Serine Hydroxymethyltransferase from the Cold Adapted Microorganism Psychromonas ingrahamii: A Low Temperature Active Enzyme with Broad Substrate Specificity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Heterologous Expression and Purification of the Recombinant Psychromonas ingrahamii SHMT
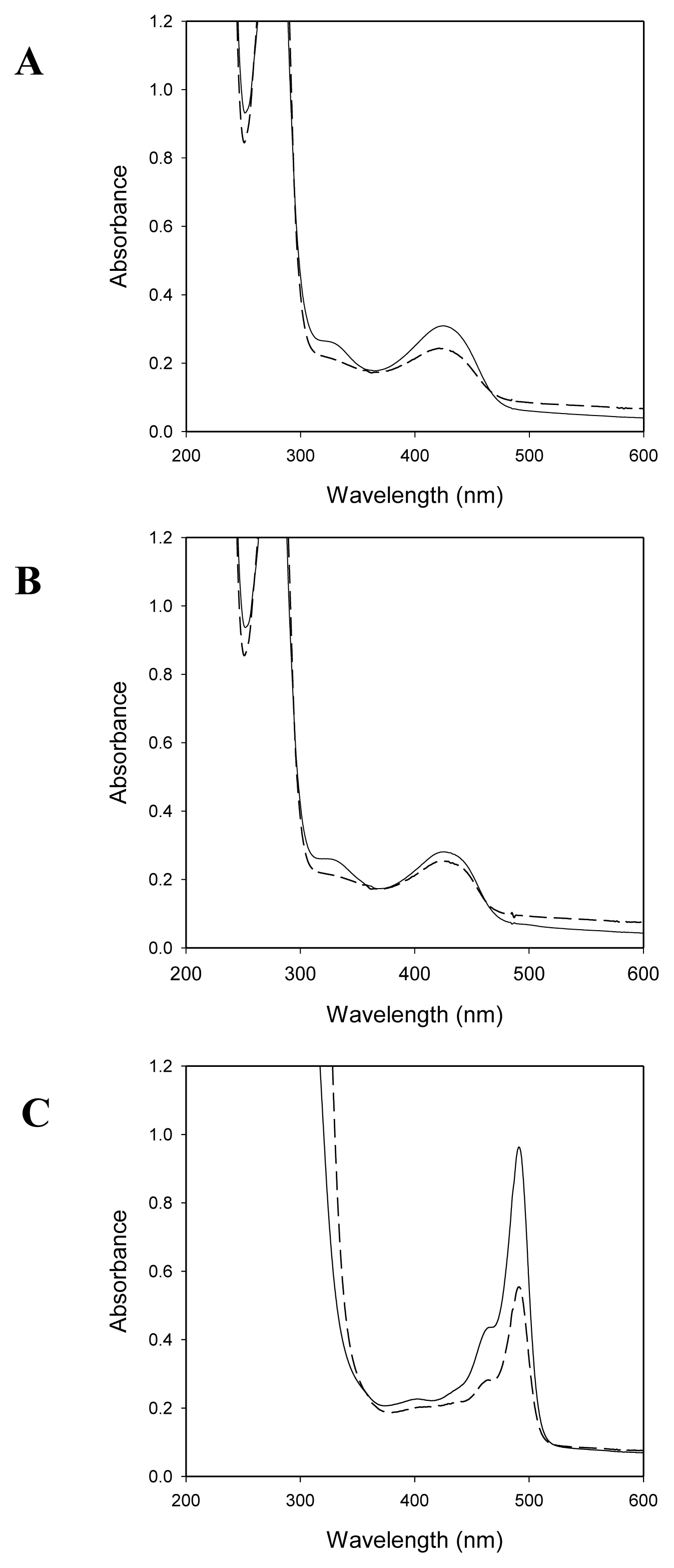
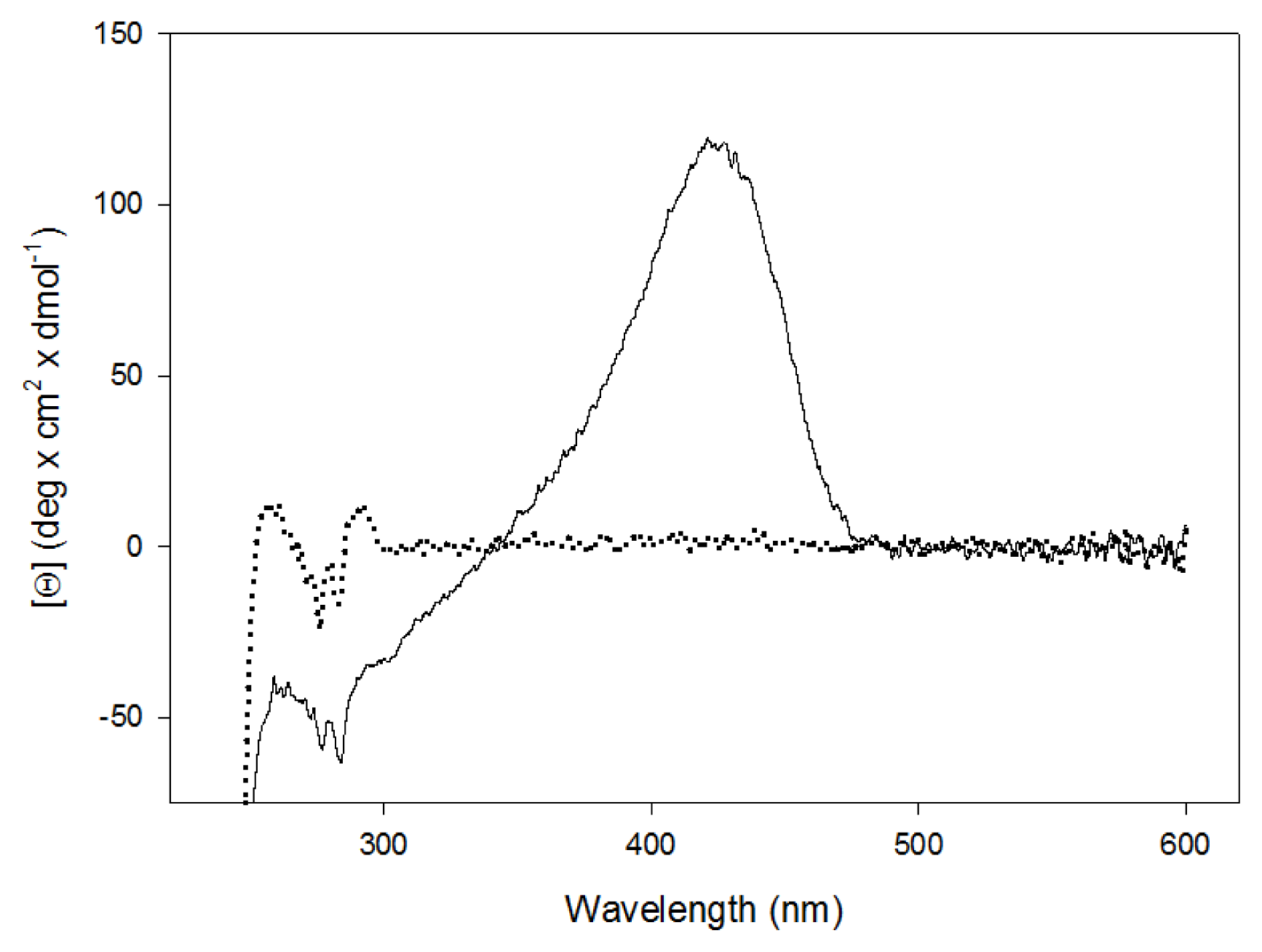
2.2. Spectroscopic Studies
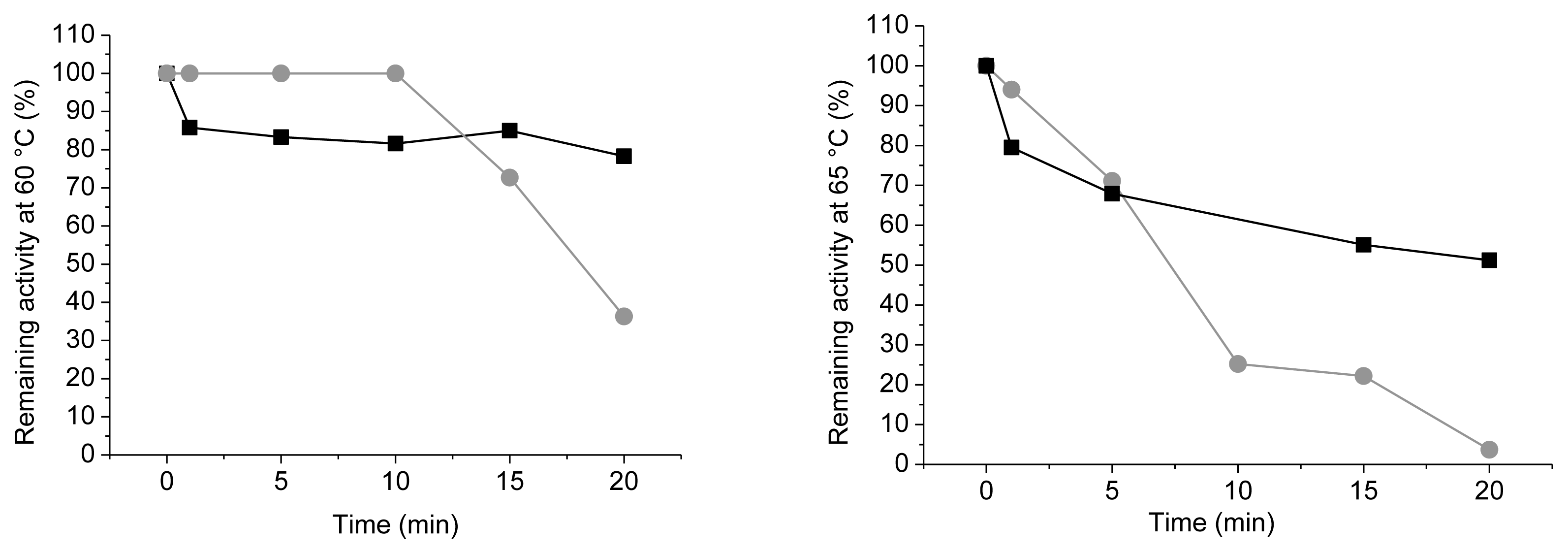
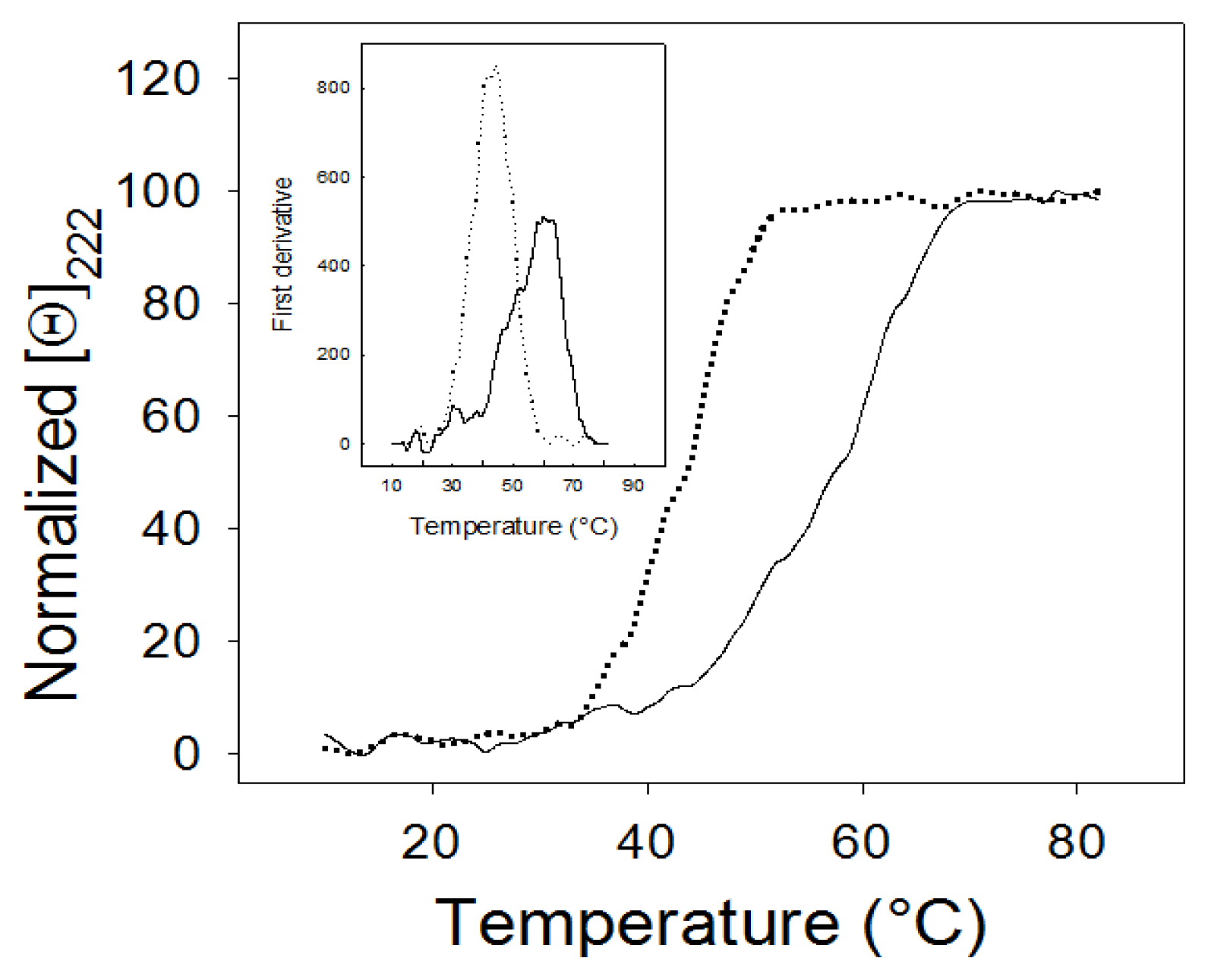
