Pro-Inflammatory S100A8 and S100A9 Proteins: Self-Assembly into Multifunctional Native and Amyloid Complexes
Abstract
:1. Introduction
2. Nomenclature
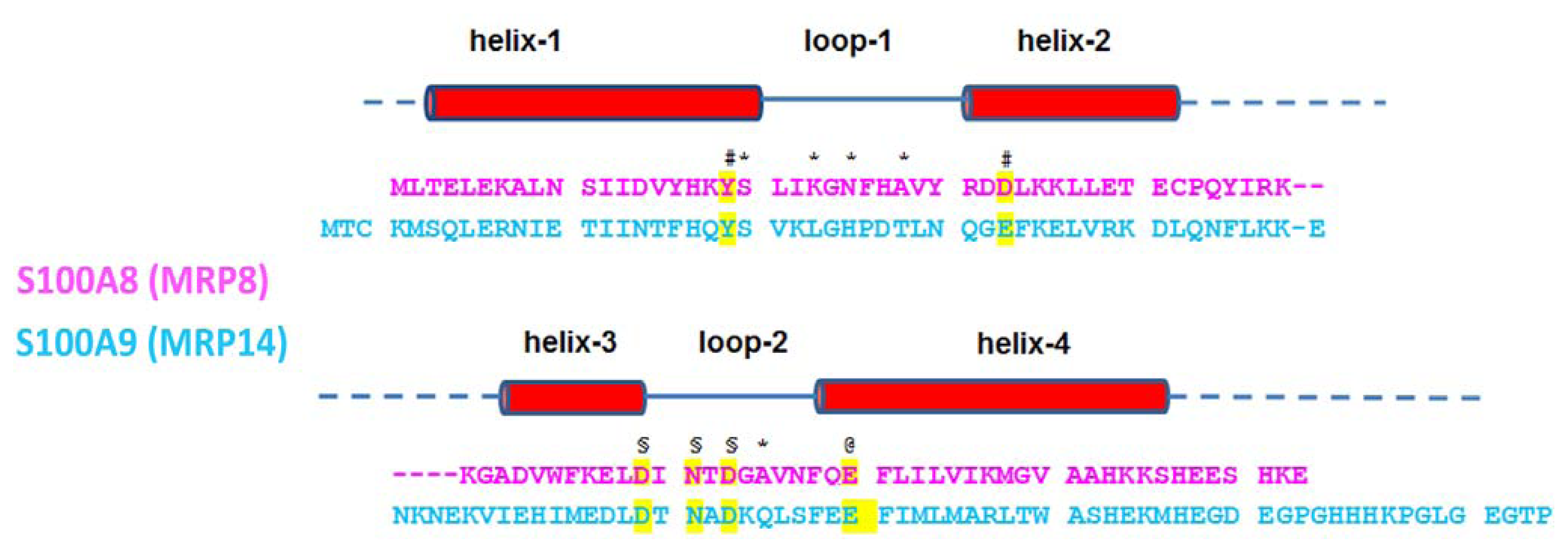
3. Primary Structure and Ca2+-Binding Sites
4. Zn2+-Binding
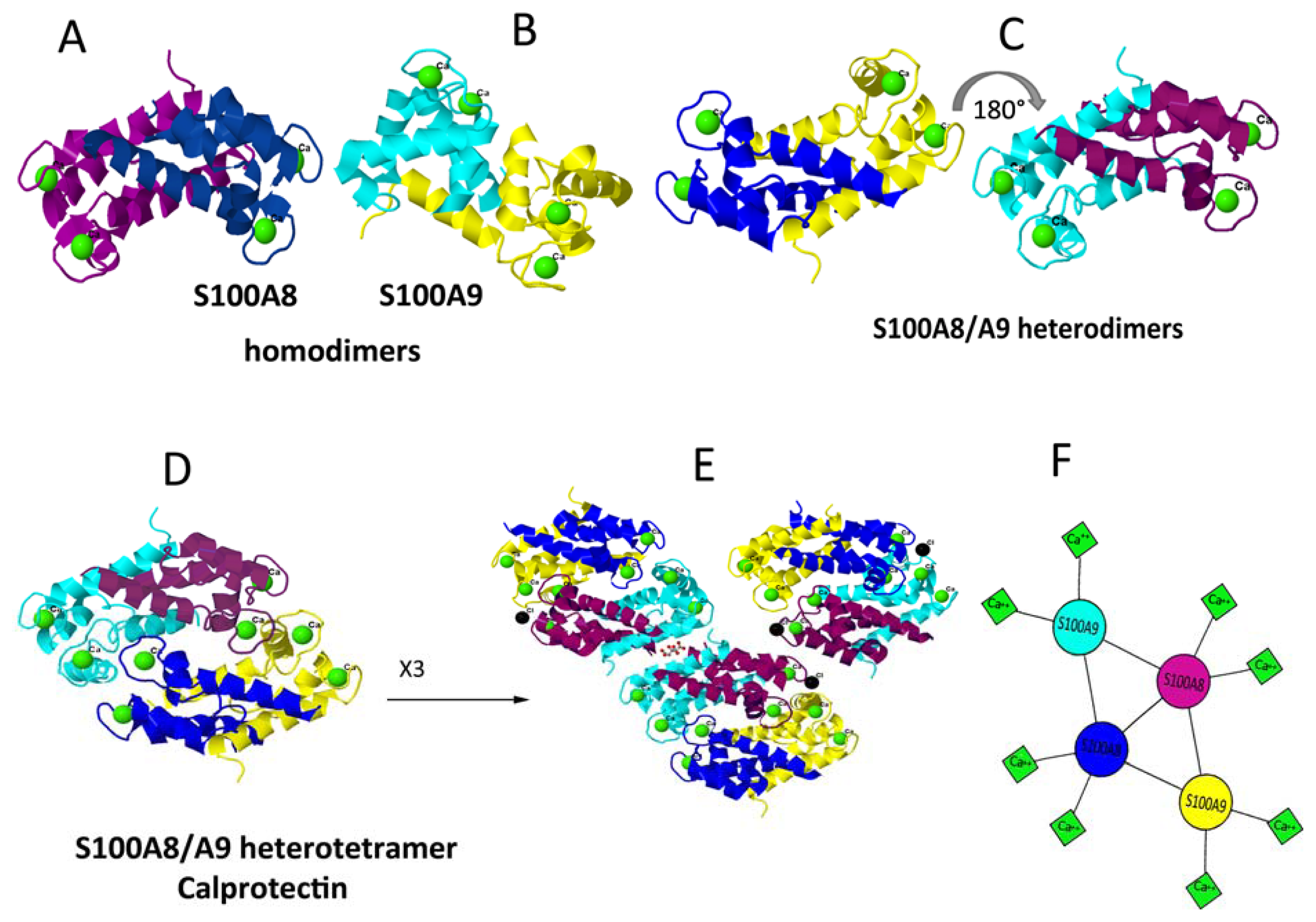
5. Dimerisation and Tetramerisation
6. Clinical Occurrence and Functional Diversity
6.1. S100A8 and S100A9 in Myeloid Cells
6.2. Role in Inflammation and Cancer
6.3. Role in Signaling Cascades
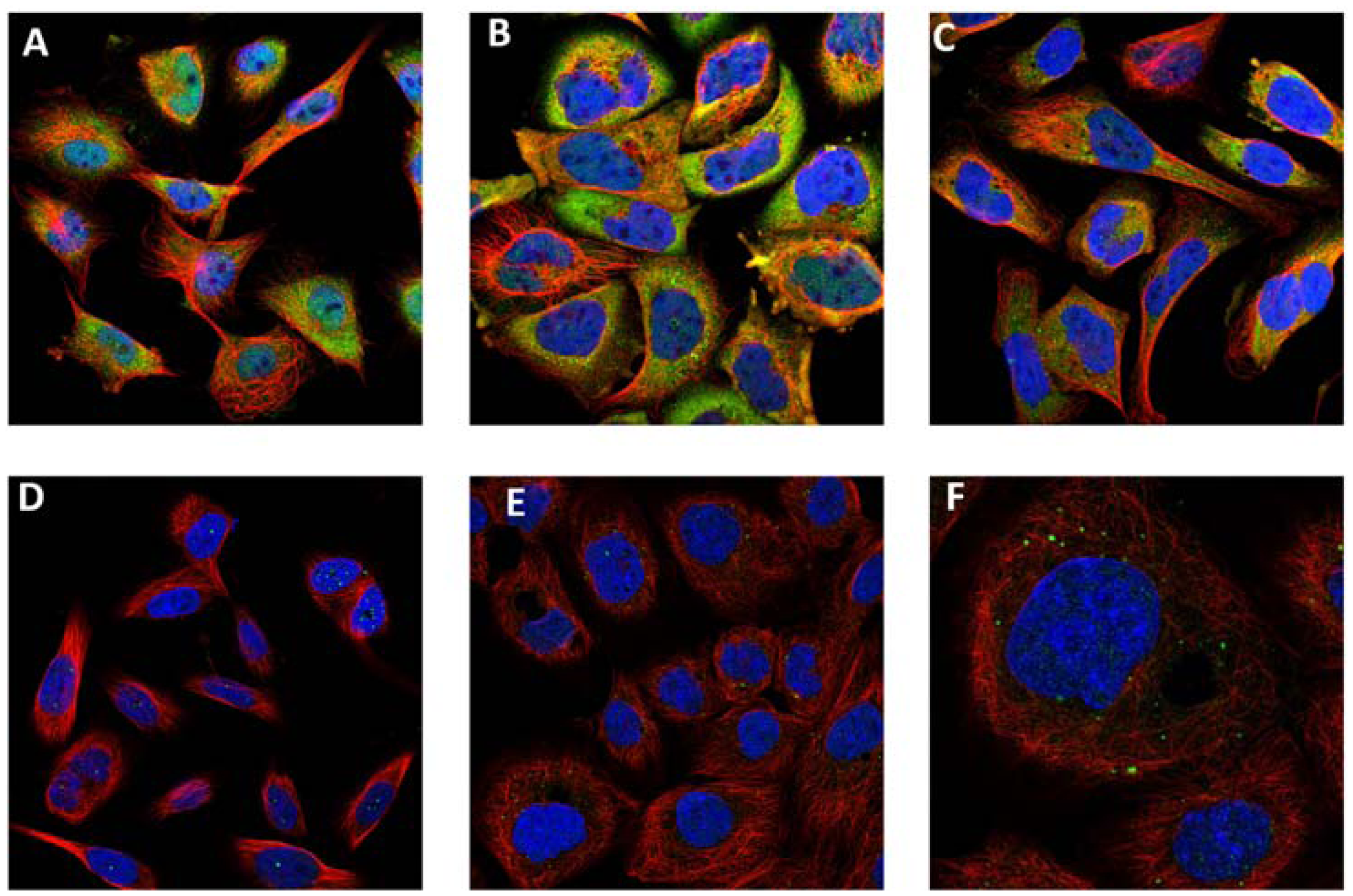
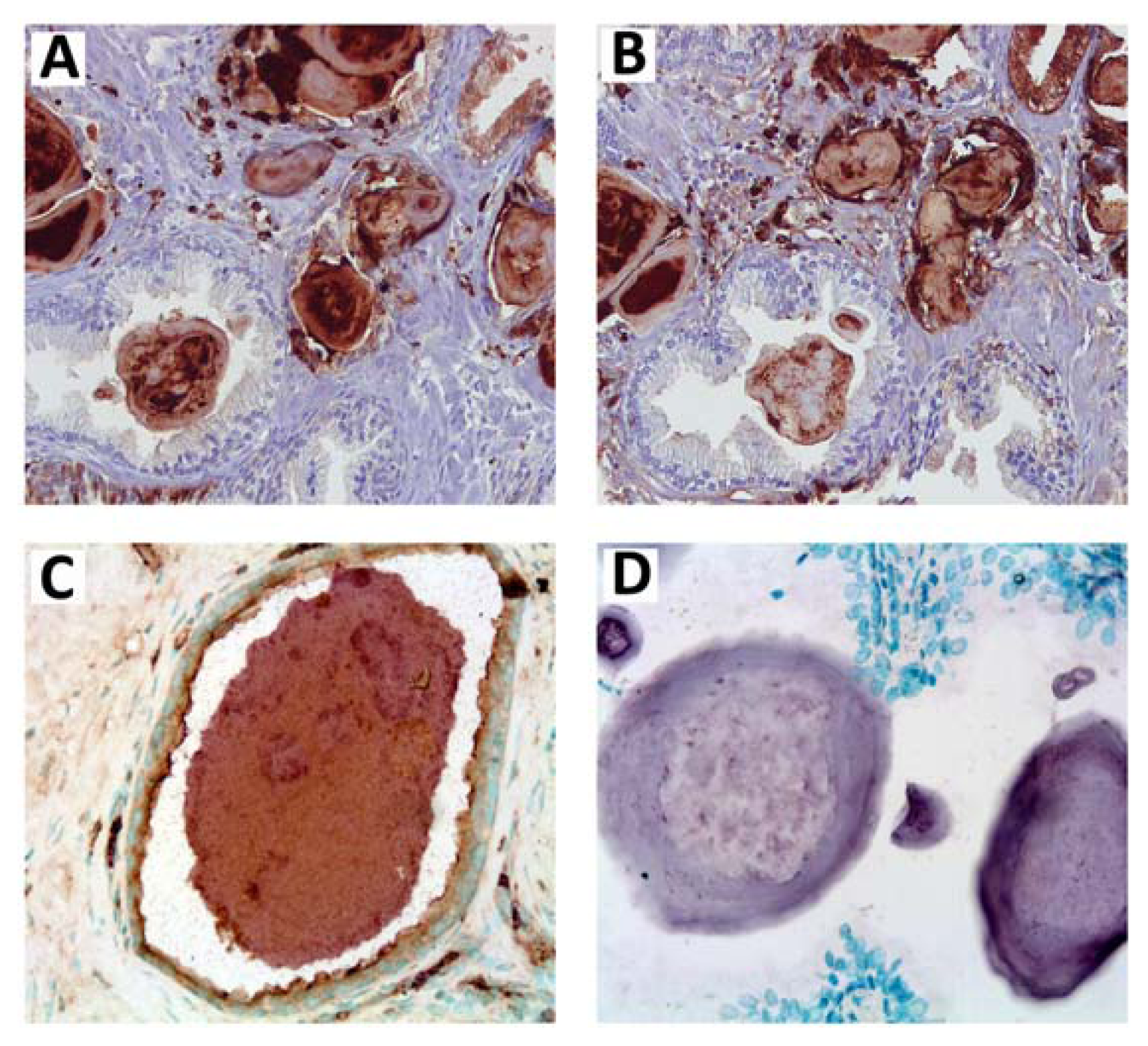
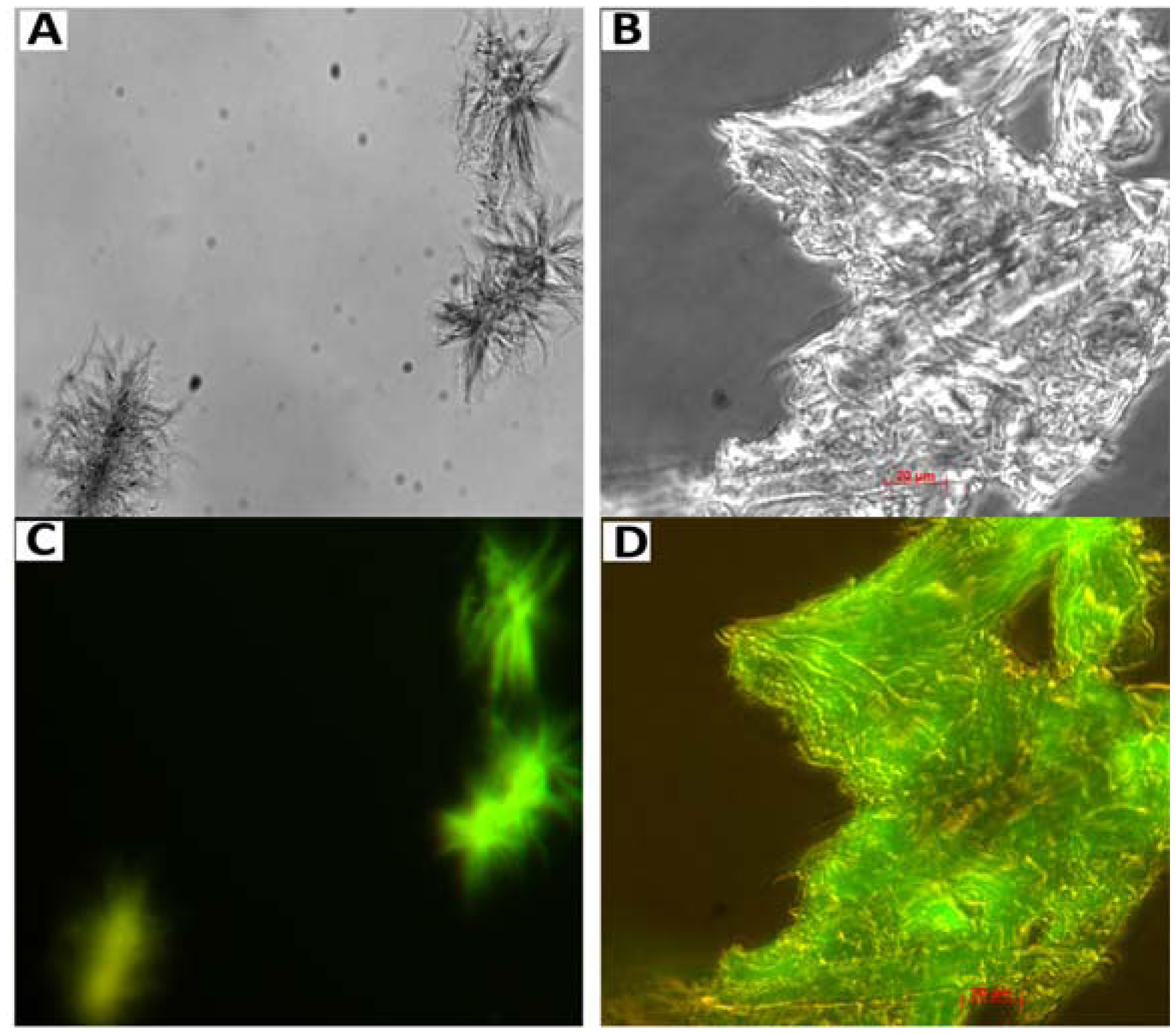
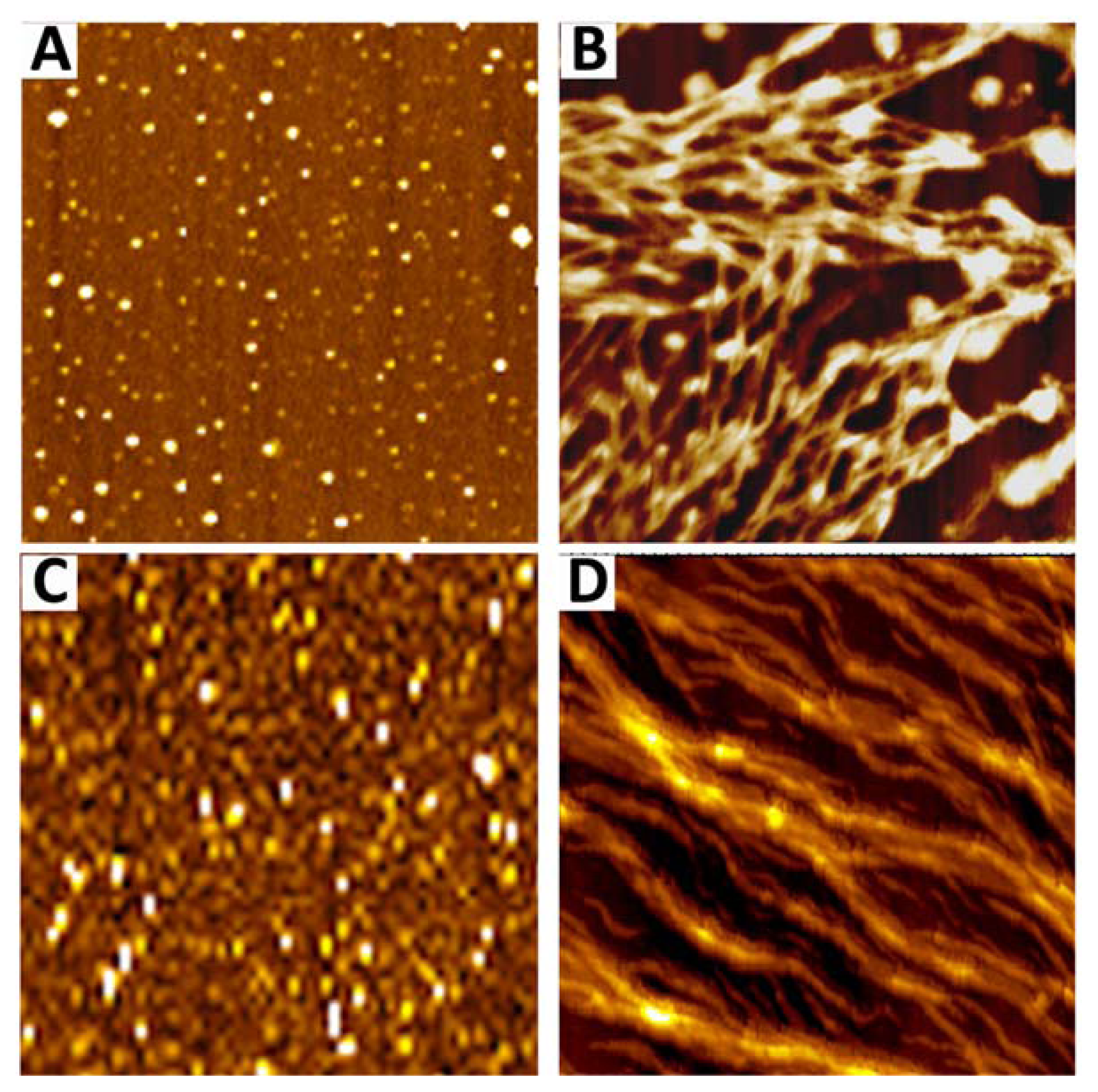
7. Amyloid Complexes
8. Conclusions
Acknowledgment
References
- Grevers, L.C.; de Vries, T.J.; Vogl, T.; Abdollahi-Roodsaz, S.; Sloetjes, A.W.; Leenen, P.J.; Roth, J.; Everts, V.; van den Berg, W.B.; van Lent, P.L. S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4: Implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheum 2011, 63, 1365–1375. [Google Scholar]
- Hiratsuka, S.; Watanabe, A.; Aburatani, H.; Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol 2006, 8, 1369–1375. [Google Scholar]
- Hoyaux, D.; Decaestecker, C.; Heizmann, C.W.; Vogl, T.; Schafer, B.W.; Salmon, I.; Kiss, R.; Pochet, R. S100 proteins in Corpora amylacea from normal human brain. Brain Res 2000, 867, 280–288. [Google Scholar]
- Salama, I.; Malone, P.S.; Mihaimeed, F.; Jones, J.L. A review of the S100 proteins in cancer. Eur. J. Surg. Oncol 2008, 34, 357–364. [Google Scholar]
- van Lent, P.L.; Grevers, L.; Blom, A.B.; Sloetjes, A.; Mort, J.S.; Vogl, T.; Nacken, W.; van den Berg, W.B.; Roth, J. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann. Rheum. Dis 2008, 67, 1750–1758. [Google Scholar]
- Vogl, T.; Ludwig, S.; Goebeler, M.; Strey, A.; Thorey, I.S.; Reichelt, R.; Foell, D.; Gerke, V.; Manitz, M.P.; Nacken, W.; et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 2004, 104, 4260–4268. [Google Scholar]
- Fritz, G.; Botelho, H.M.; Morozova-Roche, L.A.; Gomes, C.M. Natural and amyloid self-assembly of S100 proteins: Structural basis of functional diversity. FEBS J 2010, 277, 4578–4590. [Google Scholar]
