Comparison of Ultrasonic and CO2 Laser Pretreatment Methods on Enzyme Digestibility of Corn Stover
Abstract
:1. Introduction
2. Results and Discussion
2.1. Compositional Analysis of Raw Corn Stover Sample
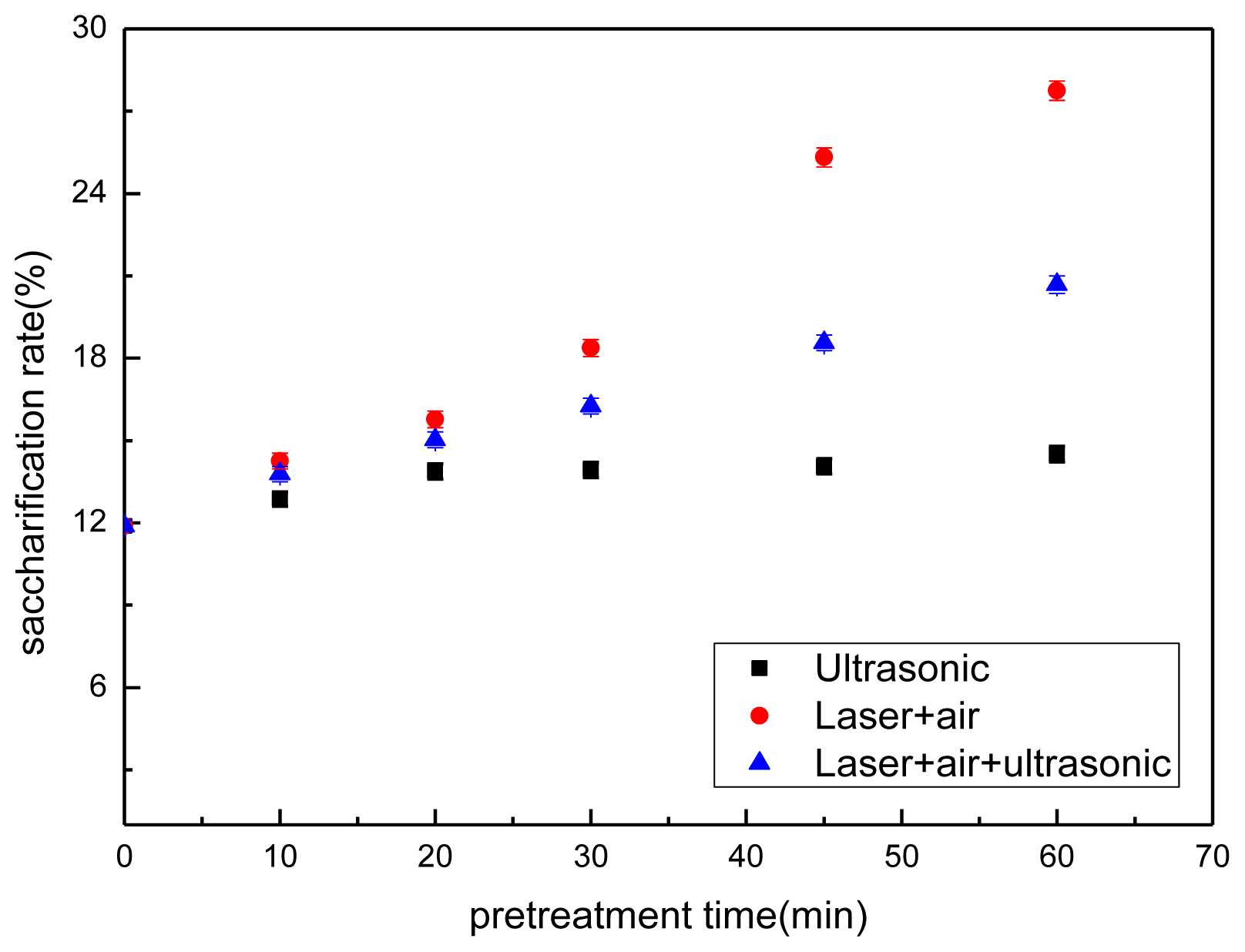
2.2. Enzymatic Hydrolysis
2.3. Effect of Pretreatment on Sugar Content Hydrolysate
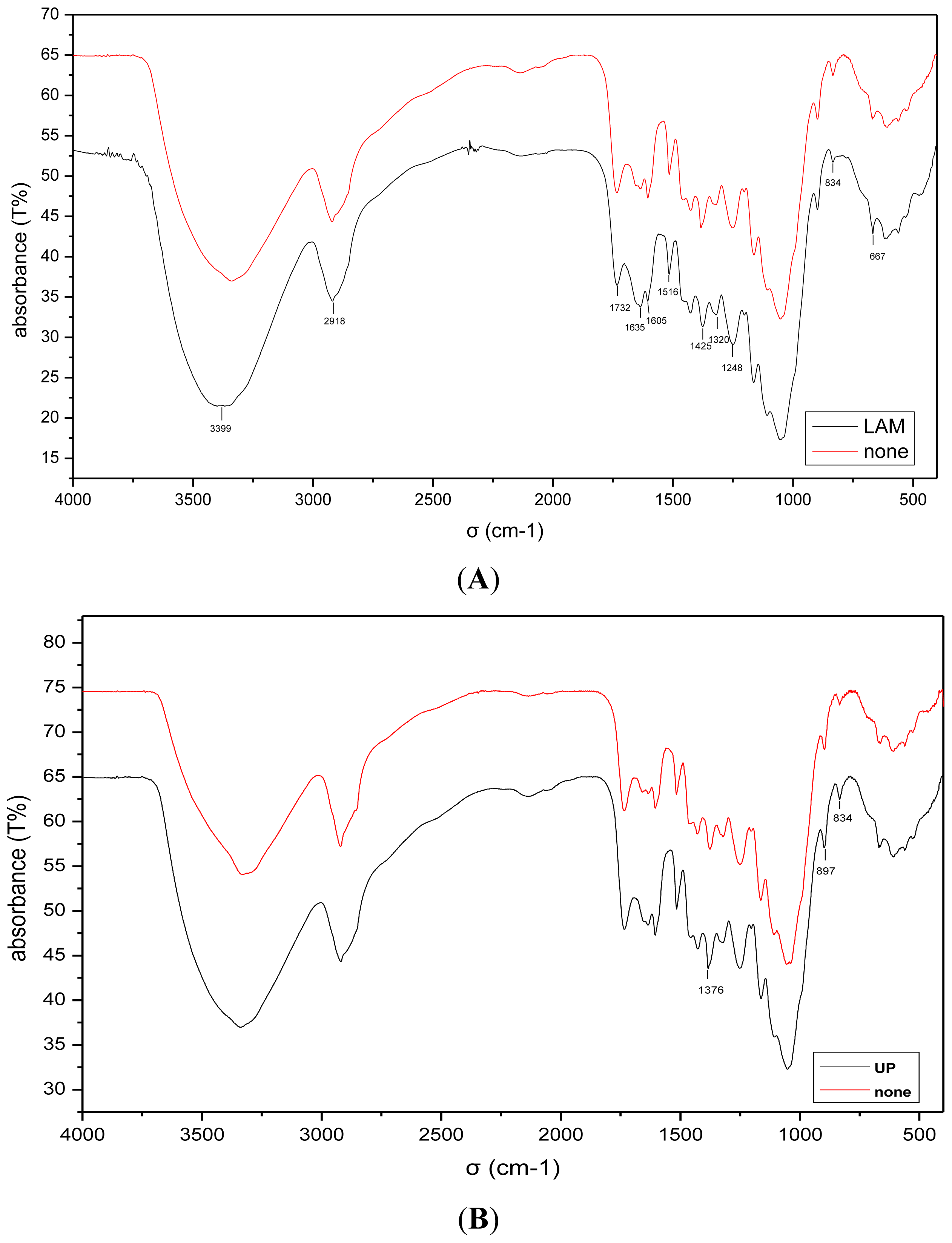
2.4. Analysis of the FT-IR Spectroscopy
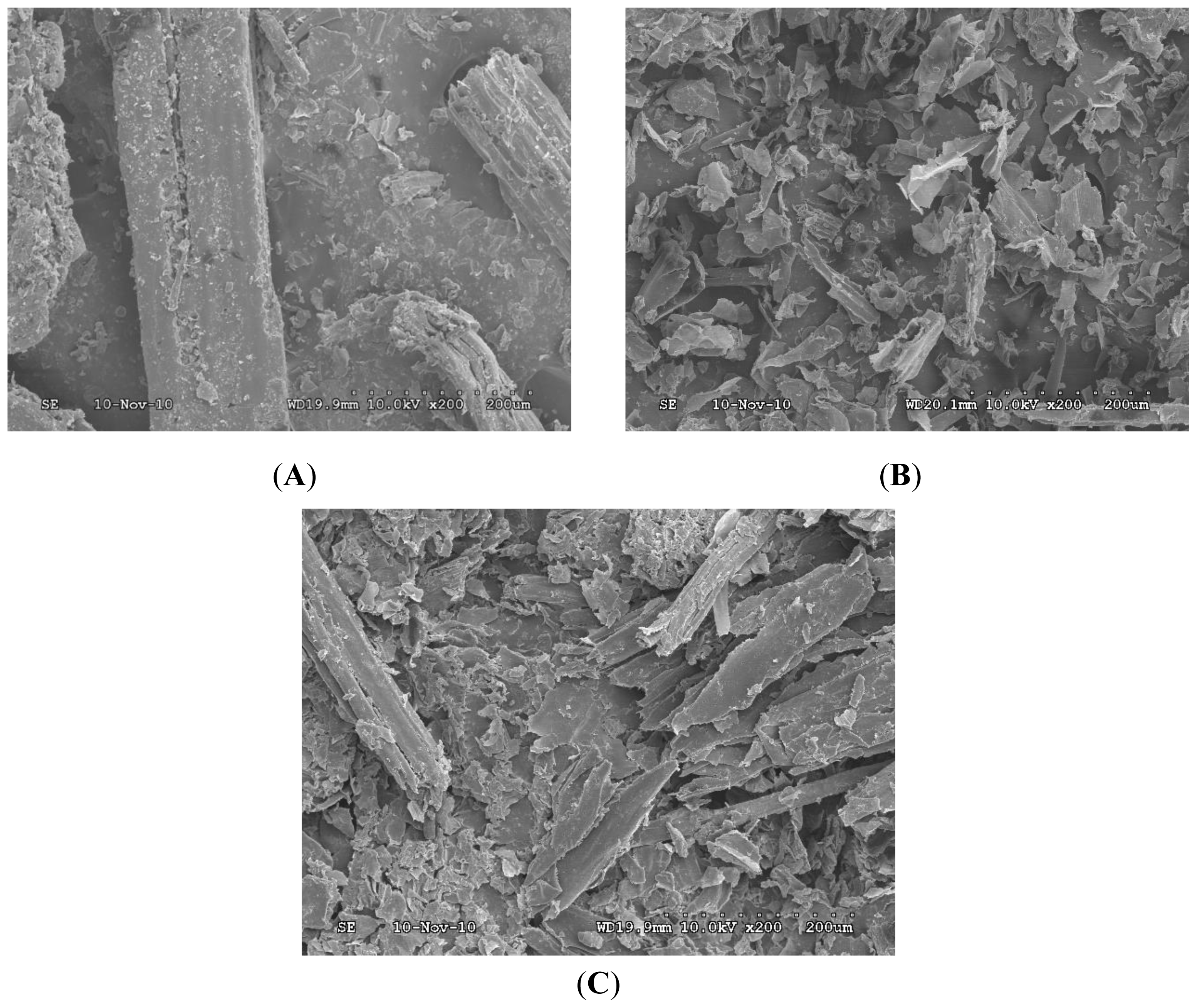
2.5. Effect of Pretreatments on Morphology
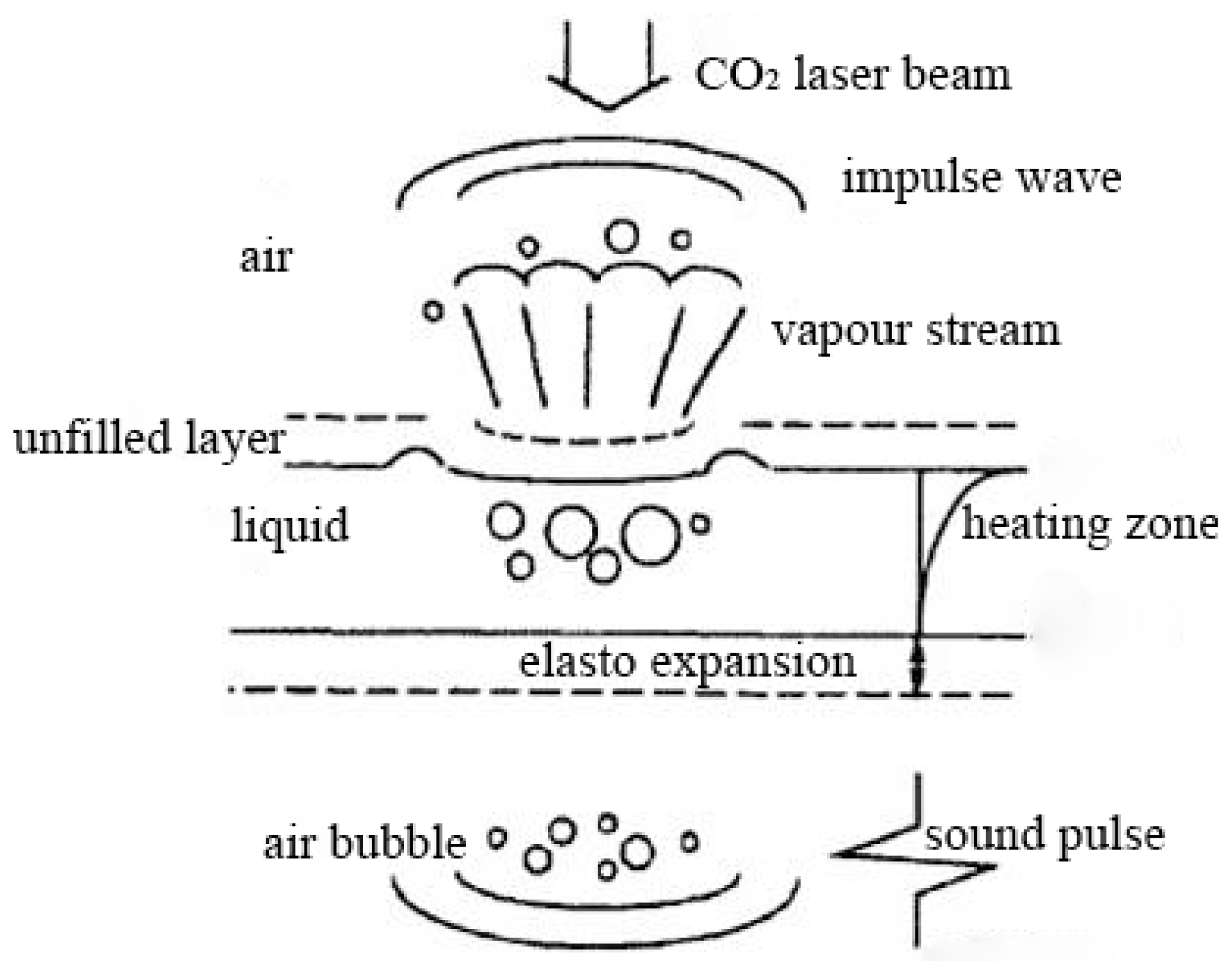
2.6. Mechanisms of LAM Pretreatment
3. Experimental Section
3.1. Materials and Equipment
3.2. Composition of Corn Stover Biomass
3.3. Pretreatment of Corn Stover Samples
3.4. Enzymatic Hydrolysis of Pretreated Corn Stover
3.5. Hydrolysate Analysis
3.6. Spectral Analysis of Pretreated Samples
3.7. Surface Morphology Observation by SEM
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Sassner, P.; Galbe, M.; Zacchi, G. Techno-economic evaluation of bioethanol production from three different lignocellulosic materials. Biomass Bioenergy 2008, 32, 422–430. [Google Scholar]
- Sun, Y.; Cheng, J.Y. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol 2002, 83, 1–11. [Google Scholar]
- Kumar, P.; Barrett, D.M.; Delwiche, M.D.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res 2009, 48, 3713–3729. [Google Scholar]
