Relationships Between Base-Catalyzed Hydrolysis Rates or Glutathione Reactivity for Acrylates and Methacrylates and Their NMR Spectra or Heat of Formation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrolysis
2.1.1. δCβ Parameter
2.1.2. Hf Parameter
2.2. GSH Reactivity
3. Experimental Section
3.1. Monomers
3.2. NMR Spectra
3.3. Hydrolysis
3.4. Heats of Formation (Hf) and Standard Enthalpy of Formation (ΔHf°)
3.5. GSH Reactivity
3.6. Multi-Regression Analysis
4. Conclusions
Acknowledgements
References
- Mabey, W.; Mill, T. Critical review of hydrolysis of organic compounds in water under environmental conditions. J. Phys. Chem. Ref. Data 1978, 7, 383–415. [Google Scholar]
- Mallik, K.L.; Das, M.N. Alkaline hydrolysis of acrylic and methacrylic esters in 60% aqueous ethanol. Naturwissenschaften 1964, 51. [Google Scholar] [CrossRef]
- Sharma, R.C.; Sharma, M.M. Kinetics of fast alkaline hydrolysis of esters. J. Appl. Chem 1969, 19, 162–166. [Google Scholar]
- Sharma, R.C.; Sharma, M.M. Kinetics of alkaline hydrolysis of esters. II. Unsaturated esters and oxalic Esters. Bull. Chem. Soc. Jpn 1970, 43, 642–645. [Google Scholar]
- Kadoma, Y.; Tanaka, M. Acid and base-catalyzed hydrolysis of bisphenol A-related compounds. Dent. Mater. J 2000, 19, 139–152. [Google Scholar]
- Freidig, A.P.; Verhaar, H.J.M.; Hermen, J.L.M. Quantitative structure-property relationships for the chemical reactivity of acrylates and methacrylates. Environ. Toxicol. Chem 1999, 18, 1133–1139. [Google Scholar]
- Chemical Substance Control Law. Available online: http://www.safe.nite.go.jp/english/db.html accessed on 12 December 2011.
- Fujisawa, S.; Kadoma, Y.; Ishihara, M.; Atsumi, T.; Yokoe, I. Dipalmitoylphosphatidylcholine (DPPC) and DPPC/cholesterol liposomes as predictors of the cytotoxicity of bis-GMA related compounds. J. Liposome Res 2004, 14, 39–49. [Google Scholar]
- Kuznetsova, N.A.; Kazantsev, O.A.; Shirshin, K.V.; Khokhlova, T.A.; Malyshev, A.P. Hydrolysis of N,N-dimethylaminoethyl methacrylate and its salts in concentrated aqueous solution. Russ. J. Appl. Chem 2003, 7, 1117–1120. [Google Scholar]
- Ishihara, M.; Fujisawa, S. A structure-activity relationship study on the mechanisms of methacrylate-induced toxicity using NMR chemical shift of β-carbon, RP-HPLC log P and semiempirical molecular descriptor. Dent. Mater. J 2009, 28, 113–120. [Google Scholar]
