A Comparative Study on the Expression, Purification and Functional Characterization of Human Adiponectin in Pichia pastoris and Escherichia coli
Abstract
:1. Introduction
2. Results
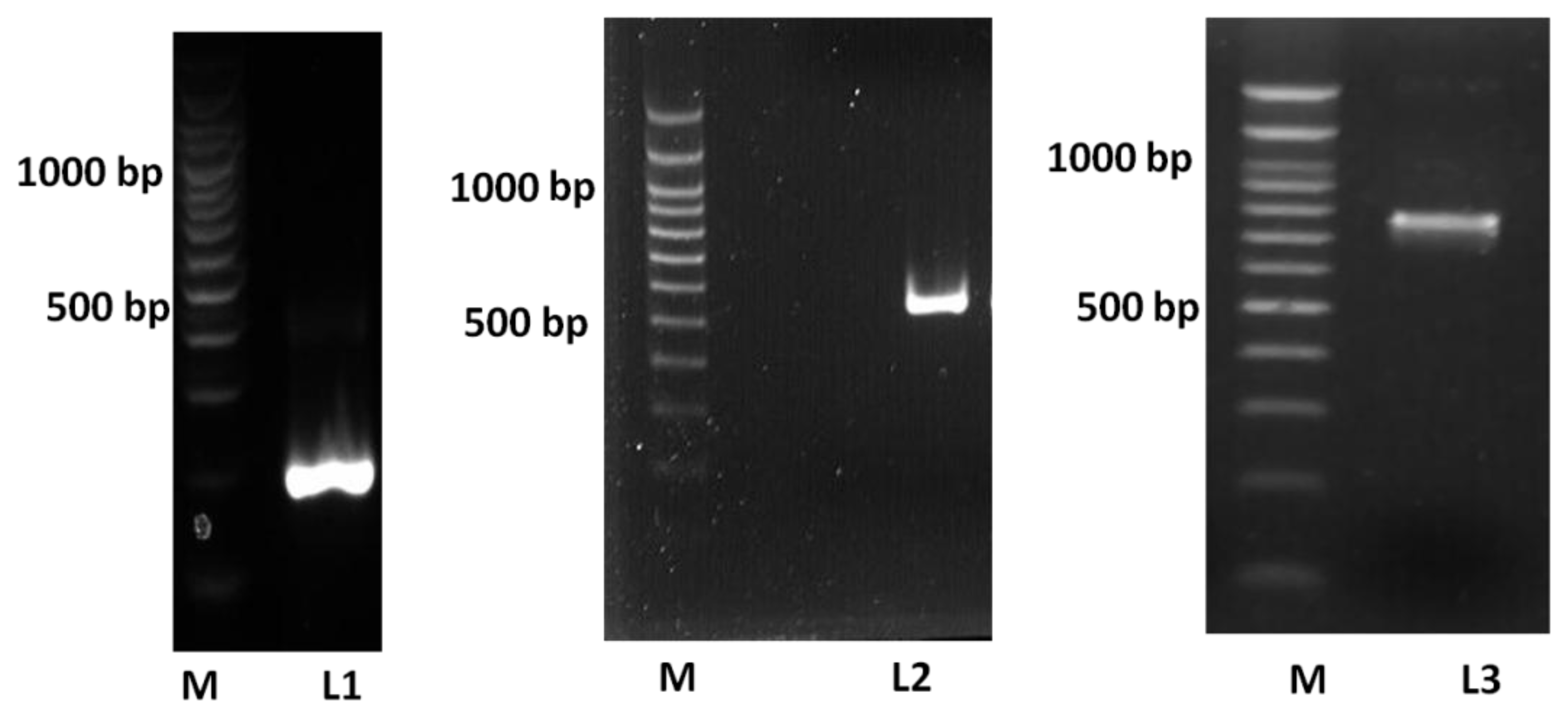
2.1. Construction and Cloning of Human ADP in P. pastoris and E. coli
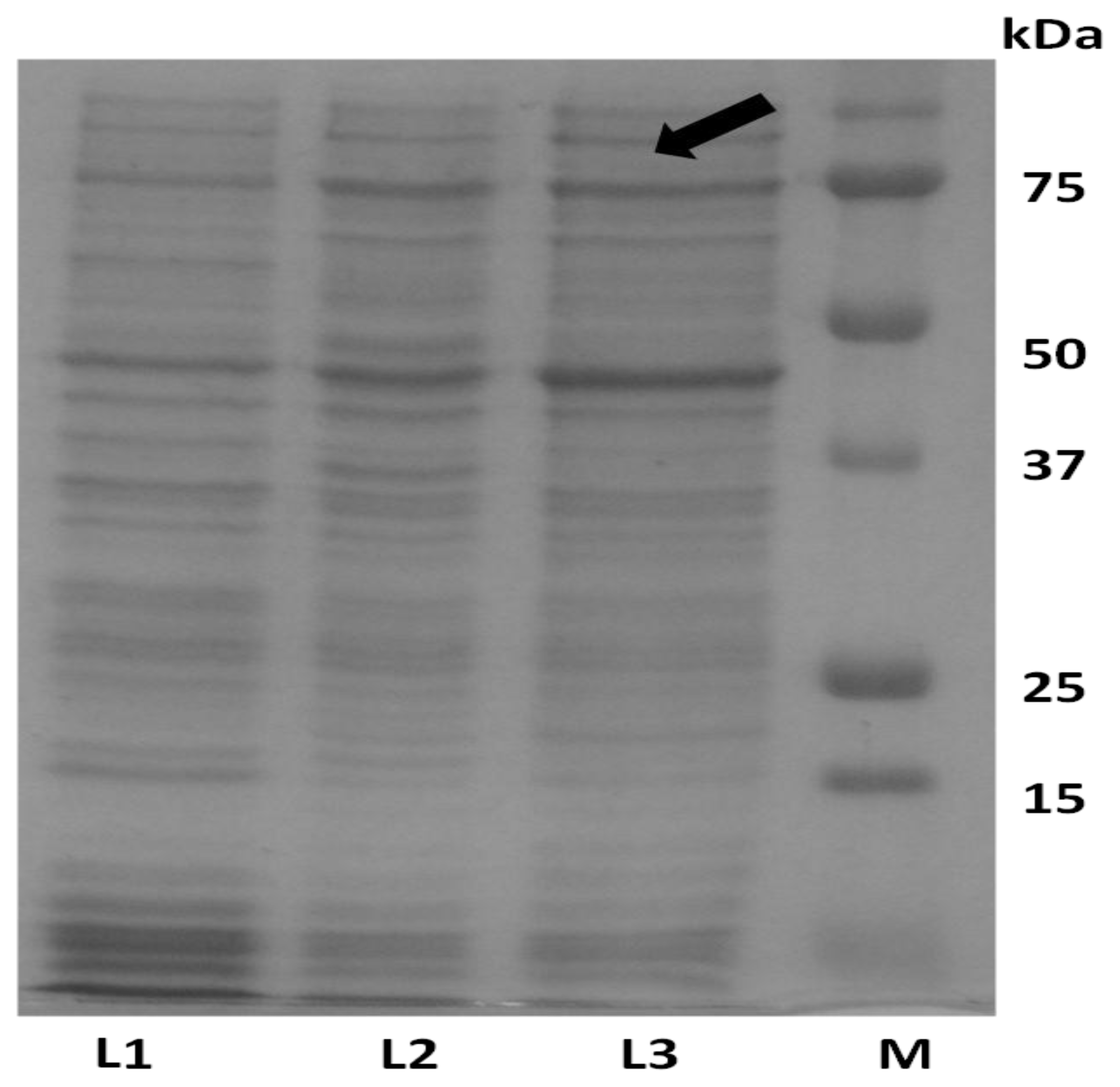
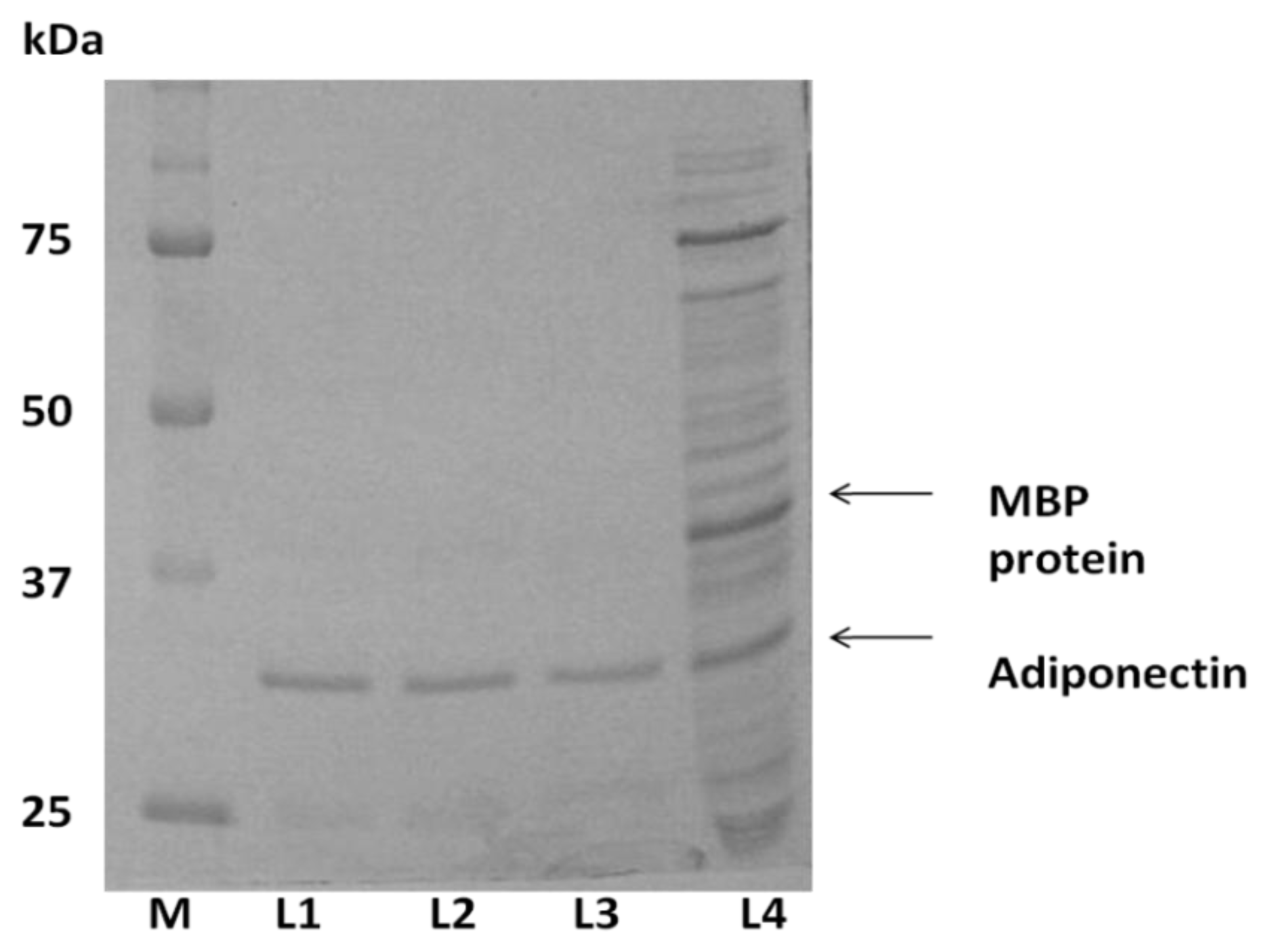
2.1. Expression of ADP in E. coli and Protein Purification
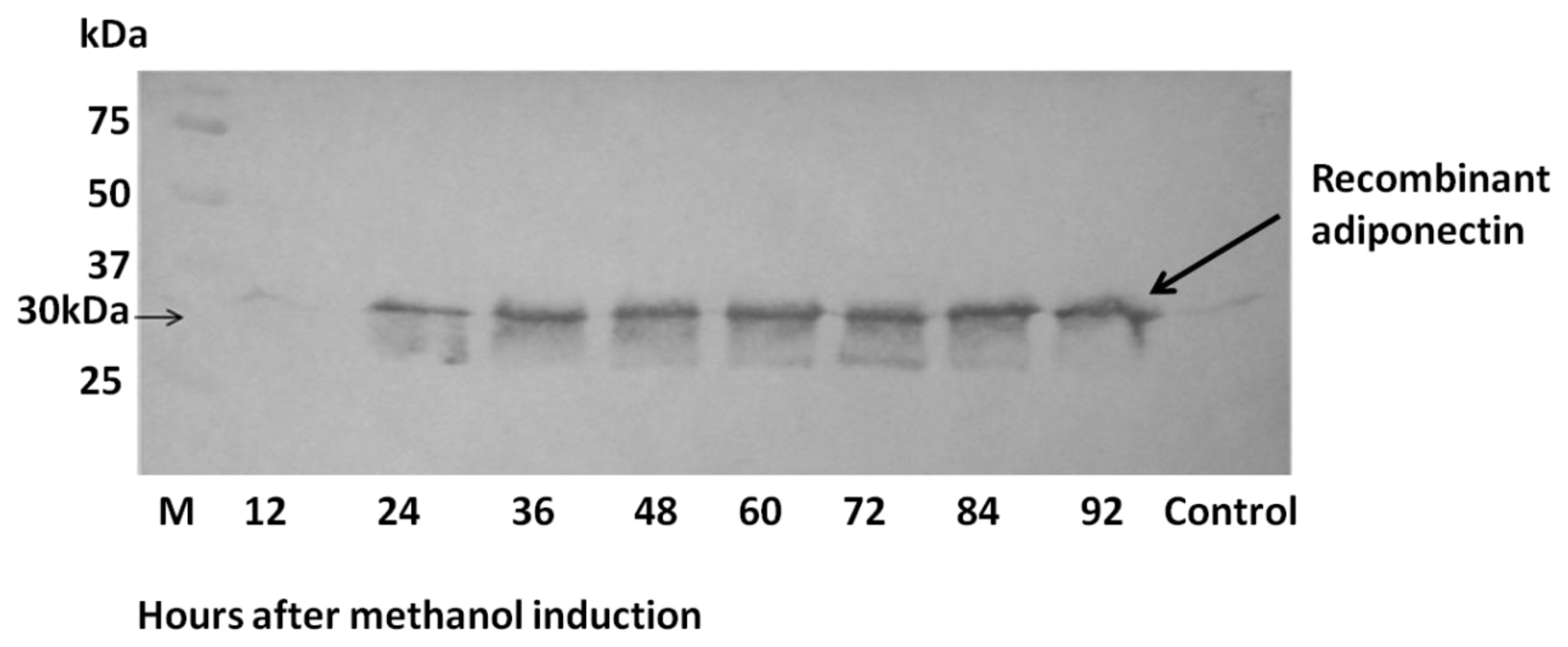
2.3. Expression of ADP in Pichia pastoris and Protein Yield Optimization
2.4. Analysis of Recombinant Adiponectin Protein Produced by E.coli and P.pastoris
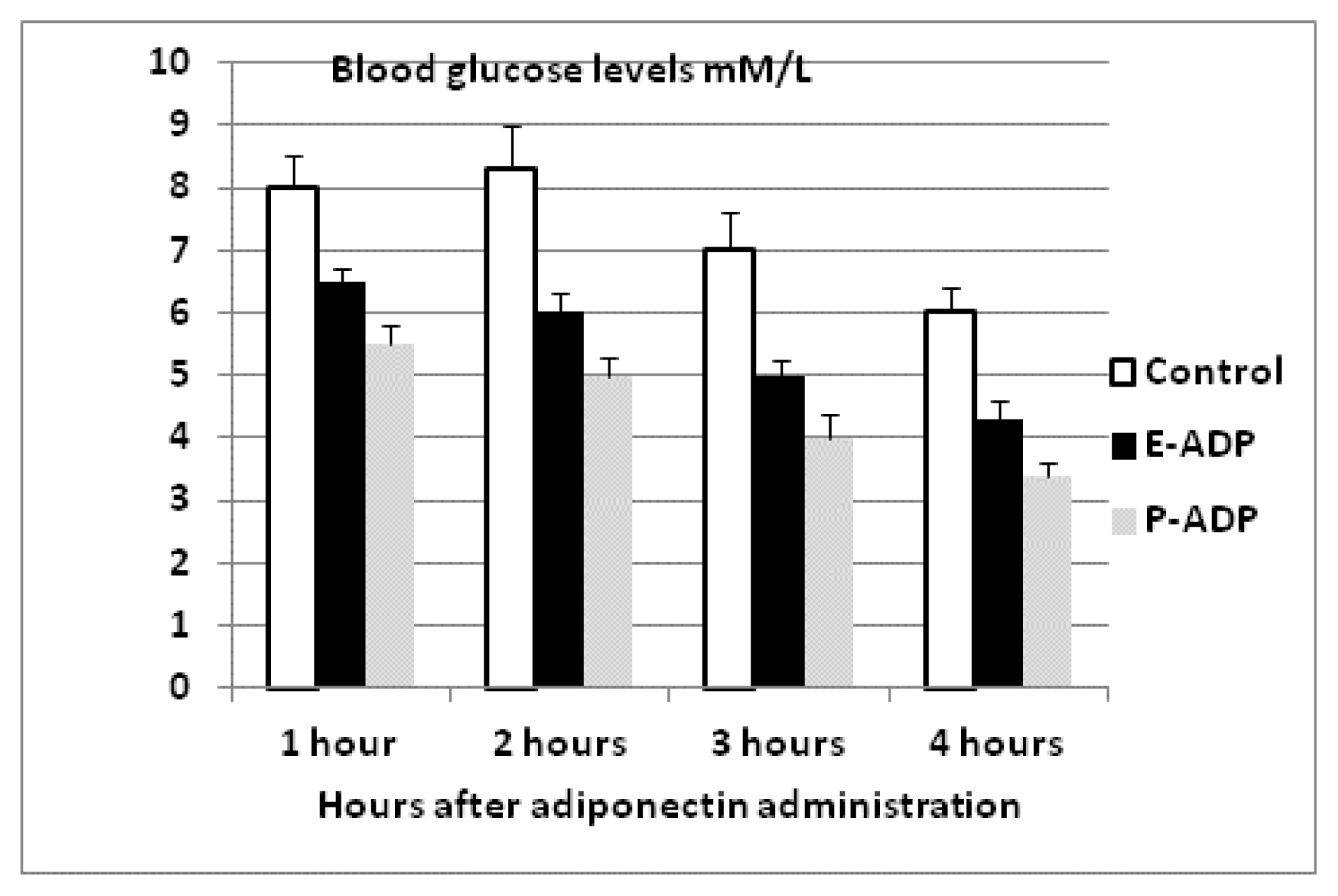
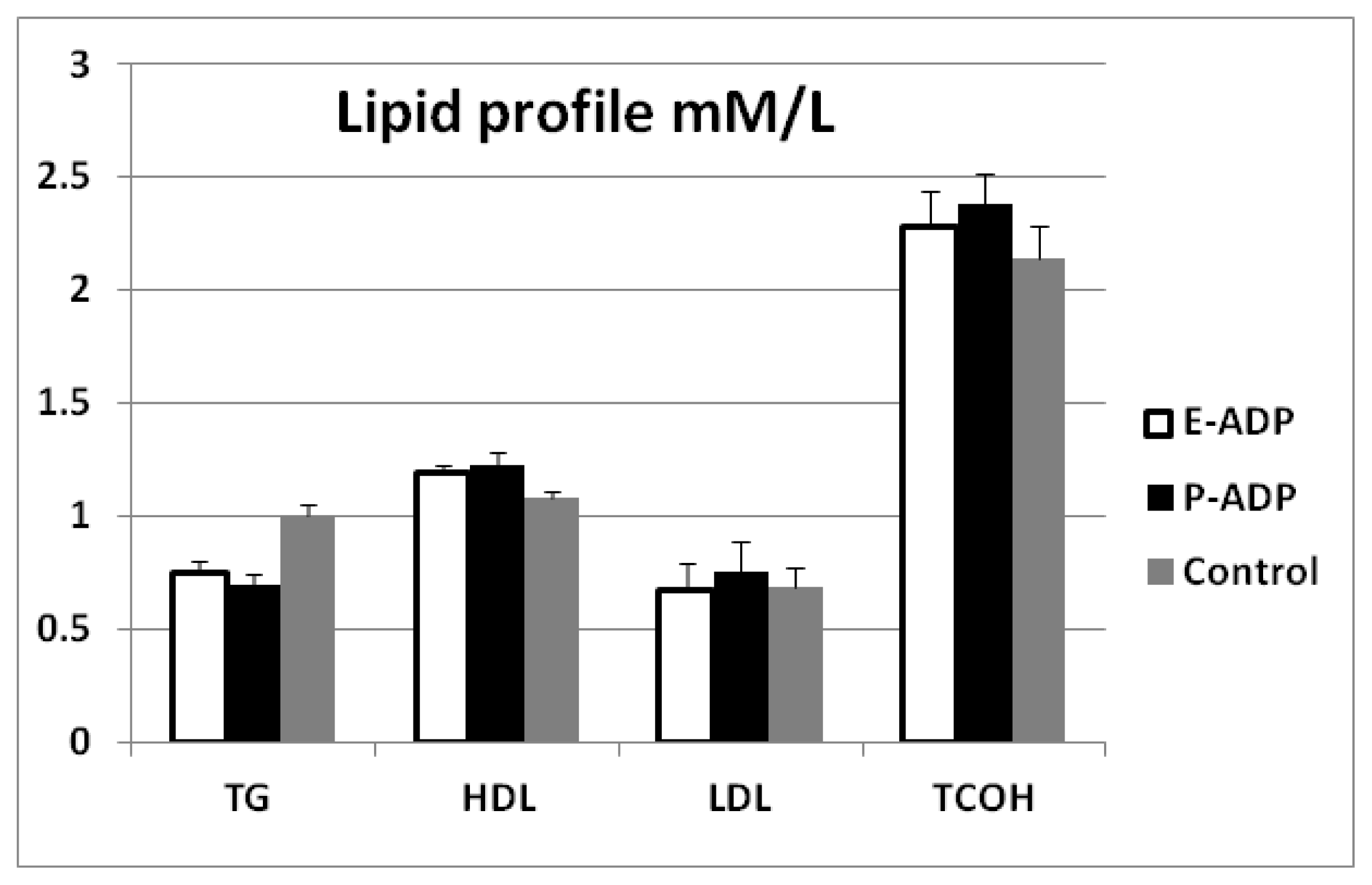
2.4. Comparison of Biological Activity Between P-ADP and E-ADP
3. Discussion
4. Experimental Section
4.1. Culture Media
4.2. Gene Construction and Cloning
4.3. Expression in E. coli
4.4. Expression and Optimization of Adiponectin Production by P. pastoris
4.5. Protein Purification
