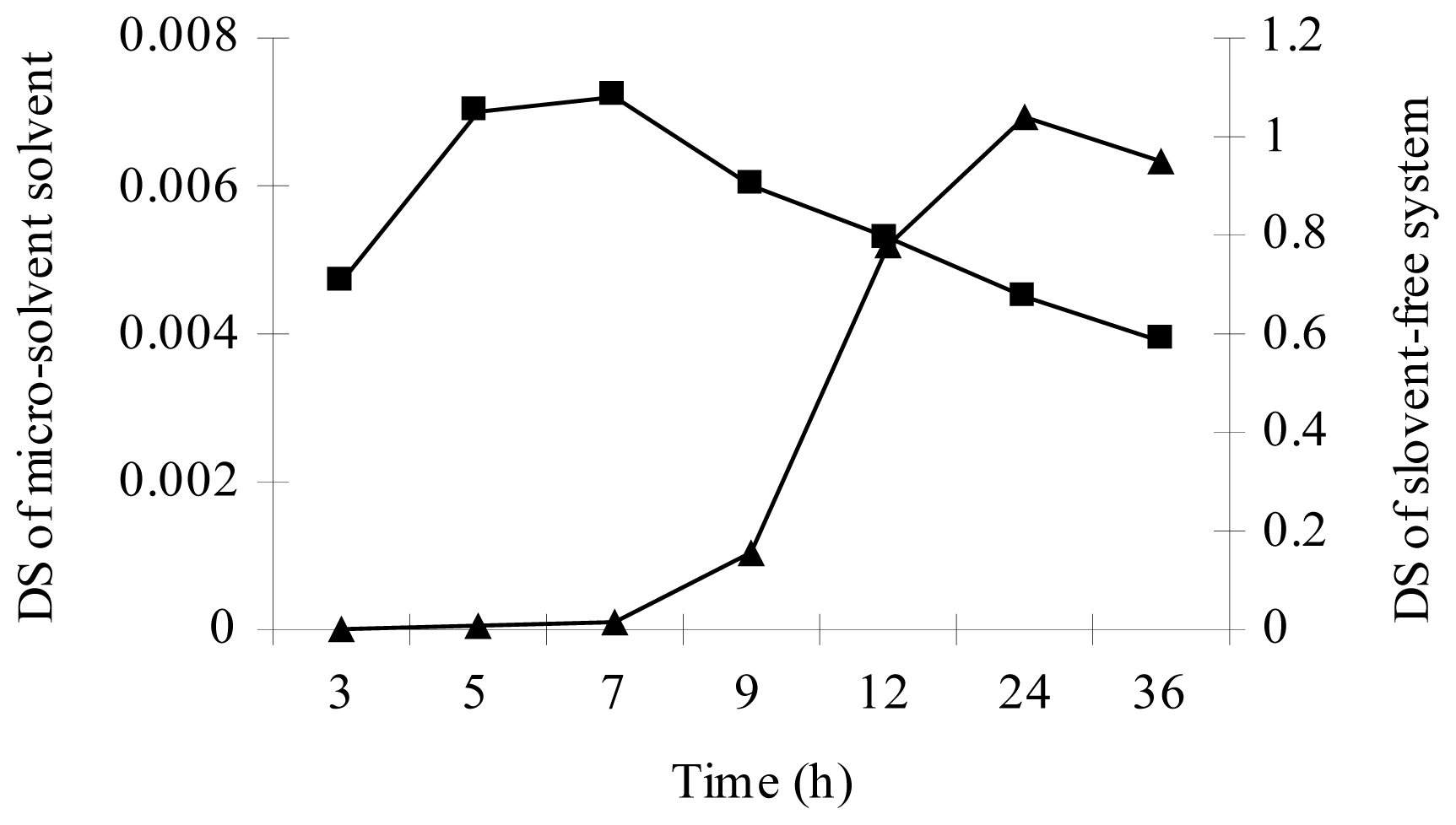
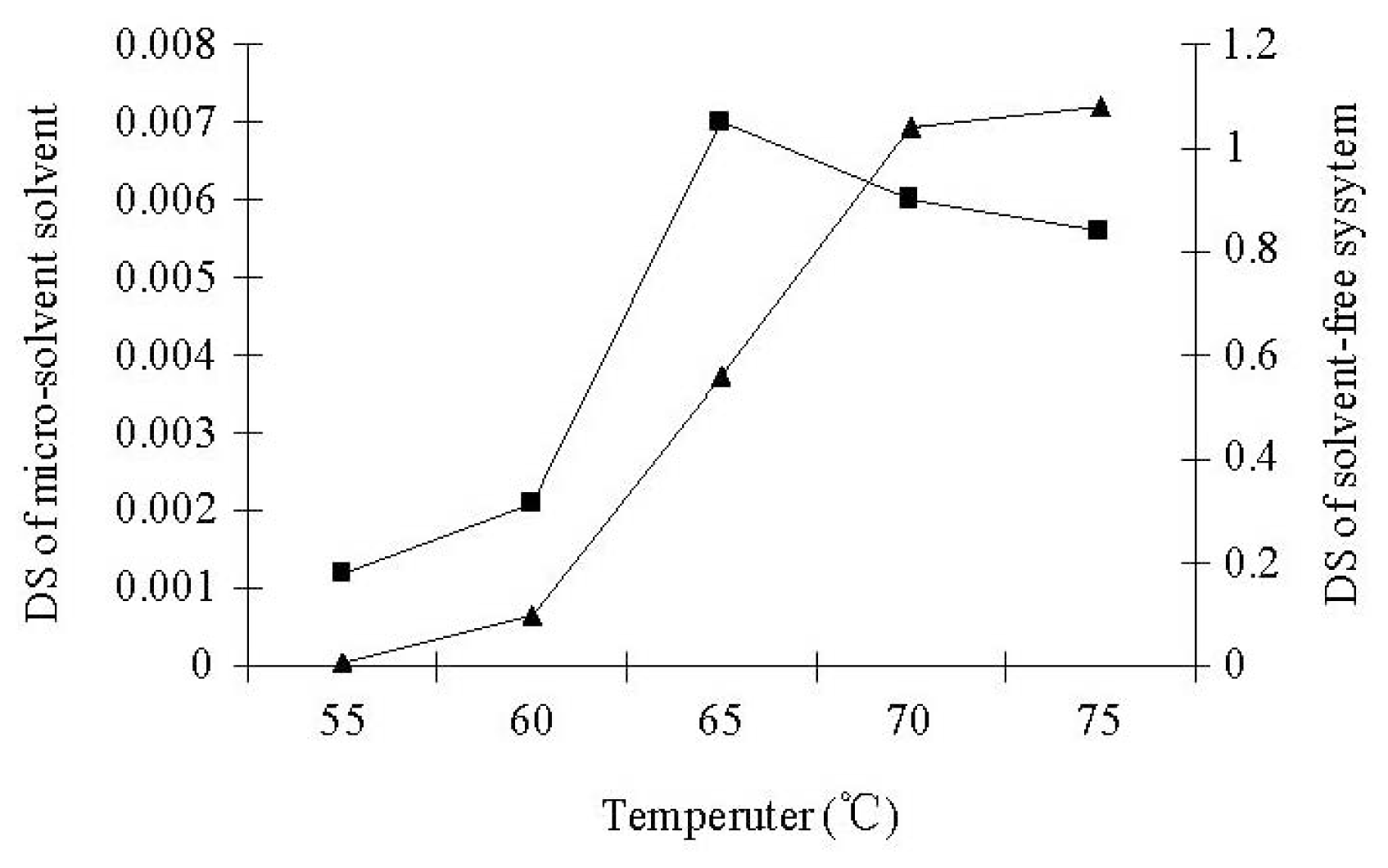
3.1. Lipase-Catalyzed Esterification under Micro-Solvent System
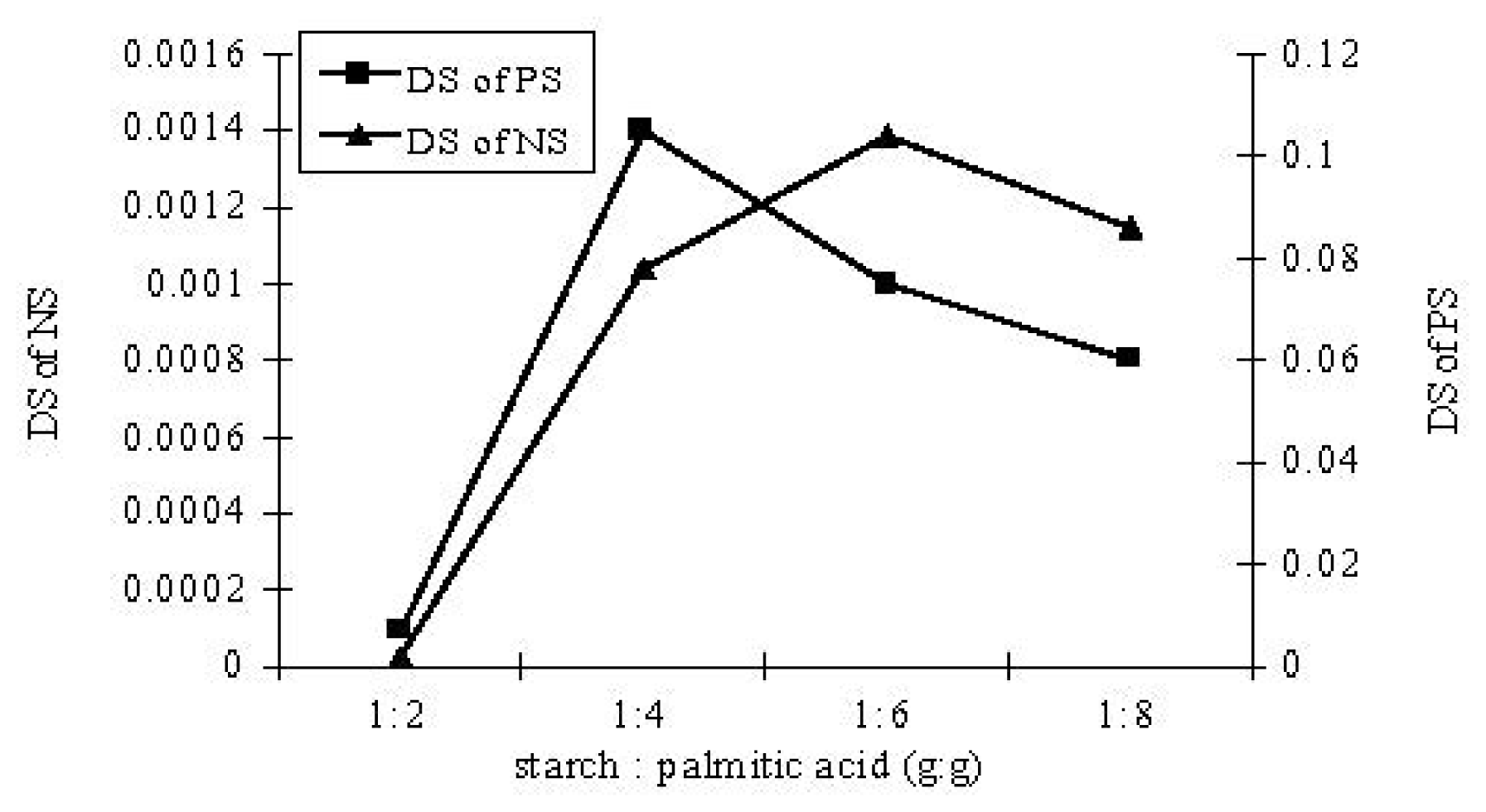
3.2. Lipase-Catalyzed Esterification under Solvent-Free System
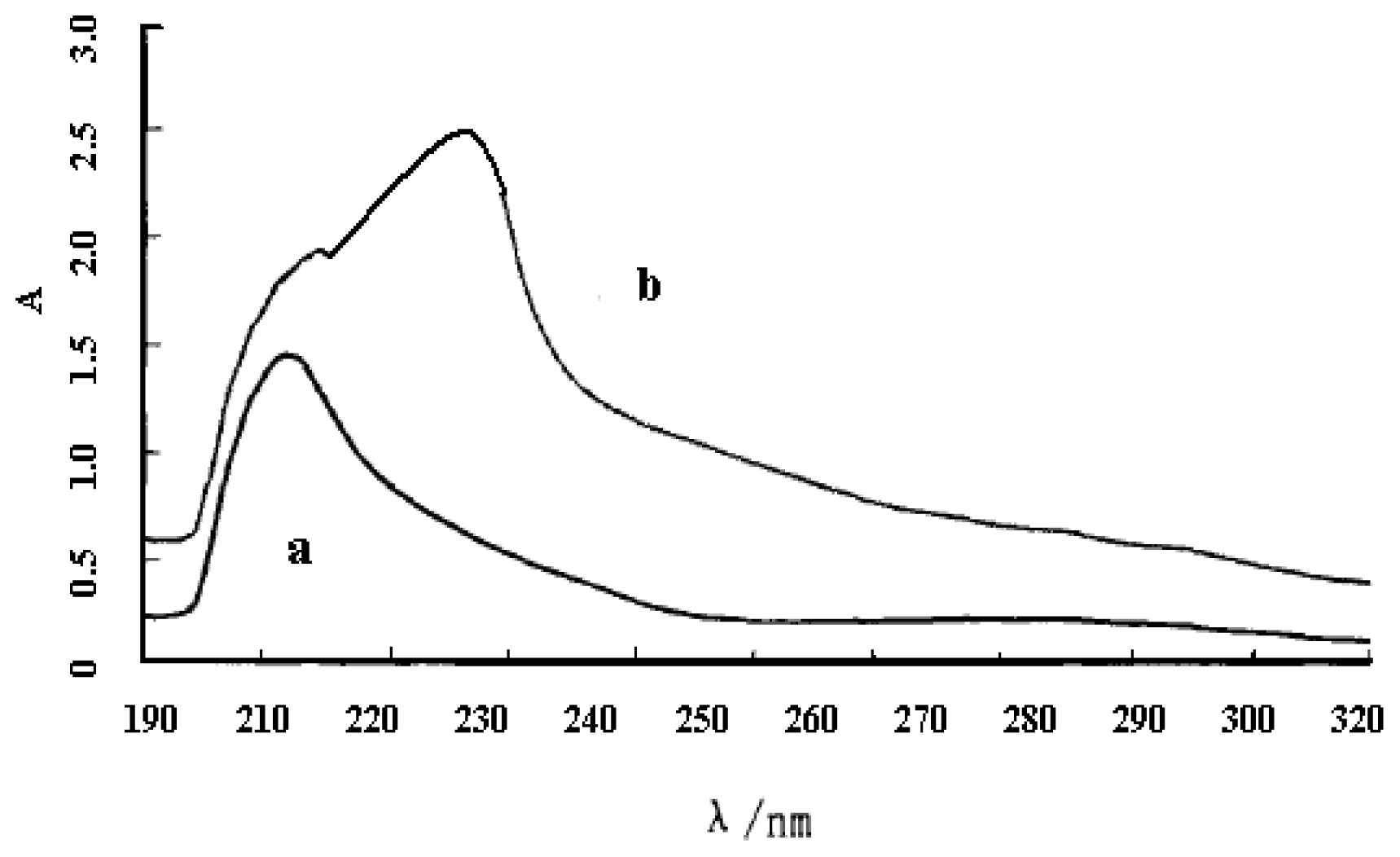
3.3. UV Spectrum Analysis