- Fujisawa, S.; Kadoma, Y. Prediction of the reduced glutathione (GSH) reactivity of dental methacrylate monomers using NMR spectra—Relationship between toxicity and GSH reactivity. Dent. Mater. J 2009, 28, 722–729. [Google Scholar]
- Fujisawa, S.; Kadoma, Y. Mechanism of action of (meth)acrylayes in hemolytic activity, in vivo toxicity and dipalmitoylphosphatidylcholine (DPPC) liposomes determined using NMR spectroscopy. Int. J. Mol. Sci 2012, 13, 758–773. [Google Scholar]
- Nakajima, M.; Sakuratani, Y.; Noguchi, Y.; Yamada, J.; Hori, K. Development of hydrolysis prediction system using reaction analysis with quantum chemical calculation. J. Comput. Aided Chem 2007, 18, 103–113. [Google Scholar]
- Hatada, K.; Kitayama, T.; Nishiura, T.; Shibuya, W. Relation between reactivities of vinyl monomers and their NMR spectra. Curr. Org. Chem 2002, 6, 121–153. [Google Scholar]
- Ishihara, M.; Fujisawa, S. Quantum-chemical descriptors for estimating hemolytic activity of aliphatic and aromatic methacrylates. Chemosphere 2008, 70, 1898–1902. [Google Scholar]
- Mill, T.; Haag, W.; Penwell, P.; Pettit, T.; Johnson, H. Environmental Fate and Exposure Studies Development of a PC-SAR for Hydrolysis: Esters, Alkyl Halides and Epoxides; EPA Contract No. 68-02-4254; SRI International: Menlo Park, CA, USA, 1987. [Google Scholar]
- Lawrence, W.H.; Bass, G.E.; Purcell, W.P.; Autian, J. Use of mathematical models in the study of structure-toxicity relationships of dental compounds: I. Esters of acrylic and methacrylic acids. J. Dent. Res 1972, 51, 526–535. [Google Scholar]
- Fujisawa, S.; Kadoma, Y.; Komoda, Y. Changes in NMR chemical shifts of methacrylates induced by their interactions with the phospholipid and the phospholipid/cholesterol liposome system. Dent. Mater. J 1990, 9, 100–107. [Google Scholar]
- Collett, T.W. Ester hydrolysis rate constant prediction from infrared interferograms. Environ. Sci. Technol 1990, 24, 1671–1676. [Google Scholar]
- Design Institute for Physical Properties Research (DIPPR). American Institute of Chemical Engineers, Project 801. 2006. Available online: http://www.aiche.org/dippr/products/801.aspx accessed on 12 December 2011.
- Vatani, A.; Mehrpooya, M.; Gharagheizi, F. Prediction of standard enthalpy of formation by a QSAR model. Int. J. Mol. Sci 2003, 8, 407–432. [Google Scholar]
