Tetrachloridocuprates(II)—Synthesis and Electron Paramagnetic Resonance (EPR) Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Chemicals
3.2. Preparation
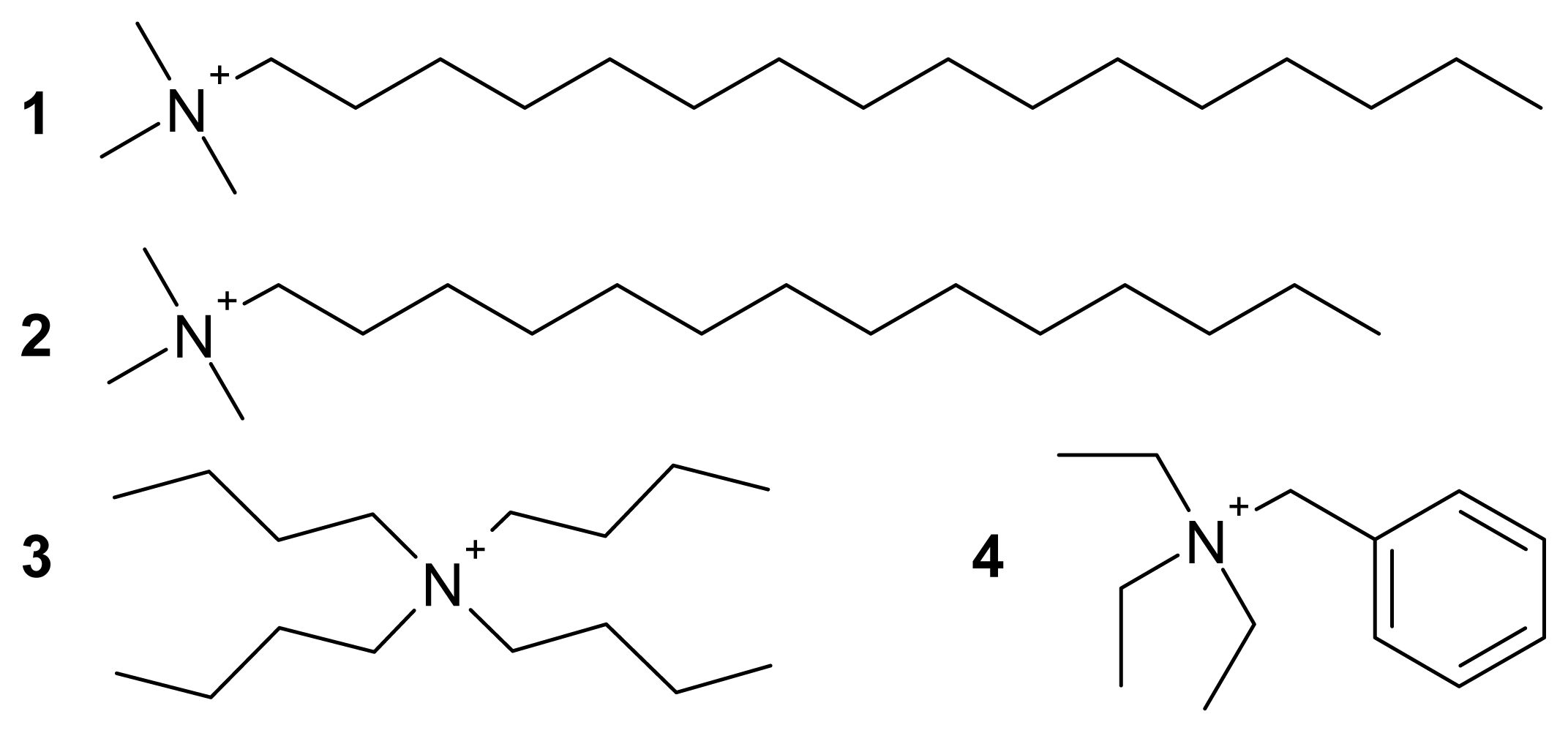
3.3. Bis(hexadecyltrimethylammonium) Tetrachloridocuprate(II), (C16H33Me3N)2[CuCl4] (1)
3.4. Bis(tetradecyltrimethylammonium) Tetrachloridocuprate(II), (C14H29Me3N)2[CuCl4] (2)
3.5. Bis(tetrabutylammonium) Tetrachloridocuprate(II), (Bu4N)2[CuCl4] (3)
3.6. Bis(benzyltriethylammonium) Tetrachloridocuprate(II), (BzlEt3N)2[CuCl4] (4)
4. Conclusions
References
- Taubert, A. Heavy elements in ionic liquids. Top. Curr. Chem 2009, 290, 127–159. [Google Scholar]
- Taubert, A. CuCl nanoplatelets from an ionic liquid-crystal precursor. Angew. Chem. Int. Ed 2004, 43, 5380–5382. [Google Scholar]
- Saito, G. Electric Conductivity and Magnetic Ionic Liquids. In Electrochemical Aspects of Ionic Liquids, 2nd ed; Ohno, H., Ed.; John Wiley & Sons, Inc: New York, NY, USA, 2011; pp. 337–346. [Google Scholar]
- Kalb, R. Use of Magnetic, Ionic Liquids as an Extraction Agent. International Application No.: PCT/EP2008/067731 2008. [Google Scholar]
- Dengler, J.E.; Doroodian, A.; Rieger, B. Protic metal-containing ionic liquids as catalysts: Cooperative effects between anion and cation. J. Organomet. Chem 2011, 696, 3831–3835. [Google Scholar]
- Bica, K.; Gaertner, P. Metal-containing ionic liquids as efficient catalysts for hydroxymethylation in water. Eur. J. Org. Chem 2008, 20, 3453–3456. [Google Scholar]
- Bica, K.; Gaertner, P. An iron-containing ionic liquid as recyclable catalyst for aryl grignard cross-coupling of aryl halides. Org. Lett 2006, 8, 733–735. [Google Scholar]
- Lin, I.J.B.; Vasam, C.S. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety. J. Organomet. Chem 2005, 690, 3498–3512. [Google Scholar]