- Ravasi, T.; Hsu, K.; Goyette, J.; Schroder, K.; Yang, Z.; Rahimi, F.; Miranda, L.P.; Alewood, P.F.; Hume, D.A.; Geczy, C. Probing the S100 protein family through genomic and functional analysis. Genomics 2004, 84, 10–22. [Google Scholar]
- Yan, W.X.; Armishaw, C.; Goyette, J.; Yang, Z.; Cai, H.; Alewood, P.; Geczy, C.L. Mast cell and monocyte recruitment by S100A12 and its hinge domain. J. Biol. Chem 2008, 283, 13035–13043. [Google Scholar]
- Potts, B.C.; Carlstrom, G.; Okazaki, K.; Hidaka, H.; Chazin, W.J. 1H NMR assignments of apo calcyclin and comparative structural analysis with calbindin D9k and S100 beta. Protein Sci 1996, 5, 2162–2174. [Google Scholar]
- Botelho, H.M.; Koch, M.; Fritz, G.; Gomes, C.M. Metal ions modulate the folding and stability of the tumor suppressor protein S100A2. FEBS J 2009, 276, 1776–1786. [Google Scholar]
- Heizmann, C.W.; Cox, J.A. New perspectives on S100 proteins: A multi-functional Ca2+-, Zn2+- and Cu2+-binding protein family. BioMetals 1998, 11, 383–397. [Google Scholar]
- Strupat, K.; Rogniaux, H.; van Dorsselaer, A.; Roth, J.; Vogl, T. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. J. Am. Soc. Mass Spectrom 2000, 11, 780–788. [Google Scholar]
- Vogl, T.; Roth, J.; Sorg, C.; Hillenkamp, F.; Strupat, K. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom 1999, 10, 1124–1130. [Google Scholar]
- Yanamandra, K.; Alexeyev, O.; Zamotin, V.; Srivastava, V.; Shchukarev, A.; Brorsson, A.C.; Tartaglia, G.G.; Vogl, T.; Kayed, R.; Wingsle, G.; et al. Amyloid formation by the pro-inflammatory S100A8/A9 proteins in the ageing prostate. PloS One 2009, 4. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.W. A solubel protein characteristics of nervous system. Biochem. Biophys. Res. Commun 1965, 19, 739–744. [Google Scholar]
- Lagasse, E.; Clerc, R.G. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol. Cell. Biol 1988, 8, 2402–2410. [Google Scholar]
- Murao, S.; Collart, F.; Huberman, E. A protein complex expressed during terminal differentiation of monomyelocytic cells is an inhibitor of cell growth. Cell Growth Differ 1990, 1, 447–454. [Google Scholar]