- Xu, J.; Thomsen, M.H.; Thomsen, A.B. Pretreatment on corn stover with low concentration of formic acid. J. Microbiol. Biotechnol 2009, 19, 845–850. [Google Scholar]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol 2010, 101, 4744–4753. [Google Scholar]
- Bommarius, A.S.; Katona, A.; Cheben, S.E.; Patel, A.S.; Ragauskas, A.J.; Knudson, K.; Pu, Y.Q. Cellulase kinetics as a function of cellulose pretreatment. Metab. Eng 2008, 10, 370–381. [Google Scholar]
- Ghazali, A.; Wan Rosli, W.D.; Law, K.N. Pre-treatment of oil palm biomass for alkaline peroxide pulping. Cellul. Chem. Technol 2009, 43, 329–336. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol 2005, 96, 673–686. [Google Scholar]
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J. Use of US croplands for biofuels increases greenhouse gases through emissions from land use change. Science 2008, 319, 1238–1244. [Google Scholar]
- Nath, A.K.; Reghu, T.; Paul, C.P.; Ittoop, M.O.; Bhargava, P. High-power transverse flow CW CO2 laser for material processing applications. Opt. Laser Technol 2005, 37, 329–335. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci 1991, 74, 3583–3597. [Google Scholar]
- David, P.; Marcia, P. Corn and Cellulosic Ethanol Cause Major Problems. Energies 2008, 1, 35–37. [Google Scholar]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y.; Mitchinson, C.; Saddler, J.N. Comparative sugar recovery and fermentation data following pretreatment of poplar wood by leading technologies. Biotechnol. Prog 2009, 25, 333–339. [Google Scholar]