4.5.1. E-ADP Purification by Amylose Resin Column
4.5.2. Cleavage, Denaturing and Re-Purification of E-ADP
4.5.3. P-ADP Purification by Nickel Column
4.6. SDS-PAGE and Western Immunoblotting
4.7. Effect of Recombinant Adiponectin on Blood Glucose and Lipids
4.8. Statistical Analysis
5. Conclusions
Acknowledgements
References
- Scherer, P.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.A. novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem 1995, 270, 26746–26749. [Google Scholar]
- Vasseur, F.D.; Meyre, D.; Froguel, P. Adiponectin, type 2 diabetes and the metabolic syndrome: lessons from human genetic studies. Expert Rev. Mol. Med 2006, 8, 1–12. [Google Scholar]
- Jang, Y.; Chae, J.S.; Koh, S.J.; Hyun, Y.J.; Kim, J.Y.; Jeong, Y.J.; Park, S.; Ahn, C.M.; Lee, J.H. The influence of the adiponectin gene on adiponectin concentrations and parameters of metabolic syndrome in non-diabetic Korean women. Clin Chim Acta 2008, 391, 85–90. [Google Scholar]
- Heid, I.; Wagner, S.; Gohlke, H.; Iglseder, B.; Mueller, J.; Cip, P.; Ladurner, G.; Reiter, R.; Stadlmayr, A.; Mackevics, V.; et al. Genetic Architecture of the APM1 Gene and Its Influence on Adiponectin Plasma Levels and Parameters of the Metabolic Syndrome in 1727 Healthy Caucasians. Diabetes 2006, 55, 375–384. [Google Scholar]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol 2000, 20, 1595–1599. [Google Scholar]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and Metabolic Syndrome Arterioscler. Thromb. Vasc. Biol 2004, 24, 29–33. [Google Scholar]
- Diez, J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol 2003, 148, 293–300. [Google Scholar]
- Marini, G.; Forno, G.; Kratje, R.; Etcheverrigaray, M. Recombinant human granulocyte-macrophage colony-stimulating factor: effect of glycosylation on pharmacokinetic parameters. Electron. J. Biotechnol 2007. [Google Scholar] [CrossRef]
- Romanos, M.A.; Scorer, C.A.; Clare, J.J. Foreign gene expression in yeast: a review. Yeast 1992, 8, 423–488. [Google Scholar]
