Effect of Fiber Esterification on Fundamental Properties of Oil Palm Empty Fruit Bunch Fiber/Poly(butylene adipate-co-terephthalate) Biocomposites
Abstract
:1. Introduction
2. Results and Discussion
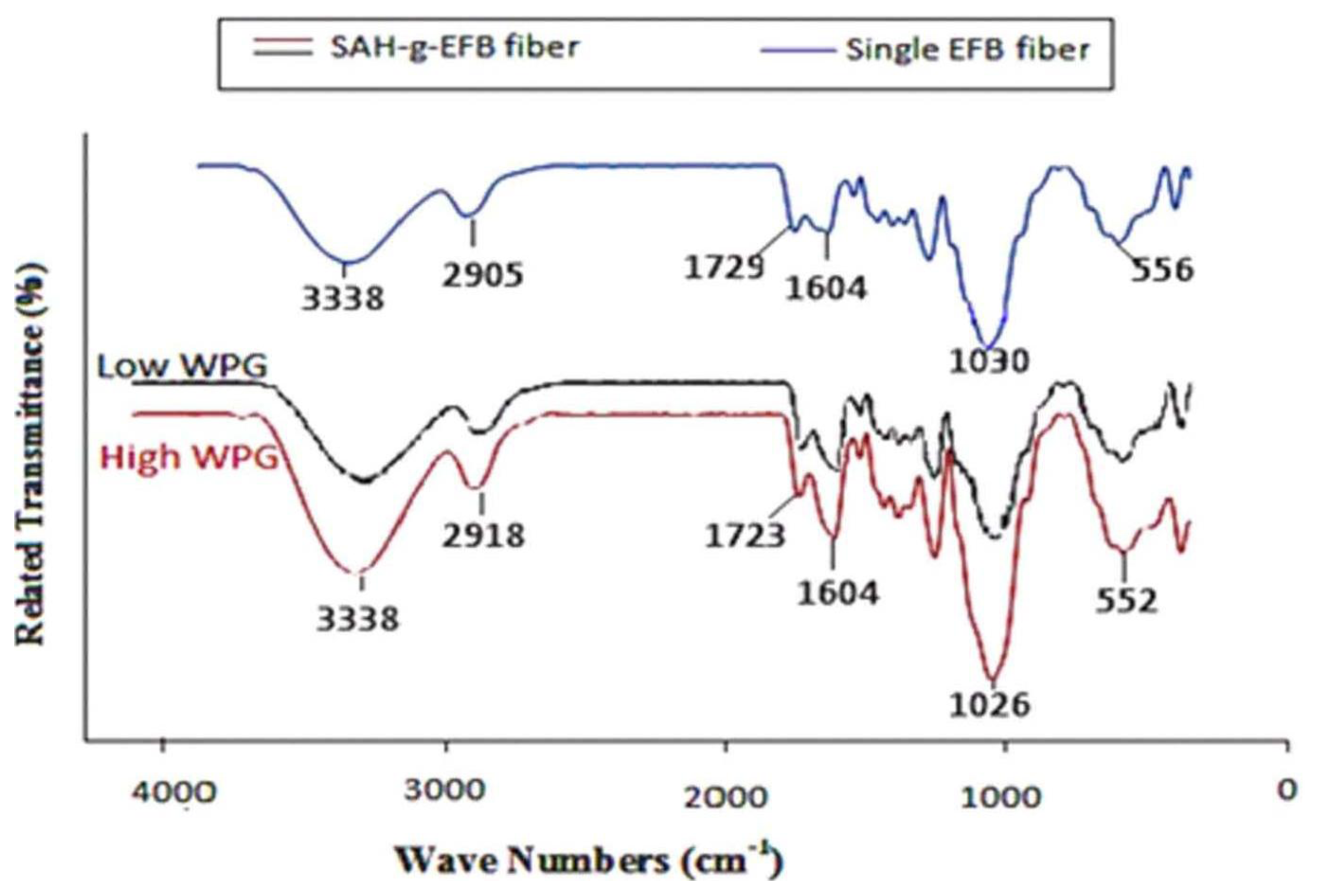
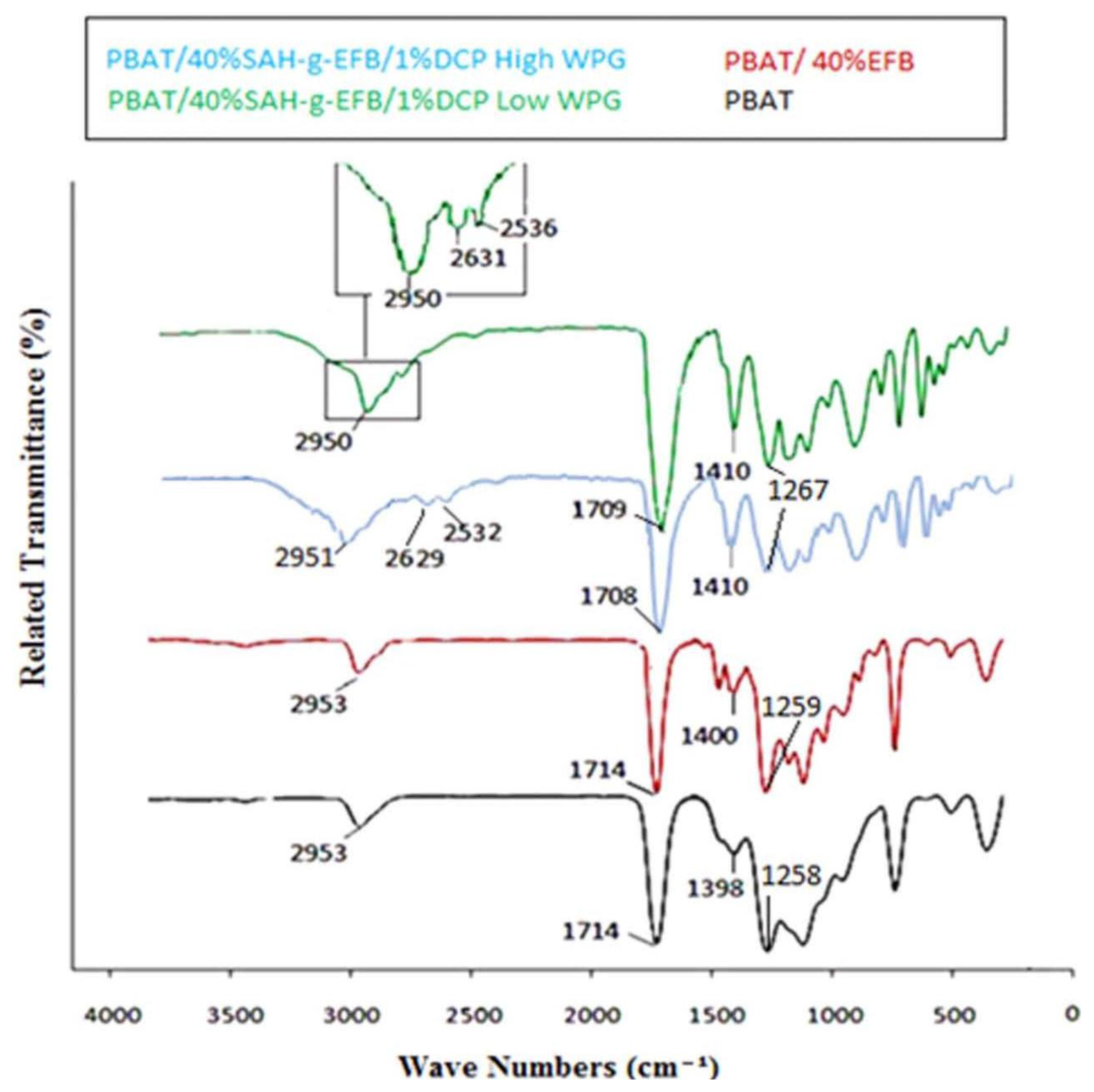
2.1. FTIR Spectroscopy
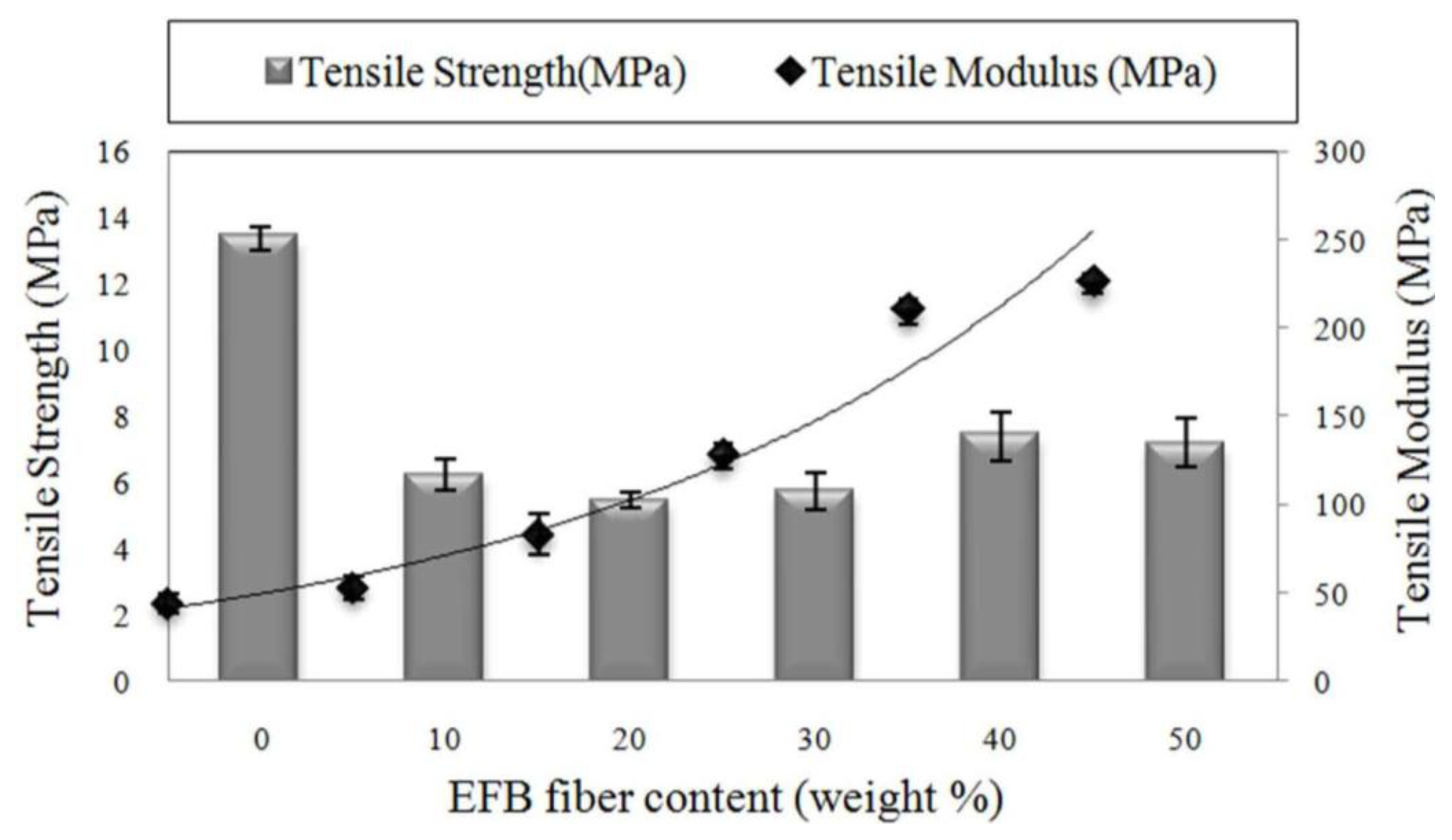
2.2. Effect of Fiber Loading on the Tensile Properties of Biocomposites
2.3. Effect of Fiber Chemical Treatment on Tensile Properties
2.4. Effect of Fiber Loading on Flexural Properties of Untreated Composites
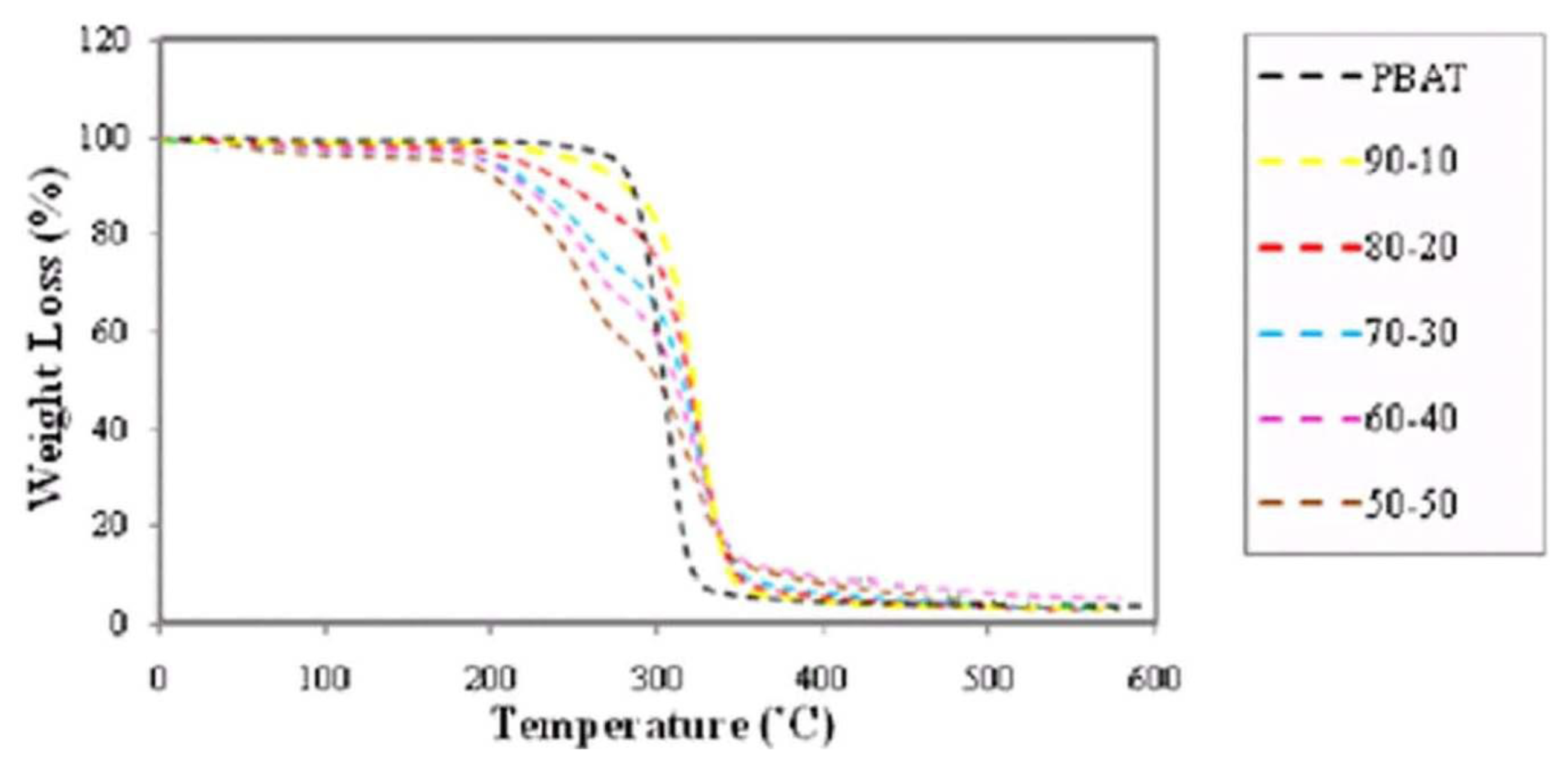
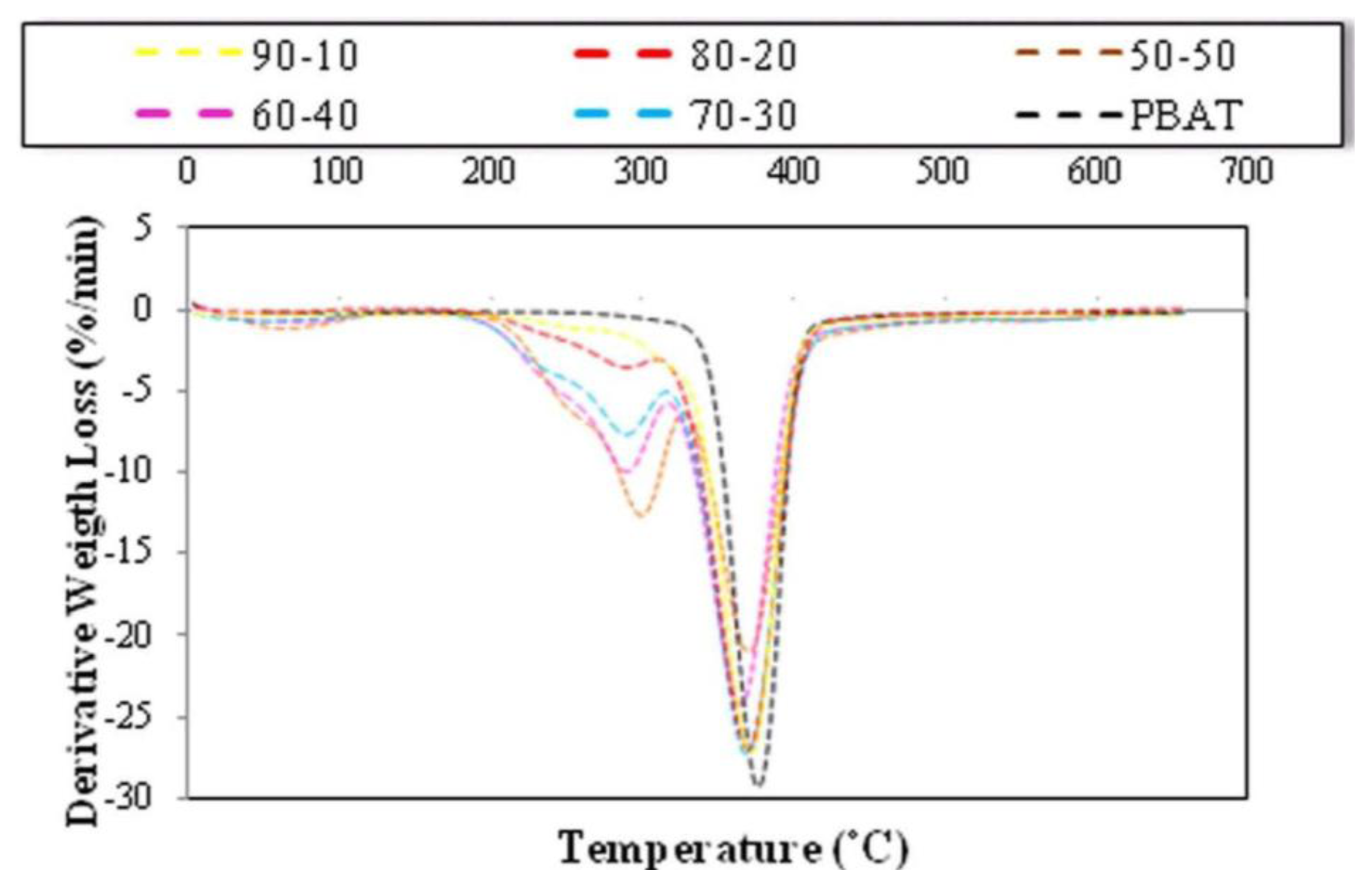
2.5. Effect of Fiber Loading and Chemical Modification on Thermal Properties of PBAT/EFB Biocomposites
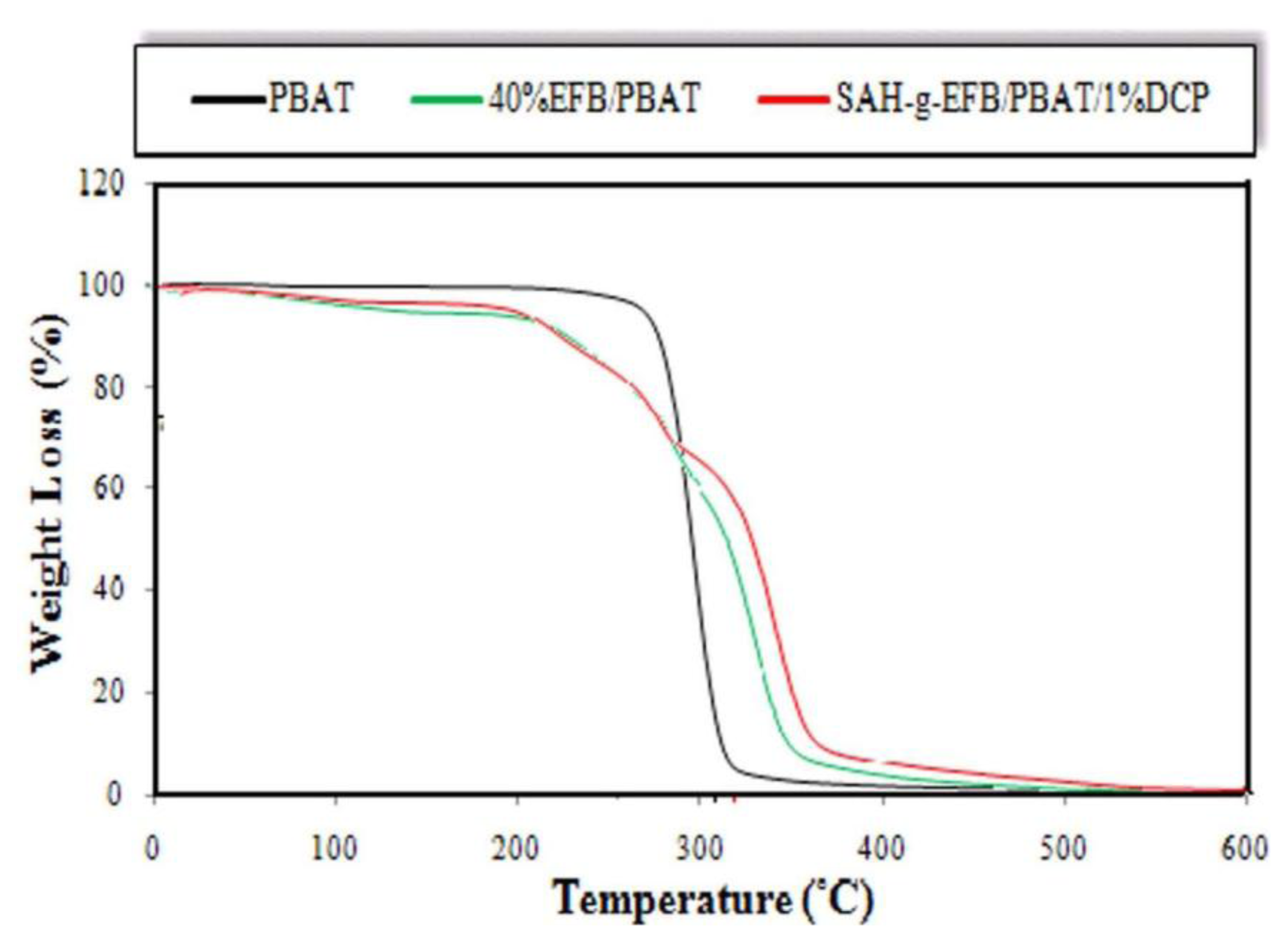
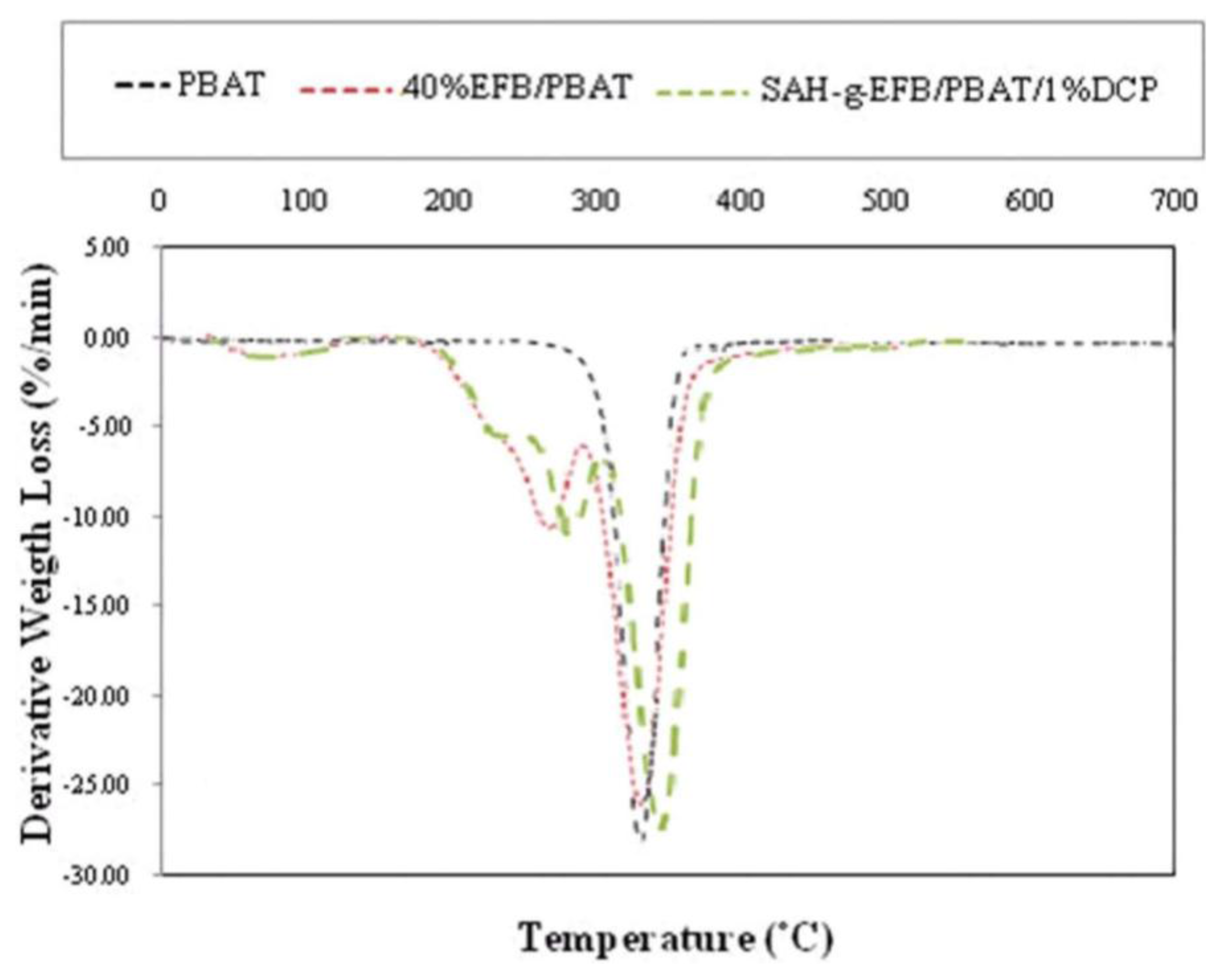
2.6. Effect of Chemical Modification on Thermal Properties of PBAT/EFB Fiber Composites
2.7. Morphological Study of PBAT/EFB Biocomposite at 40% Fiber Loading
2.8. Morphological Study of PBAT/EFB Biocomposite after Fiber Modification
2.9. Effect of Fiber Loading and Fiber Chemical Modification on Water Absorption of PBAT/EFB Biocomposites