- Zheng, M.; Li, X.; Li, L.; Yang, X.; He, Y. Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour. Technol 2009, 100, 5140–5145. [Google Scholar]
- Smidt, E.; Meissl, K. The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag 2007, 27, 268–276. [Google Scholar]
- Yu, C.T.; Chen, W.H.; Men, L.C.; Hwang, W.S. Microscopic structure features changes of rice straw treated by boiled acid solution. Ind. Crops Prod 2009, 29, 308–315. [Google Scholar]
- Tian, S.; Wang, Z.; Zuo, L.; Fan, Z. Optimization of CO2 laser-based pretreatment of corn stover using response surface methodology. Bioresour. Technol 2011, 102, 10493–10497. [Google Scholar]
- Monobe, H.; Awazu, K.; Shimizu, Y. Alignment control of columnar liquid crystals with wavelength tunable CO2 laser irradiation. Thin Solid Films 2008, 516, 2677–2681. [Google Scholar]
- Soni, R.K.; Mandloie, V.K.; Pote, M.B.; Nath, A.K. Spinning cone water film power meter for high-power CO2 lasers. Opt. Laser Technol 2007, 39, 196–201. [Google Scholar]
- Baba, Y.; Tanabe, T.; Shirai, N.; Watanabe, T.; Honda, Y.; Watanabe, T. Pretreatment of Japanese cedar wood by white rot fungi and ethanolysis for bioethanol production. Biomass Bioenergy 2011, 35, 320–324. [Google Scholar]
- Gong, G.; Liu, D.; Huang, Y. Microwave-assisted organic acid pretreatment for enzymatic hydrolysis of rice straw. Biosys. Eng 2010, 107, 67–73. [Google Scholar]