- Odink, K.; Cerletti, N.; Bruggen, J.; Clerc, R.G.; Tarcsay, L.; Zwadlo, G.; Gerhards, G.; Schlegel, R.; Sorg, C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 1987, 330, 80–82. [Google Scholar]
- Wilkinson, M.M.; Busuttil, A.; Hayward, C.; Brock, D.J.; Dorin, J.R.; van Heyningen, V. Expression pattern of two related cystic fibrosis-associated calcium-binding proteins in normal and abnormal tissues. J. Cell Sci 1988, 91, 221–230. [Google Scholar]
- Steinbakk, M.; Naess-Andresen, C.F.; Lingaas, E.; Dale, I.; Brandtzaeg, P.; Fagerhol, M.K. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet 1990, 336, 763–765. [Google Scholar]
- Lackmann, M.; Cornish, C.J.; Simpson, R.J.; Moritz, R.L.; Geczy, C.L. Purification and structural analysis of a murine chemotactic cytokine (CP-10) with sequence homology to S100 proteins. J. Biol. Chem 1992, 267, 7499–7504. [Google Scholar]
- Marenholz, I.; Lovering, R.C.; Heizmann, C.W. An update of the S100 nomenclature. Biochim. Biophys. Acta 2006, 1763, 1282–1283. [Google Scholar]
- Schafer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem. Sci 1996, 21, 134–140. [Google Scholar]
- Tufty, R.M.; Kretsinger, R.H. Troponin and parvalbumin calcium binding regions predicted in myosin light chain and T4 lysozyme. Science 1975, 187, 167–169. [Google Scholar]
- Hunter, M.J.; Chazin, W.J. High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J. Biol. Chem 1998, 273, 12427–12435. [Google Scholar]
- Leukert, N.; Vogl, T.; Strupat, K.; Reichelt, R.; Sorg, C.; Roth, J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J. Mol. Biol 2006, 359, 961–972. [Google Scholar]
- Ishikawa, K.; Nakagawa, A.; Tanaka, I.; Suzuki, M.; Nishihira, J. The structure of human MRP8, a member of the S100 calcium-binding protein family, by MAD phasing at 1.9 A resolution. Acta Crystallogr. Sect. D Biol. Crystallogr 2000, 56, 559–566. [Google Scholar]
- Itou, H.; Yao, M.; Fujita, I.; Watanabe, N.; Suzuki, M.; Nishihira, J.; Tanaka, I. The crystal structure of human MRP14 (S100A9), a Ca2+-dependent regulator protein in inflammatory process. J. Mol. Biol 2002, 316, 265–276. [Google Scholar]