3. Experimental
3.1. Materials
3.2. Fiber Chemical Treatment
3.3. Preparation of the Composites
3.4. FTIR Spectroscopy
3.5. Biocomposites characterization
3.5.1. Mechanical Testing of Biocomposites
3.5.2. Thermal Behavior (Thermogravimetric Analysis, TGA)
3.5.3. Morphological Features
3.5.4. Water Absorption Test
4. Conclusions
Acknowledgements
References
- Singh, S. Green Bio-Composites from Polyhydroxybutyrate-co-Valerate (PHBV), Wood Fiber and Talc. In Ph.D. Thesis; Michigan State University: Grand Rapids, MI, USA, 2009. [Google Scholar]
- Madera-Santana, T.J.; Misra, M.; Drzal, L.T.; Robledo, D.; Freile-Pelegrin, Y. Preparation and characterization of biodegradable agar/poly (butylene adipate-co-terephthalate) composites. Polym. Eng. Sci 2009, 49, 1117–1126. [Google Scholar]
- Satyanarayana, K.G.; Arizaga, G.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci 2009, 34, 982–1021. [Google Scholar]
- Ibrahim, N.A.; Hadithon, K.A.; Abdan, K. Effect of fiber treatment on mechanical properties of kenaf fiber-ecoflex composites. J. Reinf. Plast. Compos 2009, 30, 1029–1037. [Google Scholar]