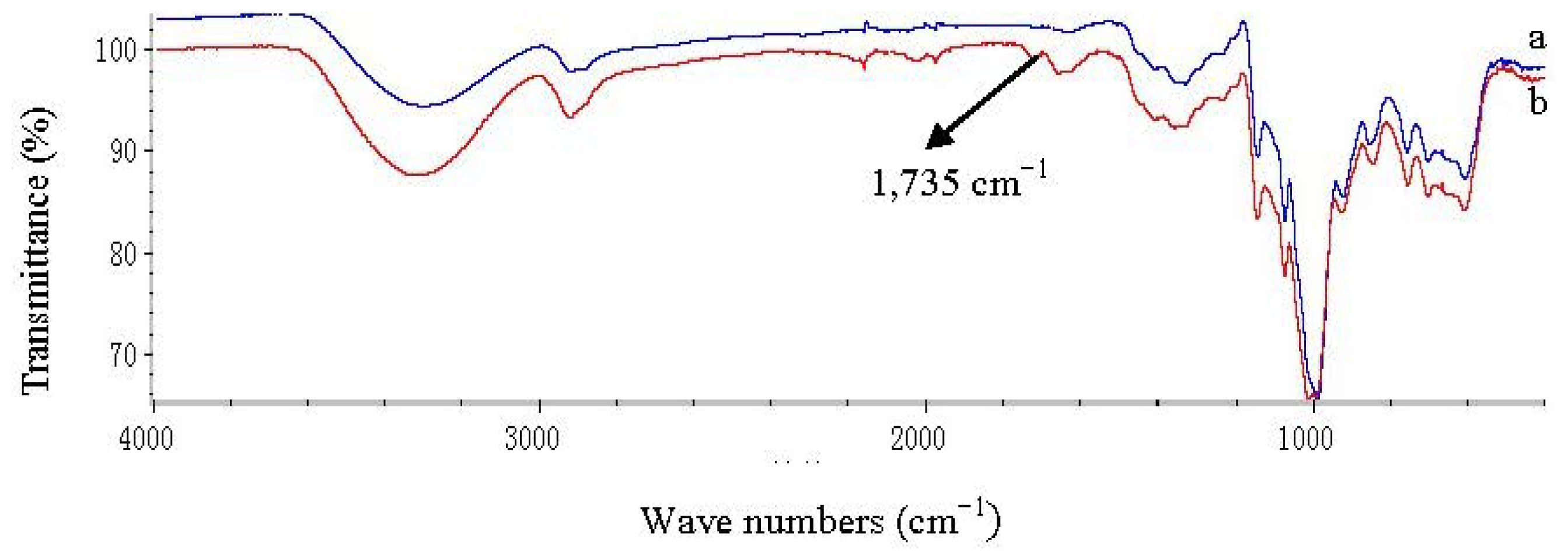
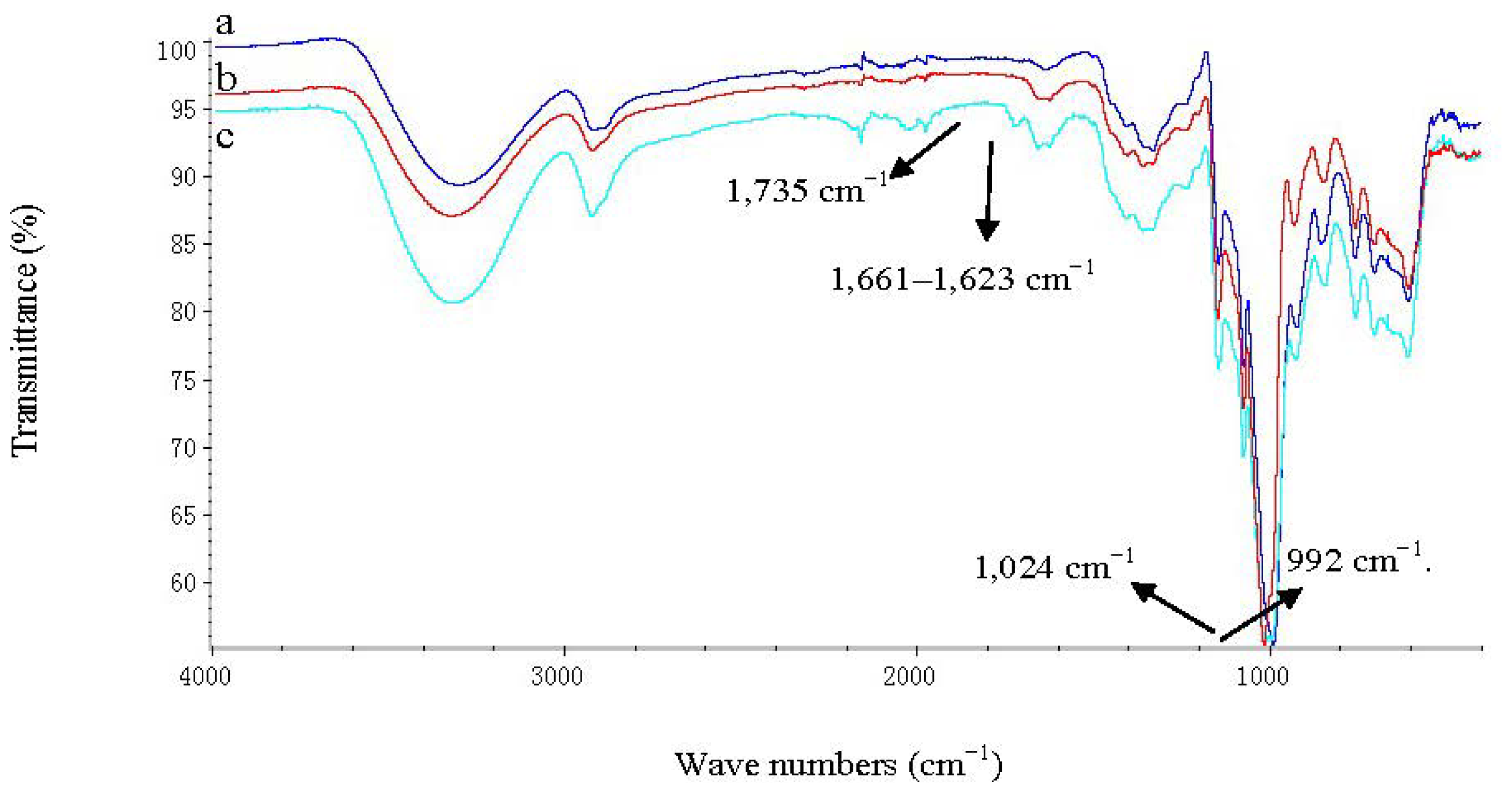
3.4. FT-IR Analysis
3.5. Emulsion Capacity and Emulsion Stability
4. Conclusions
Acknowledgments
References
- Doane, W.M. A research on starch-based biodegradable plastics. Starch 1992, 44, 293–295. [Google Scholar]