- Liu, J.; Takada, R.; Karita, S.; Watanabe, T.; Honda, Y.; Watanabe, T. Microwave-assisted pretreatment of recalcitrant softwood in aqueous glycerol. Bioresour. Technol 2010, 101, 9355–9360. [Google Scholar]
- Verma, P.; Watanabe, T.; Honda, Y.; Watanabe, T. Microwave-assisted pretreatment of woody biomass with ammonium molybdate activated by H2O2. Bioresour. Technol 2011, 102, 3941–3945. [Google Scholar]
- Taghizadeh, M.T.; Mehrdad, A. Calculation of the rate constant for the ultrasonic degradation of aqueous solutions of polyvinyl alcohol by viscometry. Ultrason. Sonochem 2003, 10, 309–313. [Google Scholar]
- Zhu, J.Y.; Pan, X.; Zalesny, R.S., Jr. Pretreatment of woody biomass for biofuel production: Energy efficiency, technologies, and recalcitrance. Appl. Microbiol. Biotechnol. 2010, 87, 847–857. [Google Scholar]
- Adney, B.; Nrel, J.B. Measurement of Cellulase Activities; LAP-006 NREL Analytical Procedure; National Renewable Energy Laboratory: Golden, CO, USA, 1996. [Google Scholar]
- Qi, B.; Chen, X.; Shen, F.; Su, Y.; Wan, Y. Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Ind. Eng. Chem. Res 2009, 48, 7346–7353. [Google Scholar]
- Hao, L.; Lawrence, J.; Phua, Y.F.; Chian, K.S.; Lim, G.C.; Zheng, H.Y. Enhanced human osteoblast cell adhesion and proliferation on 316 LS stainless steel by means of CO2 laser surface treatment. J. Biomed. Mater. Res. B 2005, 73, 148–156. [Google Scholar]