- Bowlas, C.J.; Bruce, D.W.; Seddon, K.R. Liquid-crystalline ionic liquids. Chem. Commun 1996, 14, 1625–1626. [Google Scholar]
- Neve, F.; Francescangeli, O.; Crispini, A.; Charmant, J. A2[MX4] copper(II) pyridinium salts. From ionic liquids to layered solids to liquid crystals. Chem. Mater 2001, 13, 2032–2041. [Google Scholar]
- Neve, F.; Francescangeli, O.; Crispini, A. Crystal architecture and mesophase structure of long-chain N-alkylpyridinium tetrachlorometallates. Inorg. Chim. Acta 2002, 338, 51–58. [Google Scholar]
- Neve, F.; Crispini, A. C-H···Br-M interactions at work: Tetrabromometalates of the bolaamphiphilic N,N′-dodecamethylenedipyridinium cation. Cryst. Growth Des 2001, 1, 387–393. [Google Scholar]
- Neve, F.; Crispini, A. C-H···Br-M interactions at work: Tetrabromometalates of the bolaamphiphilic N,N′-dodecamethylenedipyridinium cation (correction). Cryst. Growth Des 2001, 1, 519–519. [Google Scholar]
- Taubert, A.; Palivan, C.; Casse, O.; Gozzo, F.; Schmitt, B. Ionic liquid-crystal precursors (ILCPs) for CuCl platelets: The origin of the exothermic peak in the DSC curves. J. Phys. Chem. C 2007, 111, 4077–4082. [Google Scholar]
- Taubert, A.; Steiner, P.; Mantion, A. Ionic liquid crystal precursors for inorganic particles: Phase diagram and thermal properties of a CuCl nanoplatelet precursor. J. Phys. Chem. B 2005, 109, 15542–15547. [Google Scholar]
- Willett, R.D.; Haugen, J.A.; Lebsack, J.; Morrey, J. Thermochromism in copper(II) chlorides. Coordination geometry. Inorg. Chem 1974, 13, 2510–2513. [Google Scholar]
- Zhuravlev, O.E.; Verolainen, N.V.; Voronchikhina, L.I. Thermal stability of 1,3-disubstituted imidazolium tetrachloroferrates, magnetic ionic liquids. Russ. J. Appl. Chem 2011, 84, 1158–1164. [Google Scholar]
- Branco, A.; Branco, L.C.; Pina, F. Electrochromic and magnetic ionic liquids. Chem. Commun 2011, 47, 2300–2302. [Google Scholar]