- Malkapuram, R.; Kumar, V.; Negi, Y.S. Recent development in natural fiber reinforced polypropylene composites. J. Reinf. Plast. Compos 2009, 28, 1169–1189. [Google Scholar]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibers, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng 2000, 276/277, 1–24. [Google Scholar]
- Arif, M.F.; Yusoff, P.S.; Ahmad, M.F. Effects of chemical treatment on oil palm empty fruit bunch reinforced high density polyethylene composites. J. Reinf. Plast. Compos 2010, 29, 2105–2118. [Google Scholar]
- Araujo, J.R.; Waldman, W.R.; de Paoli, M.A. Thermal properties of high density polyethylene composites with natural fibers: Coupling agent effect. Ploym. Degrad. Stab 2008, 93, 1770–1775. [Google Scholar]
- Sreekala, M.S.; Kumaran, M.G.; Thomas, S. Oil palm fibers: Morphology, chemical composition, surface modification and mechanical properties. J. Appl. Polym. Sci 1997, 66, 821–835. [Google Scholar]
- Abdul Khalil, H.P.S.; Ismail, H.; Ahmad, M.N.; Ariffin, A.; Hassan, K. The effect of various anhydride modifications on mechanical properties and water absorption of oil palm empty fruit bunches reinforced polyester composites. Polym. Int 2001, 50, 395–402. [Google Scholar]