- McCarthy, T.J.; Hayes, E.P.; Schwartz, C.S.; Witz, G. The reactivity of selected acrylate esters toward gluthatione and deoxyribonucleosides in vitro structure-activity relationships. Fundam. Appl. Toxicol 1994, 22, 543–548. [Google Scholar]
- Putz, M.V.; Ionaşcu, C.; Putz, A.M.; Ostafe, V. Alert QSAR. Implication for electrophilic theory of chemical carcinogenesis. Int. J. Mol. Sci 2011, 12, 5098–5134. [Google Scholar]
- Hansch, C.; Kurup, A.; Garg, R.; Gao, H. Chem-bioinformatics and QSAR: A review of QSAR lacking positive hydrophobic terms. Chem. Rev 2001, 101, 619–672. [Google Scholar]
- Fujisawa, S.; Kadoma, Y. Relationship between phenol-induced cytotoxicity and experimental inhibition rate constants or theoretical parameters. Mini Rev. Med. Chem 2012, in press. [Google Scholar]
- Østergaard, J.; Larsen, C. Bioreversible derivatives of phenol. 2. Reactivity of carbonate esters with fatty acid-like structures towards hydrolysis in aqueous solutions. Molecules 2007, 12, 2396–2412. [Google Scholar]
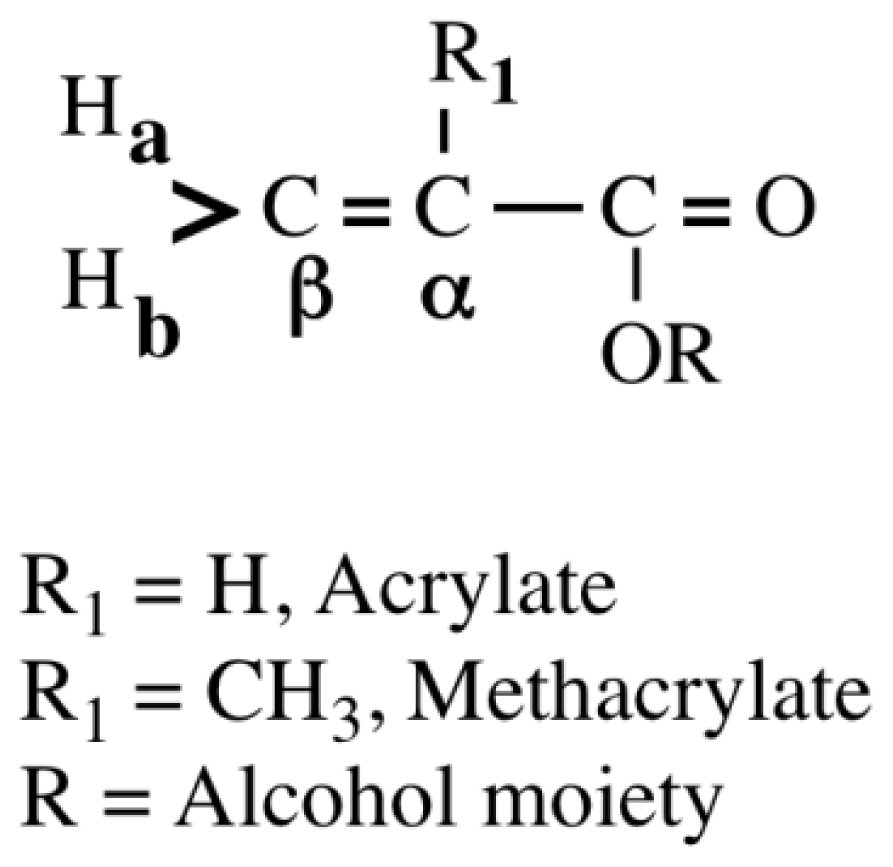
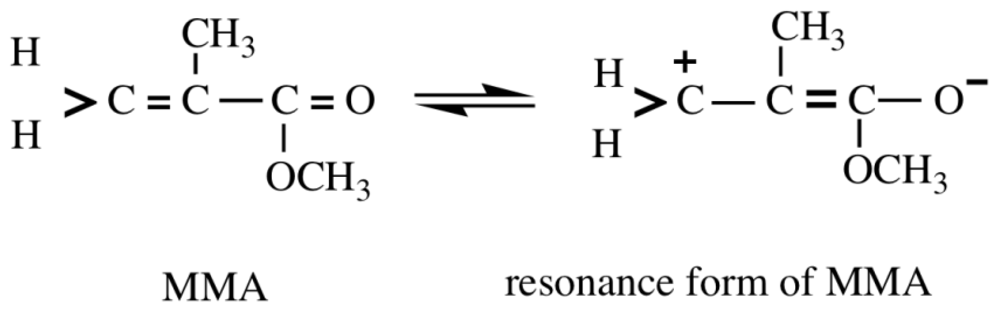
| Monomera | 13C NMR Chemical Shifts | Reported k1 at 20 °C (mol−1·s−1)c | Calculated from Equation (1) | Reported k3 at 30 °C (mol−1·s−1)d | Reported log kGSH (mol−1·min−1)c | Calculated from Equation (5) | |
|---|---|---|---|---|---|---|---|
| ppmb δCβ | ppmb δCα | ||||||
| MA | 130.56 | 128.15 | – | – | 0.198 | – | 1.68 |
| EA | 130.24 | 128.59 | 0.050 | – | 0.102 | 1.6 | 1.54 |
| nPA | 130.22 | 128.57 | – | – | – | – | 1.53 |
| nBA | 130.21 | 128.61 | – | – | 0.074 | – | 1.53 |
| isoBA | 130.23 | 128.6 | 0.020 | – | – | 1.6 | 1.54 |
| HA | 130.23 | 128.63 | 0.087 | – | – | 1.3 | 1.54 |
| MMA | 125.23 | 136.15 | 0.026 | 0.039 | 0.083 | −0.7 | −0.68 |
| EMA | 124.97 | 136.51 | – | 0.003 | – | – | −0.79 |
| isoPMA | 124.95 | 136.52 | 0.008 | 0.0002 | – | −1.0 | −0.8 |
| nBMA | 124.7 | 136.41 | (0.0027)e | nd | – | – | −0.91 |
| isoBMA | 124.98 | 136.52 | 0.007 | 0.0043 | – | −0.73 | −0.79 |
| Benzyl MA | 125.66 | 136.21 | 0.110 | 0.097 | – | −0.49 | −0.49 |
| Allyl MA | 125.46 | 136.23 | 0.059 | 0.070 | – | −0.52 | −0.58 |
| 1H NMR Chemical Shiftsb | Charge Densitye (C=O) a.u. | |||||
|---|---|---|---|---|---|---|
| Monomera | Ha ppm | Hb ppm | H ppm | |δHa–δHb|c ppm | Qσ(C=O)d | |
| MA | 5.825 | 6.406 | 0.581 | 0.581 | 0.1666 | – |
| EA | 5.807 | 6.395 | 6.113 | 0.587 | 0.1662 | 1.06 |
| nPA | 5.809 | 6.397 | 6.127 | 0.588 | – | – |
| nBA | 5.805 | 6.391 | 6.119 | 0.586 | 0.1662 | |