- Strynadka, N.C.; James, M.N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu. Rev. Biochem 1989, 58, 951–998. [Google Scholar]
- Evenas, J.; Thulin, E.; Malmendal, A.; Forsen, S.; Carlstrom, G. NMR studies of the E140Q mutant of the carboxy-terminal domain of calmodulin reveal global conformational exchange in the Ca2+-saturated state. Biochemistry 1997, 36, 3448–3457. [Google Scholar]
- Li, M.X.; Gagne, S.M.; Spyracopoulos, L.; Kloks, C.P.; Audette, G.; Chandra, M.; Solaro, R.J.; Smillie, L.B.; Sykes, B.D. NMR studies of Ca2+ binding to the regulatory domains of cardiac and E41A skeletal muscle troponin C reveal the importance of site I to energetics of the induced structural changes. Biochemistry 1997, 36, 12519–12525. [Google Scholar]
- Edgeworth, J.; Freemont, P.; Hogg, N. Ionomycin-regulated phosphorylation of the myeloid calcium-binding protein p14. Nature 1989, 342, 189–192. [Google Scholar]
- Guignard, F.; Mauel, J.; Markert, M. Phosphorylation of myeloid-related proteins MRP-14 and MRP-8 during human neutrophil activation. Eur. J. Biochem 1996, 241, 265–271. [Google Scholar]
- van den Bos, C.; Roth, J.; Koch, H.G.; Hartmann, M.; Sorg, C. Phosphorylation of MRP14, an S100 protein expressed during monocytic differentiation, modulates Ca2+-dependent translocation from cytoplasm to membranes and cytoskeleton. J. Immunol 1996, 156, 1247–1254. [Google Scholar]
- Lominadze, G.; Rane, M.J.; Merchant, M.; Cai, J.; Ward, R.A.; McLeish, K.R. Myeloid-related protein-14 is a p38 MAPK substrate in human neutrophils. J. Immunol 2005, 174, 7257–7267. [Google Scholar]
- Sohnle, P.G.; Hunter, M.J.; Hahn, B.; Chazin, W.J. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14). J. Infect. Dis 2000, 182, 1272–1275. [Google Scholar]
- Vogl, T.; Leukert, N.; Barczyk, K.; Strupat, K.; Roth, J. Biophysical characterization of S100A8 and S100A9 in the absence and presence of bivalent cations. Biochim. Biophys. Acta 2006, 1763, 1298–1306. [Google Scholar]
- Korndorfer, I.P.; Brueckner, F.; Skerra, A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting α-helices can determine specific association of two EF-hand proteins. J. Mol. Biol 2007, 370, 887–898. [Google Scholar]