- Neve, F.; Crispini, A.; Armentano, S. Synthesis, structure, and thermotropic mesomorphism of layered N-alkylpyridinium tetrahalopalladate(II) salts. Chem. Mater 1998, 10, 1904–1913. [Google Scholar]
- Neve, F.; Francescangeli, O. Layered ω-substituted alkylpyridinium salts with inorganic anions: Effects of H-bonding patterns on the layer thickness. Cryst. Growth Des 2005, 5, 163–166. [Google Scholar]
- Neve, F.; Crispini, A. Competitive interactions in carboxy-functionalized pyridinium salts: Crossover from O-H···O to O-H···X-M contacts. Cryst. Eng. Comm 2007, 9, 698–703. [Google Scholar]
- Neve, F.; Crispini, A. N,N′-Dodecamethylene-bis(pyridinium) goes lamellar. Role of C-H···I, C-H···M, and I···I interactions in the crystal structure of its hexaiododipalladate(II) derivative. Cryst. Eng. Comm 2003, 5, 265–268. [Google Scholar]
- Neve, F.; Crispini, A.; Francescangeli, O. Structural studies on layered alkylpyridinium iodopalladate networks. Inorg. Chem. 2000, 39, 1187–1194. [Google Scholar]
- Binnemans, K. Ionic liquid crystals. Chem. Rev 2005, 105, 4148–4204. [Google Scholar]
- Goossens, K.; Lava, K.; Nockemann, P.; van Hecke, K.; van Meervelt, L.; Driesen, K.; Görller-Walrand, C.; Binnemans, K.; Cardinaels, C. Pyrrolidinium ionic liquid crystals. Chem. Eur. J 2009, 15, 656–674. [Google Scholar]
- Martinez Casado, F.J.; Ramos Riesco, M.; da Silva, I.; Labrador, A.; Redondo, M.I.; Garcia Perez, M.V.; Lopez-Andres, S.; Rodriguez Cheda, J.A. Thermal and structural study of the crystal phases and mesophases in the lithium and thallium(I) propanoates and pentanoates binary systems: Formation of mixed salts and stabilization of the ionic liquid crystal phase. J. Phys. Chem. B 2010, 114, 10075–10085. [Google Scholar]