- Jahic, M.; Rotticci-Mulder, J.; Martinelle, M.; Hult, K.; Enfors, S. Modeling of growth and energy metabolism of Pichia pastoris producing a fusion protein. Bioprocess. Biosyst. Eng 2002, 24, 385–393. [Google Scholar]
- Xiao-Bo, H.; Yu-Jian, Z.; Hui-Tang, Z.; Sheng-Li, Y.; Yi, G. Cloning and expression of adiponectin and its globular domain and measurement of the biological activity in vivo. Acta Biochim. Biophys. Sin 2003, 35, 1023–1028. [Google Scholar]
- Liu, D.G.; Liu, H.L.; Song, T.J.; Huang, H.Y.; Li, X.; Tang, Q.Q. Functional expression of the globular domain of human adiponectin in Pichia pastoris. Biochem. Biophys. Res. Commun 2007, 363, 769–775. [Google Scholar]
- Avides, M.; Domingues, L.; Vicente, A.; Teixeira, J. Differentiation of human pre-adipocytes by recombinant adiponectin. Prot. Expr. Purif 2008, 59, 122–126. [Google Scholar] [Green Version]
- Takahashi, M.; Arita, Y.; Yamagata, K.; Matsukawa, Y.; Okutomi, K.; Horie, M.; Shimomura, I.; Hotta, K.; Kuriyama, H.; Kihara, S.; et al. Genomic structure and mutations in adipose-specific gene adiponectin. Int. J. Obes. Relat. Metab. Disord 2000, 24, 861–868. [Google Scholar]
- Kondo, H.; Shimomura, I.; Matsukawa, Y.; Kumada, M.; Takahashi, M.; Matsuda, M.; Ouchi, N.; Kihara, S.; Kawamoto, T.; Sumitsuji, S.; et al. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes 2002, 51, 2325–2328. [Google Scholar]
- Xita, N.; Georgiou, I.; Chatzikyriakidou, A.; Vounatsou, M.; Papassotiriou, G.; Papassotiriou, I.; Tsatsoulis, A. Effect of adiponectin gene polymorphisms on circulating adiponectin and insulin resistance indexes in women with polycystic ovary syndrome. Clin. Chem 2005, 51, 416–423. [Google Scholar]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes: Molecular structure and multimer formation of adiponectin. J. Biol. Chem 2003, 278, 40352–40363. [Google Scholar]
- Lilie, H.; Schwarz, E.; Rudolph, R. Advances in refolding of proteins produced in E. coli. Curr. Opin. Biotechnol 1998, 9, 497–501. [Google Scholar]