- Utley, D.S.; Koch, R.J.; Egbert, B.M. Histologic analysis of the thermal effect on epidermal and dermal structures following treatment with the superpulsed CO2 laser and the Erbium:YAG laser: An in vivo study. Laser Surg. Med 1999, 24, 93–102. [Google Scholar]
- Ohkubo, T.; Yabe, T.; Yoshida, K.; Uchida, S.; Funatsu, T.; Bagheri, B.; Oishi, T.; Daito, K.; Ishioka, M.; Nakayama, Y.; et al. Solar-pumped 80 W laser irradiated by a Fresnel lens. Opt. Lett 2009, 34, 175–177. [Google Scholar]

| Sample | Xylose (mg/g biomass) | Glucose (mg/g biomass) | Cellobiose (mg/g biomass) |
|---|---|---|---|
| CO2 laser pretreated hydrolysate | 15.03 | 131.20 | 4.67 |
| Ultrasonic pretreated hydrolysate | 20.61 | 101.13 | 7.07 |
| Non-pretreatment hydrolysate | 5.33 | 15.00 | trace amount |
| Wave Number(σ/cm−1) | Intensity of Absorption Band | Absorption Peak Assignment |
|---|---|---|
| 3338 | steep | OH stretching in alcohol and phenol |
| 2921 | moderate | C–H symmetrical and asymmetrical stretching in –CH3 and –CH2– Organic acid COO– asymmetrical stretch |
| 1650–1630 | semi-steep | Lignin and aromatic ring conjugated C=O stretch |
| 1509–1515 | moderate | Lignin and other aromatic ring skeletal stretch –CH2– scissoring deformation in carbonhydrates and fatty compounds |
| 1462 | infirm | C–H deformations (asym. in –CH3 and –CH2–) in lignin and carbonhydrates |