- Shogren, R.L.; Fanta, G.F.; Doane, W.M. Development of starch based plastics—A reexamination of selected polymer systems in historical perspective. Starch 1994, 45, 276–280. [Google Scholar]
- Miladinov, V.D.; Hanna, M.A. Starch esterification by reactive extrusion. Ind. Crop. Prod 2000, 11, 51–57. [Google Scholar]
- Apostolos, A.; Peter, J.H. Enzymatic acylation of starch. Bioresour. Technol 2011, 115, 41–47. [Google Scholar]
- Jorge, A.; Hassina, H.; Geneviève, M.B.; François, S.; Isabelle, A.; Elisabeth, B. Free-solvent synthesis and properties of higher fatty esters of starch—Part 2. Starch Stärke 1999, 51, 302–307. [Google Scholar]
- Ambuj, D.S.; Edward, W.M. Properties of fatty-acid esters of starch. J. Appl. Polym. Sci 1995, 58, 1647–1656. [Google Scholar]
- Bai, Y.J.; Shi, Y.C. Structure and preparation of octenyl succinic esters of granular starch, microporous starch and soluble maltodextrin. Carbohydr. Polym 2011, 83, 520–527. [Google Scholar]
- Marcin, L. Biocatalytic Esterification of Common Polysaccharides-Starch Modification Using Lipases. Proceedings of the 14th International Electronic Conference on Synthetic Organic Chemistry, Santiago, Chile, 1–30 November 2010; Available online: http://www.sciforum.net & http://www.usc.es/congresos/ecsoc/ accessed on 3 November 2010.