- Pajvani, U.; Du, X.; Combs, T.; Berg, A.; Rajala, M.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P. Structure-Function Studies of the Adipocyte-secreted Hormone Acrp30/Adiponectin. J. Biol. Chem 2003, 278, 9073–9085. [Google Scholar]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest 2006, 116, 1784–1792. [Google Scholar]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev 2005, 26, 439–451. [Google Scholar]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of aiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar]
- Yang, B.; Brown, K.; Chen, L.; Carrick, K.; Clifton, L.; Mc Nulty, J.; Winegar, D.; Strum, J.; Stimpson, S.; Pahel, G. Serum adiponectin as a biomarker for in vivo PPARgamma activation and PPARgamma agonist-induced efficacy on insulin sensitization/lipid lowering in rats. BMC Pharmacol 2004, 4, 23. [Google Scholar]
- Li, C.J.; Sun, H.W.; Zhu, F.L.; Chen, L.; Rong, Y.Y.; Zhang, Y.; Zhang, M. Local adiponectin treatment reduces atherosclerotic plaque size in rabbits. J. Endocrinol 2007, 193, 137–145. [Google Scholar]
- Menzaghi, C.; Trischitta, V.; Doria, A. Genetic Influences of Adiponectin on Insulin Resistance, Type 2 Diabetes, and Cardiovascular Disease. Diabetes 2007, 56, 1198–1209. [Google Scholar]
- Garaulet, M.; Viguerie, N.; Porubsky, S.; Klimcakova, E.; Clement, K.; Langin, D.; Stich, V. Adiponectin Gene Expression and Plasma Values in Obese Women during Very-Low-Calorie Diet. Relationship with Cardiovascular Risk Factors and Insulin Resistance. J. Clin. Endocrinol. Metab 2004, 89, 756–760. [Google Scholar]
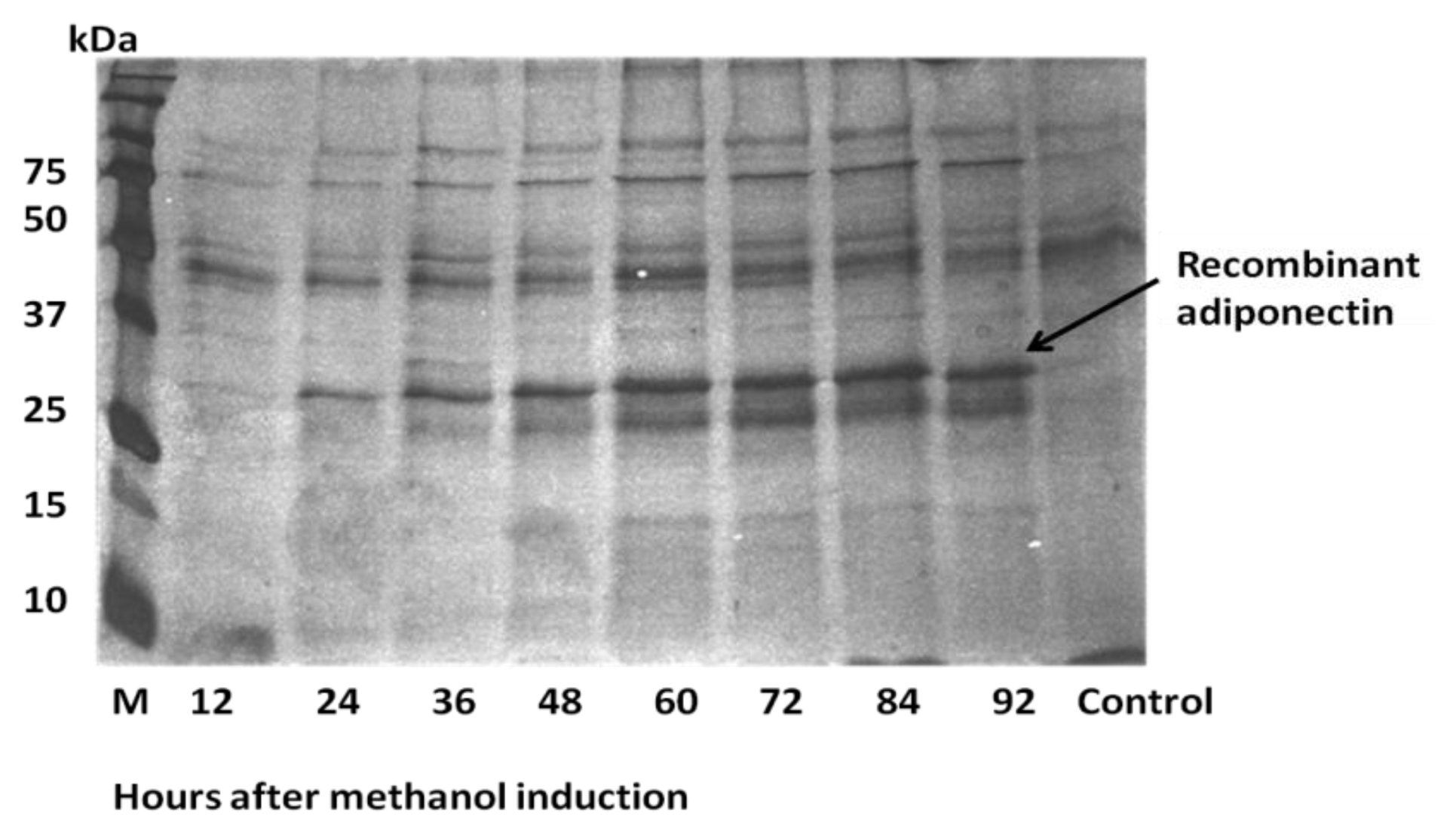

| Hours after methanol Induction | Culture density (OD600) | Total cells mass (g) | Protein concentration (μg/mL) |
|---|---|---|---|
| 12 | 27.75 | 1.02 | 5 |
| 24 | 36.57 | 1.49 | 22 |
| 36 | 35.5 | 1.51 | 25 |
| 48 | 52.8 | 1.60 | 93 |
| 60 | 49.25 | 1.60 | 111 |
| 72 | 51.33 | 1.80 | 78 |
| 86 | 49.0 | 1.62 | 48 |
| 96 | 43.15 | 1.78 | 77 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rothan, H.A.; Teh, S.H.; Haron, K.; Mohamed, Z. A Comparative Study on the Expression, Purification and Functional Characterization of Human Adiponectin in Pichia pastoris and Escherichia coli . Int. J. Mol. Sci. 2012, 13, 3549-3562. https://doi.org/10.3390/ijms13033549
Rothan HA, Teh SH, Haron K, Mohamed Z. A Comparative Study on the Expression, Purification and Functional Characterization of Human Adiponectin in Pichia pastoris and Escherichia coli . International Journal of Molecular Sciences. 2012; 13(3):3549-3562. https://doi.org/10.3390/ijms13033549
Chicago/Turabian StyleRothan, Hussin A., Ser Huy Teh, Kamariah Haron, and Zulqarnain Mohamed. 2012. "A Comparative Study on the Expression, Purification and Functional Characterization of Human Adiponectin in Pichia pastoris and Escherichia coli " International Journal of Molecular Sciences 13, no. 3: 3549-3562. https://doi.org/10.3390/ijms13033549