- Kerkhoff, C.; Vogl, T.; Nacken, W.; Sopalla, C.; Sorg, C. Zinc binding reverses the calcium-induced arachidonic acid-binding capacity of the S100A8/A9 protein complex. FEBS Lett 1999, 460, 134–138. [Google Scholar]
- Bianchi, M.; Niemiec, M.J.; Siler, U.; Urban, C.F.; Reichenbach, J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J. Allergy Clin. Immunol 2011, 127, 1243–1252. [Google Scholar]
- Kehl-Fie, T.E.; Chitayat, S.; Hood, M.I.; Damo, S.; Restrepo, N.; Garcia, C.; Munro, K.A.; Chazin, W.J.; Skaar, E.P. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 2011, 10, 158–164. [Google Scholar]
- Wilder, P.T.; Varney, K.M.; Weiss, M.B.; Gitti, R.K.; Weber, D.J. Solution structure of zinc- and calcium-bound rat S100B as determined by nuclear magnetic resonance spectroscopy. Biochemistry 2005, 44, 5690–5702. [Google Scholar]
- Ostendorp, T.; Diez, J.; Heizmann, C.W.; Fritz, G. The crystal structures of human S100B in the zinc- and calcium-loaded state at three pH values reveal zinc ligand swapping. Biochim. Biophys. Acta 2011, 1813, 1083–1091. [Google Scholar]
- Brodersen, D.E.; Nyborg, J.; Kjeldgaard, M. Zinc-binding site of an S100 protein revealed. Two crystal structures of Ca2+-bound human psoriasin (S100A7) in the Zn2+-loaded and Zn2+-free states. Biochemistry 1999, 38, 1695–1704. [Google Scholar]
- Moroz, O.V.; Burkitt, W.; Wittkowski, H.; He, W.; Ianoul, A.; Novitskaya, V.; Xie, J.; Polyakova, O.; Lednev, I.K.; Shekhtman, A.; et al. Both Ca2+ and Zn2+ are essential for S100A12 protein oligomerization and function. BMC Biochem 2009, 10. [Google Scholar] [CrossRef]
- Fritz, G.; Heizmann, C.W. 3D Structures of the Calcium and Zinc Binding S100 Proteins. In Handbook of Metalloproteins; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Yu, W.H.; Fraser, P.E. S100β interaction with tau is promoted by zinc and inhibited by hyperphosphorylation in Alzheimer’s disease. J. Neurosci 2001, 21, 2240–2246. [Google Scholar]
- Roth, J.; Burwinkel, F.; van den Bos, C.; Goebeler, M.; Vollmer, E.; Sorg, C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood 1993, 82, 1875–1883. [Google Scholar]