| isoBA | 5.813 | 6.4 | 6.113 | 0.587 | 0.1662 | 1.04 |
| Hexyl A | 5.804 | 6.391 | 6.12 | 0.587 | – | 1.03 |
| Benzyl A | 5.83 | 6.349 | 6.162 | 0.519 | – | – |
| MMA | 5.55 | 6.1 | – | 0.55 | 0.1638 | 0.94 |
| EMA | 5.541 | 6.09 | – | 0.555 | 0.1634 | – |
| nPMA | 5.54 | 6.1 | – | 0.56 | – | – |
| nBMA | 5.532 | 6.091 | – | 0.559 | 0.1634 | – |
| isoBMA | 5.543 | 6.108 | – | 0.565 | – | 0.91 |
| Benzyl MA | 5.572 | 6.153 | – | 0.581 | – | 0.88 |
| Allyl MA | 5.574 | 6.138 | – | 0.564 | – | 0.94 |
| Hexyl MA | 5.537 | 6.092 | – | 0.555 | – | – |
| Monomera | Heat of Formation (Hf) (kcal mol−1)b | Enthalpy of Formation (ΔHf°) (kJ mol−1)c | Reported k1 (mol−1sec−1)d 20 °C | Calculated from Equation (2) | Calculated from Equation (3) | Reported k2 (mol−1s−1)e 30 °C | Calculated from Equation (4) |
|---|---|---|---|---|---|---|---|
| MA | −67.387 | −362.2 | – | 0.054 | 0.052 | 0.015 | 0.016 |
| EA | −72.173 | −379.59 | 0.05 | 0.040 | 0.044 | 0.013 | 0.011 |
| nPA | −77.404 | −407.17 | – | 0.025 | 0.029 | – | 0.005 |
| nBA | −82.791 | −433.45 | – | 0.011 | 0.020 | – | nd |
| isoBA | −82.435 | −438.95 | 0.02 | 0.012 | 0.018 | – | nd |
| MMA | −74.768 | −399.13 | 0.026 | 0.032 | 0.036 | 0.008 | 0.009 |
| EMA | −79.542 | −421.34 | – | 0.020 | 0.026 | 0.003 | 0.003 |
| nPMA | −84.767 | −446.7 | – | 0.006 | 0.014 | – | nd |
| nBMA | −90.156 | −471.39 | – | nd | 0.0004 | – | nd |
| isoBMA | −89.832 | −465.16 | 0.007 | nd | 0.006 | – | nd |
| Benzyl MA | −49.295 | – | 0.11 | 0.1 | 0.091 | – | 0.021 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fujisawa, S.; Kadoma, Y. Relationships Between Base-Catalyzed Hydrolysis Rates or Glutathione Reactivity for Acrylates and Methacrylates and Their NMR Spectra or Heat of Formation. Int. J. Mol. Sci. 2012, 13, 5789-5800. https://doi.org/10.3390/ijms13055789
Fujisawa S, Kadoma Y. Relationships Between Base-Catalyzed Hydrolysis Rates or Glutathione Reactivity for Acrylates and Methacrylates and Their NMR Spectra or Heat of Formation. International Journal of Molecular Sciences. 2012; 13(5):5789-5800. https://doi.org/10.3390/ijms13055789
Chicago/Turabian StyleFujisawa, Seiichiro, and Yoshinori Kadoma. 2012. "Relationships Between Base-Catalyzed Hydrolysis Rates or Glutathione Reactivity for Acrylates and Methacrylates and Their NMR Spectra or Heat of Formation" International Journal of Molecular Sciences 13, no. 5: 5789-5800. https://doi.org/10.3390/ijms13055789