- Suisse, J.-M.; Douce, L.; Bellemin-Laponnaz, S.; Maisse-Francois, A.; Welter, R.; Miyake, Y.; Shimizu, Y. Liquid crystal imidazolium salts: Towards materials for catalysis and molecular electronics. Eur. J. Inorg. Chem 2007, 3899–3905. [Google Scholar]
- Dobbs, W.; Suisse, J.-M.; Douce, L.; Welter, R. Electrodeposition of silver particles and gold nanoparticles from ionic liquid-crystal precursors. Angew. Chem. Int. Ed 2006, 45, 4179–4182. [Google Scholar]
- Lee, C.K.; Peng, H.H.; Lin, I.J.B. Liquid crystals of N,N′-dialkylimidazolium salts comprising palladium(II) and copper(II) ions. Chem. Mater 2004, 16, 530–536. [Google Scholar]
- Herber, R.H.; Nowik, I.; Kostner, M.E.; Kahlenberg, V.; Kreutz, C.; Laus, G.; Schottenberger, H. Mössbauer spectroscopy and x-ray diffraction study of 57Fe-labeled tetrachloroferrate(III)-based magnetic ionic liquids. Int. J. Mol. Sci 2011, 12, 6397–6406. [Google Scholar]
- De Pedro, I.; Rojas, D.P.; Albo, J.; Luis, P.; Irabien, A.; Blanco, J.A.; Fernandez, J.R. Long-range magnetic ordering in magnetic ionic liquid: Emim[FeCl4]. J. Phys. Condens. Matter 2010, 22, 296006:1–296006:4. [Google Scholar]
- Sasaki, T.; Zhong, C.; Tada, M.; Iwasawa, Y. Immobilized metal ion-containing ionic liquids: Preparation, structure and catalytic performance in Kharasch addition reaction. Chem. Commun 2005, 19, 2506–2508. [Google Scholar]
- Thiel, K.; Klamroth, T.; Strauch, P.; Taubert, A. On the interaction of ascorbic acid and the tetrachlorocuprate ion [CuCl4]2− in CuCl nanoplatelet formation from an ionic liquid precursor (ILP). Phys. Chem. Chem. Phys 2011, 13, 13537–13543. [Google Scholar]
- Farra, R.; Thiel, K.; Winter, A.; Klamroth, T.; Pöppl, A.; Kelling, A.; Schilde, U.; Taubert, A.; Strauch, P. Tetrahalidocuprates(II)—Structure and EPR spectroscopy. Part 1: Tetrabromidocuprates(II). New J. Chem 2011, 35, 2793–2803. [Google Scholar]