- Foell, D.; Roth, J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum 2004, 50, 3762–3771. [Google Scholar]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med 2007, 13, 1042–1049. [Google Scholar]
- Loser, K.; Vogl, T.; Voskort, M.; Lueken, A.; Kupas, V.; Nacken, W.; Klenner, L.; Kuhn, A.; Foell, D.; Sorokin, L.; et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med 2010, 16, 713–717. [Google Scholar]
- van Lent, P.L.; Grevers, L.C.; Blom, A.B.; Arntz, O.J.; van de Loo, F.A.; van der Kraan, P.; Abdollahi-Roodsaz, S.; Srikrishna, G.; Freeze, H.; Sloetjes, A.; et al. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum 2008, 58, 3776–3787. [Google Scholar]
- Vogl, T. University of Muenster, Muenster: Germany, Unpublished work; 2010.
- Propper, C.; Huang, X.; Roth, J.; Sorg, C.; Nacken, W. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J. Biol. Chem 1999, 274, 183–188. [Google Scholar]
- PDB Protein Databank. Available online: http://www.rcsb.org accessed on 27 November 2011.
- Ishikawa, K.; Nakagawa, A.; Tanaka, I.; Suzuki, M.; Nishihira, J. Image of 1mr8. The structure of human MRP8, a member of the S100 calcium-binding protein family, by MAD phasing at 1.9 A resolution. Acta Crystallogr. Sect. D Biol. Crystallogr 2000, 56, 559–566, created with Jmol: An open-source Java viewer for chemical structures in 3D.. [Google Scholar]
- Itou, H.; Yao, M.; Fujita, I.; Watanabe, N.; Suzuki, M.; Nishihira, J.; Tanaka, I. Image of 1irj. The crystal structure of human MRP14 (S100A9), a Ca2+-dependent regulator protein in inflammatory process. J. Mol. Biol 2002, 316, 265–276, created with Jmol: An open-source Java viewer for chemical structures in 3D.. [Google Scholar]
- Korndoerfer, I.P.; Brueckner, F.; Skerra, A. Images from 1xk4. The crystal structure of human calprotectin illustrates how sequence variation and small conformational changes can determine specific hetero-association of two EF hand proteins. created with Jmol: An open-source Java viewer for chemical structures in 3D..
- Jmol: An open-source Java viewer for chemical structures in 3D. Available online: http://www.jmol.org accessed on 27 November 2011.
- Vogl, T. University of Muenster: Muenster, Germany, Unpublished work; 2011.