- Roseley, A.S.M. Morphology of Chemically Refined and Chemically Treated Oil Palm Empty Fruit Bunch Fiber. Master Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2007. [Google Scholar]
- Rozman, H.D.; Saad, M.J.; Mohd Ishak, Z.A. Flexural and impact properties of oil palm empty fruit bunch (EFB)-polypropylene composites—the effect of maleic anhydride chemical modification of EFB. Polym. Test 2003, 22, 335–341. [Google Scholar]
- Tronc, E.; Hernandez-Escobar, C.A.; Ibarra-Gomez, R.; Estrada-Monje, A.; Navarrete-Bolanos, J.; Zaragoza-Contreras, E.A. Blue agave fiber esterification for the reinforcement of thermoplastic composites. Carbohydr. Polym 2007, 67, 245–255. [Google Scholar]
- Mohanty, S.; Verma, K.; Nayak, S.; Sudhansu, S.K. Dynamic mechanical and thermal properties of MAPE treated jute/HDPE composites. Compos. Sci. Technol 2006, 66, 538–547. [Google Scholar]
- Luz, S.M.; Del Tio, J.; Rocha, G.J.M.; Gonçalves, A.R.; Del’Arco, A.P., Jr. Cellulose and cellulignin from sugarcane bagasse reinforced polypropylene composites: Effect of acetylation on mechanical and thermal properties. Compos. Part A 2008, 39, 1362–1369. [Google Scholar]