| 1421 | steep | Aromatic skeletal vibrations combined with C–H in-plane deformations |
| 1325 | moderate | C–H vibration in cellulose and C1–O vibration in syringyl derivatives |
| 1265 | moderate | Aromatic skeletal vibrations, guaiacyl, C=O stretch |
| 1160 | faint | C–O–C vibration in cellulose and hemicellulose |
| 1117–1124 | infirm | C–H aromatic ring, syringyl C–O stretch in cellulose and hemicellulose |
| 1049 | moderate | Si–O stretch in amorphous SiO2 |
| 898 | faint | C–H deformation in cellulose and saccharide |
| 666 | faint | Single-plane vibration of substituted aromatics |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tian, S.-Q.; Wang, Z.-Y.; Fan, Z.-L.; Zuo, L.-L. Comparison of Ultrasonic and CO2 Laser Pretreatment Methods on Enzyme Digestibility of Corn Stover. Int. J. Mol. Sci. 2012, 13, 4141-4152. https://doi.org/10.3390/ijms13044141
Tian S-Q, Wang Z-Y, Fan Z-L, Zuo L-L. Comparison of Ultrasonic and CO2 Laser Pretreatment Methods on Enzyme Digestibility of Corn Stover. International Journal of Molecular Sciences. 2012; 13(4):4141-4152. https://doi.org/10.3390/ijms13044141
Chicago/Turabian StyleTian, Shuang-Qi, Zhen-Yu Wang, Zi-Luan Fan, and Li-Li Zuo. 2012. "Comparison of Ultrasonic and CO2 Laser Pretreatment Methods on Enzyme Digestibility of Corn Stover" International Journal of Molecular Sciences 13, no. 4: 4141-4152. https://doi.org/10.3390/ijms13044141