- Hessian, P.A.; Edgeworth, J.; Hogg, N. MRP-8 and MRP-14, two abundant Ca2+-binding proteins of neutrophils and monocytes. J. Leukoc. Biol 1993, 53, 197–204. [Google Scholar]
- Mork, G.; Schjerven, H.; Mangschau, L.; Soyland, E.; Brandtzaeg, P. Proinflammatory cytokines upregulate expression of calprotectin (L1 protein, MRP-8/MRP-14) in cultured human keratinocytes. Br. J. Dermatol 2003, 149, 484–491. [Google Scholar]
- McNeill, E.; Conway, S.J.; Roderick, H.L.; Bootman, M.D.; Hogg, N. Defective chemoattractant-induced calcium signalling in S100A9 null neutrophils. Cell Calcium 2007, 41, 107–121. [Google Scholar]
- Manitz, M.P.; Horst, B.; Seeliger, S.; Strey, A.; Skryabin, B.V.; Gunzer, M.; Frings, W.; Schonlau, F.; Roth, J.; Sorg, C.; et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol. Cell. Biol 2003, 23, 1034–1043. [Google Scholar]
- Hobbs, J.A.; May, R.; Tanousis, K.; McNeill, E.; Mathies, M.; Gebhardt, C.; Henderson, R.; Robinson, M.J.; Hogg, N. Myeloid cell function in MRP-14 (S100A9) null mice. Mol. Cell. Biol 2003, 23, 2564–2576. [Google Scholar]
- Rammes, A.; Roth, J.; Goebeler, M.; Klempt, M.; Hartmann, M.; Sorg, C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J. Biol. Chem 1997, 272, 9496–9502. [Google Scholar]
- Goebeler, M.; Roth, J.; van den Bos, C.; Ader, G.; Sorg, C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem. J 1995, 309, 419–424. [Google Scholar]
- Frosch, M.; Strey, A.; Vogl, T.; Wulffraat, N.M.; Kuis, W.; Sunderkotter, C.; Harms, E.; Sorg, C.; Roth, J. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum 2000, 43, 628–637. [Google Scholar]
- Viemann, D.; Strey, A.; Janning, A.; Jurk, K.; Klimmek, K.; Vogl, T.; Hirono, K.; Ichida, F.; Foell, D.; Kehrel, B.; et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 2005, 105, 2955–2962. [Google Scholar]
- Viemann, D.; Barczyk, K.; Vogl, T.; Fischer, U.; Sunderkotter, C.; Schulze-Osthoff, K.; Roth, J. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood 2007, 109, 2453–2460. [Google Scholar]
- Kane, D.; Roth, J.; Frosch, M.; Vogl, T.; Bresnihan, B.; FitzGerald, O. Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum 2003, 48, 1676–1685. [Google Scholar]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 2009, 5. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol 2007, 81, 28–37. [Google Scholar]
- Ehrchen, J.M.; Sunderkotter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol 2009, 86, 557–566. [Google Scholar]
- Healy, A.M.; Pickard, M.D.; Pradhan, A.D.; Wang, Y.; Chen, Z.; Croce, K.; Sakuma, M.; Shi, C.; Zago, A.C.; Garasic, J.; et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 2006, 113, 2278–2284. [Google Scholar]
- Altwegg, L.A.; Neidhart, M.; Hersberger, M.; Muller, S.; Eberli, F.R.; Corti, R.; Roffi, M.; Sutsch, G.; Gay, S.; von Eckardstein, A.; et al. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: A novel, early, and sensitive marker of acute coronary syndromes. Eur. Heart J 2007, 28, 941–948. [Google Scholar]
- Morrow, D.A.; Wang, Y.; Croce, K.; Sakuma, M.; Sabatine, M.S.; Gao, H.; Pradhan, A.D.; Healy, A.M.; Buros, J.; McCabe, C.H.; et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am. Heart J 2008, 155, 49–55. [Google Scholar]
- Gebhardt, C.; Nemeth, J.; Angel, P.; Hess, J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol 2006, 72, 1622–1631. [Google Scholar]
- Emberley, E.D.; Murphy, L.C.; Watson, P.H. S100 proteins and their influence on pro-survival pathways in cancer. Biochem. Cell Biol 2004, 82, 508–515. [Google Scholar]
- Heizmann, C.W.; Ackermann, G.E.; Galichet, A. Pathologies involving the S100 proteins and RAGE. Subcell. Biochem 2007, 45, 93–138. [Google Scholar]
- Hermani, A.; de Servi, B.; Medunjanin, S.; Tessier, P.A.; Mayer, D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp. Cell. Res 2006, 312, 184–197. [Google Scholar]
- Hermani, A.; Hess, J.; de Servi, B.; Medunjanin, S.; Grobholz, R.; Trojan, L.; Angel, P.; Mayer, D. Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin. Cancer Res 2005, 11, 5146–5152. [Google Scholar]
- Ott, H.W.; Lindner, H.; Sarg, B.; Mueller-Holzner, E.; Abendstein, B.; Bergant, A.; Fessler, S.; Schwaerzler, P.; Zeimet, A.; Marth, C.; et al. Calgranulins in cystic fluid and serum from patients with ovarian carcinomas. Cancer Res 2003, 63, 7507–7514. [Google Scholar]
- The Human Protein Atlas. Available online: http://www.proteinatlas.org accessed on 28 November 2011.
- Ghavami, S.; Rashedi, I.; Dattilo, B.M.; Eshraghi, M.; Chazin, W.J.; Hashemi, M.; Wesselborg, S.; Kerkhoff, C.; Los, M. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J. Leukoc. Biol 2008, 83, 1484–1492. [Google Scholar]
- Turovskaya, O.; Foell, D.; Sinha, P.; Vogl, T.; Newlin, R.; Nayak, J.; Nguyen, M.; Olsson, A.; Nawroth, P.P.; Bierhaus, A.; et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 2008, 29, 2035–2043. [Google Scholar]
- Moon, A.; Yong, H.Y.; Song, J.I.; Cukovic, D.; Salagrama, S.; Kaplan, D.; Putt, D.; Kim, H.; Dombkowski, A.; Kim, H.R. Global gene expression profiling unveils S100A8/A9 as candidate markers in H-ras-mediated human breast epithelial cell invasion. Mol. Cancer Res 2008, 6, 1544–1553. [Google Scholar]
- Cheng, P.; Corzo, C.A.; Luetteke, N.; Yu, B.; Nagaraj, S.; Bui, M.M.; Ortiz, M.; Nacken, W.; Sorg, C.; Vogl, T.; et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med 2008, 205, 2235–2249. [Google Scholar]
- Sinha, P.; Okoro, C.; Foell, D.; Freeze, H.H.; Ostrand-Rosenberg, S.; Srikrishna, G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol 2008, 181, 4666–4675. [Google Scholar]
- Ichikawa, M.; Williams, R.; Wang, L.; Vogl, T.; Srikrishna, G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol. Cancer Res 2011, 9, 133–148. [Google Scholar]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol 2009, 9, 162–174. [Google Scholar]
- Ostrand-Rosenberg, S. Cancer and complement. Nat. Biotechnol 2008, 26, 1348–1349. [Google Scholar]