- Mohanty, S.; Verma, K.; Nayak, S.; Sudhansu, S.K.; Tripathy, S. Influence of fiber treatment on the performance of sisal-polypropylene composites. Appl. Polym. Sci 2004, 94, 1336–1345. [Google Scholar]
- Rozman, H.D.; Lim, P.P.; Abusamah, A.; Kumar, R.N.; Ismail, H.; Ishak, Z.M. The physical properties of oil palm Empty Fruit Bunch (EFB) composites made from various thermoplastics. Int. J. Polym. Mater 1999, 44, 179–195. [Google Scholar]
- Rozman, H.D.; Ismail, H.; Jaffri, R.M.; Aminullah, A.; Mohd Ishak, Z.A. Mechanical properties of polyethylene-oil palm empty fruit bunch composites. Polym. Plast. Technol. Eng 1998, 37, 495–507. [Google Scholar]
- Abdul Khalil, H.P.S.; Poh, B.T.; Jawaid, M. The effect of soil burial degradation of oil palm trunk fiber-filled recycled polypropylene composites. J. Reinf. Plast. Compos 2010, 29, 1653–1663. [Google Scholar]
- Ibrahim, N.A.; Wan Yunus, W.M.Z.; Abu-Ilaiwi, F.A.F.; Rahman, M.Z.A.; Ahmad, M.B.; Dahlan, K.Z.M. Graft copolymerization of methyl methacrylate onto oil palm empty fruit bunch fiber using H2O2/Fe2+ as an initiator. J. Appl. Polym. Sci. 2003, 89, 2233–2238. [Google Scholar]