- Akhila, R.T.; Emilia, A. Enzymatic modification of cassava starch by bacterial lipase. Bioprocess Biosyst. Eng 2006, 29, 65–71. [Google Scholar]
- Aburto, J.; Alric, I.; Thiebaud, S.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Synthesis, characterization, and biodegradability of fatty-acid esters of amylose and starch. J. Appl. Polym. Sci 1999, 74, 1440–1451. [Google Scholar]
- Aburto, J.; Thiebaud, S.; Alric, I.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Properties of octanoated starch and its blends with polyethylene. Carbohydr. Polym 1997, 34, 101–112. [Google Scholar]
- Fang, J.M.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterisation of a series of chemically modified potato starches. Carbohydr. Polym 2002, 47, 245–252. [Google Scholar]
- Thiebaud, S.; Aburto, J.; Alric, I.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Properties of fatty-acid esters of starch and their blends with LDPE. J. Appl. Polym. Sci 1997, 65, 705–721. [Google Scholar]
- Habib, H.; Moncef, C.; Youssef, G.; Adel, S. Solvent-free lipase-catalyzed synthesis of long-chain starch esters using microwave heating: Optimization by response surface methodology. Carbohydr. Polym 2010, 79, 466–474. [Google Scholar]
- Kshirsagar, A.C.; Singhal, R.S. Optimization of starch oleate derivatives from native corn and hydrolyzed corn starch by response surface methodology. Carbohydr. Polym 2007, 69, 455–461. [Google Scholar]
- Zhou, J.P.; Zhang, L. Solubility of cellulose in NaOH/Urea aqueous solution. Polymer 2000, 32, 866–870. [Google Scholar]
- Apostolos, A.; Nina, B.; Sabine, L.F.; Bernhard, H.; Peter, J.H. Lipase-catalysed acylation of starch and determination of the degree of substitution by methanolysis and GC. BMC Biotechnol 2010, 10, 1–25. [Google Scholar]
- Kiyoshi, K.; Setsuko, T.; Tomoko, S.; Kazuhito, K. Complex formation, thermal properties, and in-vitro digestibility of gelatinized potato starch fatty acid mixtures. Food Hydrocoll 2012, 27, 228–234. [Google Scholar]
- Zhao, W.X.; Zheng, W.W.; Li, J.H.; Lin, H.H. Synthesis and characterization of starch fatty acid esters. Mod. Chem. Ind 2007, 27, 281–283. [Google Scholar]
- Huang, M.F.; Yu, J.G.; Ma, X.F. Studies on the properties of montmorillonite-reinforced thermoplastic starch composites. Polymer 2004, 45, 7017–7023. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xin, J.-Y.; Wang, Y.; Liu, T.; Lin, K.; Chang, L.; Xia, C.-G. Biosysthesis of Corn Starch Palmitate by Lipase Novozym 435. Int. J. Mol. Sci. 2012, 13, 7226-7236. https://doi.org/10.3390/ijms13067226
Xin J-Y, Wang Y, Liu T, Lin K, Chang L, Xia C-G. Biosysthesis of Corn Starch Palmitate by Lipase Novozym 435. International Journal of Molecular Sciences. 2012; 13(6):7226-7236. https://doi.org/10.3390/ijms13067226
Chicago/Turabian StyleXin, Jia-Ying, Yan Wang, Tie Liu, Kai Lin, Le Chang, and Chun-Gu Xia. 2012. "Biosysthesis of Corn Starch Palmitate by Lipase Novozym 435" International Journal of Molecular Sciences 13, no. 6: 7226-7236. https://doi.org/10.3390/ijms13067226
APA StyleXin, J.-Y., Wang, Y., Liu, T., Lin, K., Chang, L., & Xia, C.-G. (2012). Biosysthesis of Corn Starch Palmitate by Lipase Novozym 435. International Journal of Molecular Sciences, 13(6), 7226-7236. https://doi.org/10.3390/ijms13067226