- van Zoelen, M.A.; Vogl, T.; Foell, D.; van Veen, S.Q.; van Till, J.W.; Florquin, S.; Tanck, M.W.; Wittebole, X.; Laterre, P.F.; Boermeester, M.A.; et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am. J. Respir. Crit. Care Med 2009, 180, 1098–1106. [Google Scholar]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol 2007, 81, 1–5. [Google Scholar]
- Zreiqat, H.; Belluoccio, D.; Smith, M.M.; Wilson, R.; Rowley, L.A.; Jones, K.; Ramaswamy, Y.; Vogl, T.; Roth, J.; Bateman, J.F.; et al. S100A8 and S100A9 in experimental osteoarthritis. Arthritis Res. Ther 2010, 12. [Google Scholar] [CrossRef]
- Schelbergen, R.F.; Blom, A.B.; van den Bosch, M.H.; Sloetjes, A.; Abdollahi-Roodsaz, S.; Schreurs, B.W.; Mort, J.S.; Vogl, T.; Roth, J.; van den Berg, W.B.; et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on toll-like receptor 4. Arthritis Rheum 2011. [Google Scholar] [CrossRef]
- van Lent, P.; Blom, A.; Schelbergen, R.; Sloetjes, A.; Lafeber, F.; Lems, W.; Cats, H.; Vogl, T.; Roth, J.; van den Berg, W. Active involvement of “alarmins” S100A8 and S100A9 in regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum 2011. [Google Scholar] [CrossRef]
- Broome, A.M.; Ryan, D.; Eckert, R.L. S100 protein subcellular localization during epidermal differentiation and psoriasis. J. Histochem. Cytochem 2003, 51, 675–685. [Google Scholar]
- Wittkowski, H.; Kuemmerle-Deschner, J.B.; Austermann, J.; Holzinger, D.; Goldbach-Mansky, R.; Gramlich, K.; Lohse, P.; Jung, T.; Roth, J.; Benseler, S.M.; et al. MRP8 and MRP14, phagocyte-specific danger signals, are sensitive biomarkers of disease activity in cryopyrin-associated periodic syndromes. Ann. Rheum. Dis 2011, 70, 2075–2081. [Google Scholar]
- Bjork, P.; Bjork, A.; Vogl, T.; Stenstrom, M.; Liberg, D.; Olsson, A.; Roth, J.; Ivars, F.; Leanderson, T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 2009, 7. [Google Scholar] [CrossRef]
- Boyd, J.H.; Kan, B.; Roberts, H.; Wang, Y.; Walley, K.R. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ. Res 2008, 102, 1239–1246. [Google Scholar]
- Novitskaya, V.; Grigorian, M.; Kriajevska, M.; Tarabykina, S.; Bronstein, I.; Berezin, V.; Bock, E.; Lukanidin, E. Oligomeric forms of the metastasis-related Mts1 (S100A4) protein stimulate neuronal differentiation in cultures of rat hippocampal neurons. J. Biol. Chem 2000, 275, 41278–41286. [Google Scholar]
- Moroz, O.V.; Antson, A.A.; Dodson, E.J.; Burrell, H.J.; Grist, S.J.; Lloyd, R.M.; Maitland, N.J.; Dodson, G.G.; Wilson, K.S.; Lukanidin, E.; et al. The structure of S100A12 in a hexameric form and its proposed role in receptor signalling. Acta Crystallogr. Sect. D Biol. Crystallogr 2002, 58, 407–413. [Google Scholar]
- Ostendorp, T.; Leclerc, E.; Galichet, A.; Koch, M.; Demling, N.; Weigle, B.; Heizmann, C.W.; Kroneck, P.M.H.; Fritz, G. Structural and functional insights into RAGE activation by multimeric S100B. EMBO J 2007, 26, 3868–3878. [Google Scholar]
- de Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Gronberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar]
- Jager, G.J.; Ruijter, E.T.; de la Rosette, J.J.; van de Kaa, C.A. Amyloidosis of the seminal vesicles simulating tumor invasion of prostatic carcinoma on endorectal MR images. Eur. Radiol 1997, 7, 552–554. [Google Scholar]
- Christian, J.D.; Lamm, T.C.; Morrow, J.F.; Bostwick, D.G. Corpora amylacea in adenocarcinoma of the prostate: Incidence and histology within needle core biopsies. Mod. Pathol 2005, 18, 36–39. [Google Scholar]
- Bostwick, D.G.; Amin, M.B.; Dundore, P.; Marsh, W.; Schultz, D.S. Architectural patterns of high-grade prostatic intraepithelial neoplasia. Hum. Pathol 1993, 24, 298–310. [Google Scholar]
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp. Gerontol 2005, 40, 121–128. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar]
- Stefani, M. Biochemical and biophysical features of both oligomer/fibril and cell membrane in amyloid cytotoxicity. FEBS J 2010, 277, 4602–4613. [Google Scholar]
- Klimas, R.; Bennett, B.; Gardner, W.A., Jr. Prostatic calculi: A review. Prostate 1985, 7, 91–96. [Google Scholar]
- Thomas, B.A. Vital factors in the management of prostatic obstruction. Ann. Surg 1927, 86, 563–576. [Google Scholar]
- Drach, G.W.; Fair, W.R.; Meares, E.M.; Stamey, T.A. Classification of benign diseases associated with prostatic pain: Prostatitis or prostatodynia? J. Urol 1978, 120, 266. [Google Scholar]
- Kayed, R.; Head, E.; Sarsoza, F.; Saing, T.; Cotman, C.W.; Necula, M.; Margol, L.; Wu, J.; Breydo, L.; Thompson, J.L.; et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener 2007, 2. [Google Scholar] [CrossRef]
- McCormick, M.M.; Rahimi, F.; Bobryshev, Y.V.; Gaus, K.; Zreiqat, H.; Cai, H.; Lord, R.S.; Geczy, C.L. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J. Biol. Chem 2005, 280, 41521–41529. [Google Scholar]
- Gharibyan, A.L.; Raveh, D.; Morozova-Roche, L.A. S100A8/A9 Amyloidosis in the Ageing Prostate: Relating ex vivo and in vitro Studies. In Amyloid Proteins: Methods and Protocols (Methods in Molecular Biology), 2nd ed; Sigurdsson, E.M., Ed.; Humana Press: New York NY, USA, 2012; p. 849. [Google Scholar]
- Nacken, W.; Roth, J.; Sorg, C.; Kerkhoff, C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc. Res. Tech 2003, 60, 569–580. [Google Scholar]
- Otzen, D.; Nielsen, P.H. We find them here, we find them there: Functional bacterial amyloid. Cell. Mol. Life Sci 2008, 65, 910–927. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vogl, T.; Gharibyan, A.L.; Morozova-Roche, L.A. Pro-Inflammatory S100A8 and S100A9 Proteins: Self-Assembly into Multifunctional Native and Amyloid Complexes. Int. J. Mol. Sci. 2012, 13, 2893-2917. https://doi.org/10.3390/ijms13032893
Vogl T, Gharibyan AL, Morozova-Roche LA. Pro-Inflammatory S100A8 and S100A9 Proteins: Self-Assembly into Multifunctional Native and Amyloid Complexes. International Journal of Molecular Sciences. 2012; 13(3):2893-2917. https://doi.org/10.3390/ijms13032893
Chicago/Turabian StyleVogl, Thomas, Anna L. Gharibyan, and Ludmilla A. Morozova-Roche. 2012. "Pro-Inflammatory S100A8 and S100A9 Proteins: Self-Assembly into Multifunctional Native and Amyloid Complexes" International Journal of Molecular Sciences 13, no. 3: 2893-2917. https://doi.org/10.3390/ijms13032893