- Rozman, H.D.; Musa, L.; Abubakar, A. Rice husk-polyester composites: The effect of chemical modification of rice husk on the mechanical and dimensional stability properties. Appl. Polym. Sci 2005, 97, 1237–1247. [Google Scholar]
- Rozman, H.D.; Hilmi, A.; Abubakar, K.R. Polyurethane (PU)-oil palm empty fruit bunch (EFB) composites: The effect of EFBG reinforcement in mat form and isocyanate treatment on the mechanical properties. Polym. Test 2004, 23, 559–565. [Google Scholar]
- Sykacek, E.; Hrabalova, M.; Frech, H.; Mundigler, N. Extrusion of five biopolymers reinforced with increasing wood flour concentration on a production machine, injection moulding and mechanical performance. Compos. Part A 2009, 40, 1272–1282. [Google Scholar]
- Hill, C.A.S.; Abdul Khalil, H.P.S. Effect of fiber treatments on mechanical properties of coir or oil palm fiber reinforced polyester composites. Appl. Polym. Sci 2000, 78, 1685–1697. [Google Scholar]
- Abu Bakar, B.A. Mechanical, Thermal And Processing Properties Of Oil Palm Empty Fruit Bunch-Filled Impact Modified Unplasticised Poly(Vinyl Chloride) Composites. Master Thesis, Universiti Teknologi Malaysia, Johor Bahru, Johor, Malaysia, 2006. [Google Scholar]
- Hassan, M.L.; Rowell, R.M.; Fadl, N.A.; Yacoub, S.F.; Alfred, W.C. Thermo-plasticization of Bagasse. II. Dimensional stability and mechanical properties of esterified bagasse composite. Appl. Polym. Sci 1999, 76, 515–586. [Google Scholar]
- Atikler, U. Preparation and Characterization of Polypropylene-Cellulose Composites. Master Thesis, Izmir Institute of Technology, Izmir, Turkey, 2004. [Google Scholar]
- Abdul Khalil, H.P.S.; Azura, M.; Issam, A.M.; Said, M.R.; Adawi, T. Oil Palm Empty Fruit Bunches (OPEFB) reinforced in new unsaturated polyester composite. Reinf. Plast. Compos 2008, 27, 1817–1826. [Google Scholar]
- ASTM Standard D638-99: Standard Test Method for Tensile Properties of Plastic; American Society for Testing and Materials: New York, NY, USA, 1999.
- ASTM Standard D790-07: Standard Test Method for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulation Materials; American Society for Testing and Materials: Philadelphia, PA, USA, 1997.
- ASTM Standard D570-98: Standard Test Method for water Absorption of Plastics; American Society for Testing and Materials: New York, NY, USA, 1998.