- Smith, D.W. Chlorocuprates(II). Coord. Chem. Rev 1976, 21, 93–158. [Google Scholar]
- Liu, H.; Wang, X.; Hu, W.; Guo, L; Ouyang, S. Preparation and characterization of (C4H9NH3)2CuX4 (X-Cl, Br). Chem. J. Internet 2004, 6, 066039ne. [Google Scholar]
- Choi, S.; Larrabee, J.A. Thermochromic tetrachlorocuprate(II). J. Chem. Educ 1989, 66, 774–776. [Google Scholar]
- Xiao, Z.-L.; Chen, H.-Z.; Shi, M.-M.; Wu, G.; Zhou, R.-J.; Yang, Z.-S.; Wang, M.; Tang, B.-Z. Preparation and characterization of organic-inorganic hybrid perovskite (C4H9NH3)2CuCl4. Mater. Sci. Eng. B 2005, 117, 313–316. [Google Scholar]

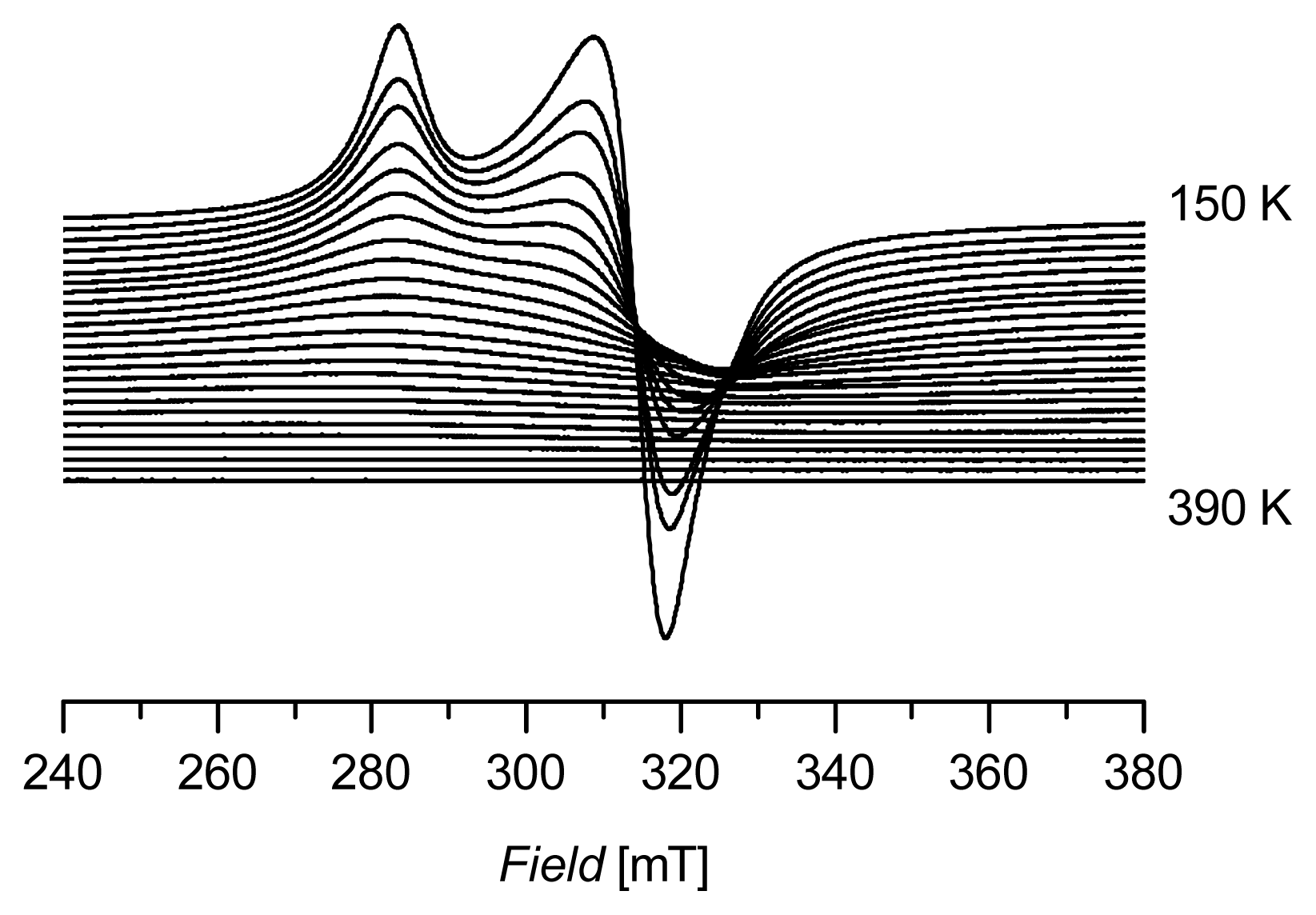
| Compound | g║ | g┴ | gav |
|---|---|---|---|
| (C16H33Me3N)2[CuCl4] (1) | 2.365 ± 0.005 | 2.145 ± 0.005 | 2.218 ± 0.005 |
| (C14H29Me3N)2[CuCl4] (2) | 2.366 ± 0.005 | 2.141 ± 0.005 | 2.216 ± 0.005 |
| (Bu4N)2[CuCl4] (3) | 2.394 ± 0.005 | 2.081 ± 0.005 | 2.185 ± 0.005 |
| (BzlEt3N)2[CuCl4] (4) | 2.424 ± 0.005 | 2.089 ± 0.005 | 2.200 ± 0.005 |
| (BuPy)2[Cu/ZnCl4] [33] | 2.300 ± 0.005 | 2.070 ± 0.005 | 2.147 ± 0.005 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Winter, A.; Zabel, A.; Strauch, P. Tetrachloridocuprates(II)—Synthesis and Electron Paramagnetic Resonance (EPR) Spectroscopy. Int. J. Mol. Sci. 2012, 13, 1612-1619. https://doi.org/10.3390/ijms13021612
Winter A, Zabel A, Strauch P. Tetrachloridocuprates(II)—Synthesis and Electron Paramagnetic Resonance (EPR) Spectroscopy. International Journal of Molecular Sciences. 2012; 13(2):1612-1619. https://doi.org/10.3390/ijms13021612
Chicago/Turabian StyleWinter, Alette, André Zabel, and Peter Strauch. 2012. "Tetrachloridocuprates(II)—Synthesis and Electron Paramagnetic Resonance (EPR) Spectroscopy" International Journal of Molecular Sciences 13, no. 2: 1612-1619. https://doi.org/10.3390/ijms13021612