| PBAT/40 wt% EFB-g-SAH (High WPG *) | PBAT/40 wt% EFB-g-SAH(Low WPG *) | |||||||
|---|---|---|---|---|---|---|---|---|
| Tensile Strength (MPa) | Tensile Modulus (MPa) | Tensile Strength (MPa) | Tensile Modulus (MPa) | |||||
| Peroxide Content (%) | BPO *** | DCP | BPO | DCP | BPO | DCP | BPO | DCP |
| 0 | 8.4 (0.77)** | 8.4 (0.77) | 109 (9.11) | 109 (9.18) | 8.8 (0.31) | 8.8 (0.31) | 154 (8.11) | 154 (8.10) |
| 0.5 | 9.0 (0.53) | 8.8 (0.73) | 139 (7.83) | 156 (6.53) | 9.9 (0.26) | 10.2 (0.36) | 194 (8.95) | 197 (9.42) |
| 1 | 9.6 (0.44) | 9.6 (0.91) | 143 (8.30) | 176 (8.94) | 10.1 (0.44) | 11.0 (0.29) | 200 (9.05) | 220 (9.10) |
| 1.5 | 9.8 (0.60) | 9.2 (0.61) | 134 (8.25) | 176 (7.20) | 10.2 (0.63) | 10.4 (0.62) | 198 (7.78) | 202 (7.51) |
| Samples | Flexural Strength (MPa) | Flexural Modulus (MPa) | ||
|---|---|---|---|---|
| PBAT | 5.9 | (0.31) * | 90 | (4.28) |
| PBAT/EFB 10 wt% of fiber | 7.8 | (0.26) | 140 | (5.12) |
| PBAT/EFB 20 wt% of fiber | 9.7 | (0.44) | 214 | (9.61) |
| PBAT/EFB 30 wt% of fiber | 10.0 | (0.58) | 371 | (2.32) |
| PBAT/EFB 40 wt% of fiber | 10.3 | (0.63) | 465 | (3.51) |
| PBAT/EFB 50 wt% of fiber | 9.5 | (0.76) | 596 | (3.50) |
| PBAT/40 wt% EFB-g-SAH ** | 10.4 | (0.92) | 473 | (2.91) |
| PBAT/40 wt%EFB-g-SAH/DCP *** | 11.1 | (1.04) | 518 | (2.21) |
| Specimens | First peak (°C) | Second peak (°C) | Third peak (°C) |
|---|---|---|---|
| PBAT | – | – | 379.6 |
| PBAT/EFB10% | – | – | 386.1 |
| PBAT/EFB20% | – | 305.5 | 383.3 |
| PBAT/EFB30% | 88.0 | 307.5 | 382.2 |
| PBAT/EFB40% | 88.8 | 307.0 | 380.9 |
| PBAT/EFB50% | 79.5 | 307.2 | 379.4 |
| Sample | Water content (%) | Initial degradation temperature (°C) * | T10% | T50% | T80% | Final degradation temperature (°C) | Ash content (%) * |
|---|---|---|---|---|---|---|---|
| PBAT | – | 311.5 | 352.8 | 381.3 | 398.4 | 413.3 | 4.4 |
| PBAT/EFB10% | – | 280.4 | 339.7 | 382.8 | 399.2 | 431.7 | 6.7 |
| PBAT/EFB20% | 1.3 | 248.8 | 302.3 | 378.0 | 398.6 | 432.5 | 9.1 |
| PBAT/EFB30% | 2 | 210.2 | 276.7 | 373.6 | 401.5 | 440.2 | 11.2 |
| PBAT/EFB40% | 3.9 | 153.3 | 275.2 | 371.1 | 400.2 | 443.6 | 12.2 |
| PBAT/EFB50% | 3.4 | 148.2 | 233.3 | 360.2 | 398.8 | 447.7 | 17.7 |
| Sample | Water content (%) | Initial degradation temperature (°C) * | T10% | T50% | T80% | Final degradation temperature (°C) | Ash content (%) ** |
|---|---|---|---|---|---|---|---|
| Neat PBAT | – | 311.5 | 352.8 | 381.3 | 398.4 | 413.3 | 4.4 |
| PBAT/40%EFB | 3.9 | 153.3 | 275.2 | 371.1 | 400.2 | 443.6 | 12.2 |
| PBAT/EFB-g-SAH/1%DCP | 2.0 | 136.8 | 267.1 | 378.7 | 446.4 | 450.2 | 14.8 |
| Specimens | First peak (°C) | Second peak (°C) | Third peak (°C) | Fourth peak (°C) |
|---|---|---|---|---|
| PBAT | – | – | – | 379.6 |
| PBAT/40%EFB | 88.8 | – | 307.0 | 380.9 |
| PBAT/40%EFB-g-SAH/1%DCP | 86.5 | 249.7 | 316.6 | 388.2 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Siyamak, S.; Ibrahim, N.A.; Abdolmohammadi, S.; Yunus, W.M.Z.W.; Rahman, M.Z.A. Effect of Fiber Esterification on Fundamental Properties of Oil Palm Empty Fruit Bunch Fiber/Poly(butylene adipate-co-terephthalate) Biocomposites. Int. J. Mol. Sci. 2012, 13, 1327-1346. https://doi.org/10.3390/ijms13021327
Siyamak S, Ibrahim NA, Abdolmohammadi S, Yunus WMZW, Rahman MZA. Effect of Fiber Esterification on Fundamental Properties of Oil Palm Empty Fruit Bunch Fiber/Poly(butylene adipate-co-terephthalate) Biocomposites. International Journal of Molecular Sciences. 2012; 13(2):1327-1346. https://doi.org/10.3390/ijms13021327
Chicago/Turabian StyleSiyamak, Samira, Nor Azowa Ibrahim, Sanaz Abdolmohammadi, Wan Md Zin Wan Yunus, and Mohamad Zaki AB Rahman. 2012. "Effect of Fiber Esterification on Fundamental Properties of Oil Palm Empty Fruit Bunch Fiber/Poly(butylene adipate-co-terephthalate) Biocomposites" International Journal of Molecular Sciences 13, no. 2: 1327-1346. https://doi.org/10.3390/ijms13021327