2.3. Thermal Stability
2.4. Catalytic Properties of piSHMT
2.5. Thermal Denaturation Experiments
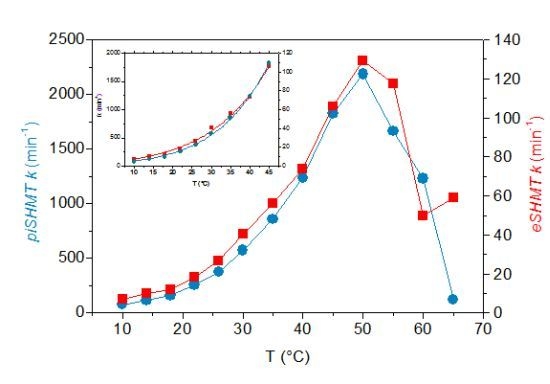
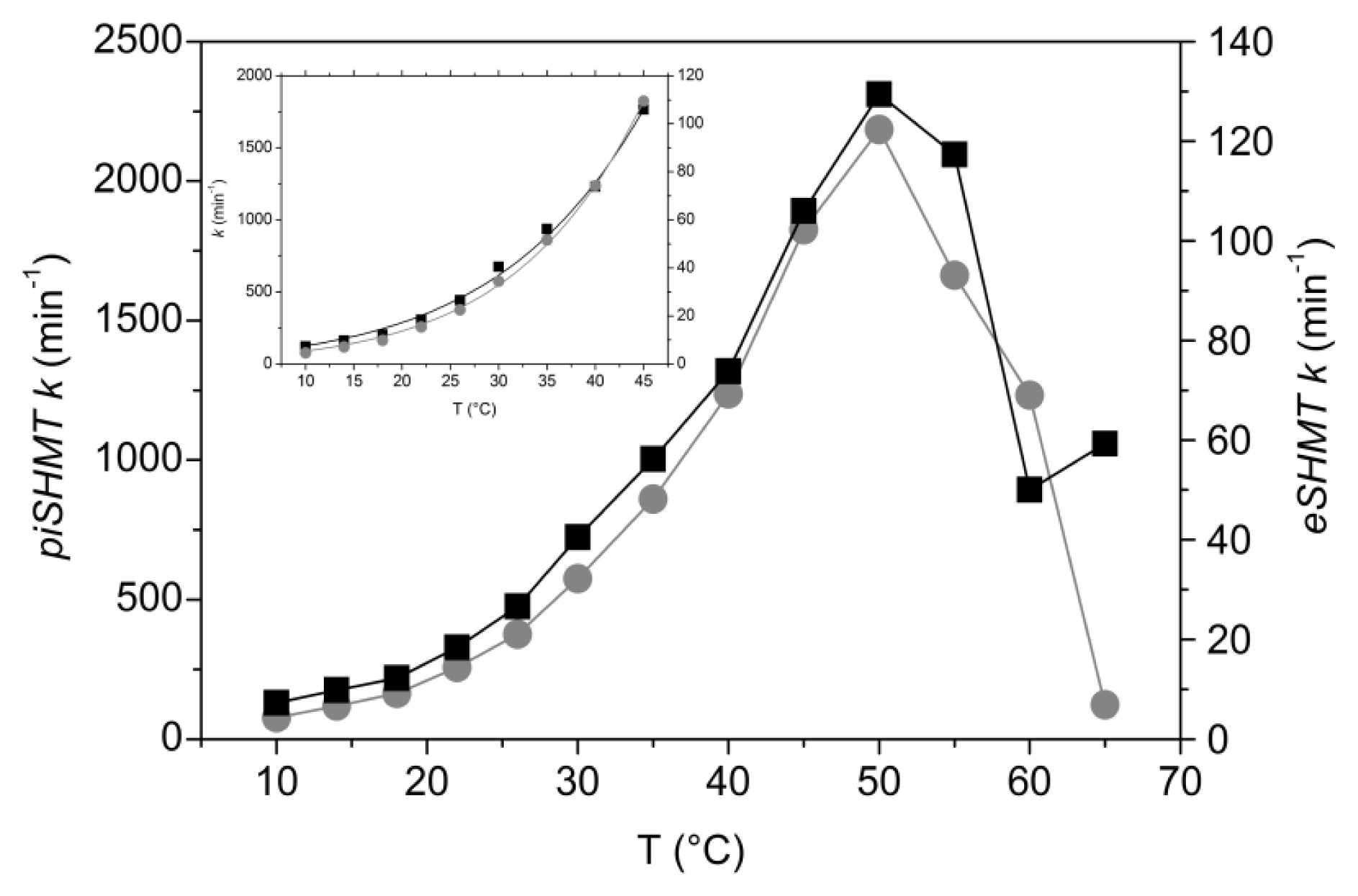
2.6. Temperature Dependence of Enzyme Activity
2.7. Psychrophilicity of Psychromonas ingrahamii SHMT
3. Experimental Section
3.1. Materials
3.2. Cloning of the Gene Encoding the Psychromonas ingrahamii SHMT
3.3. Expression and Purification of Psychromonas ingrahamii SHMT
3.4. Preparation of Apoenzyme
3.5. Spectroscopic Measurements
3.6. Activity Assays
3.7. Data Analysis
3.8. Thermal Denaturation Experiments
3.9. Heat Inactivation
3.10. Temperature Dependence of Enzyme Activity
4. Conclusions
Acknowledgments
References
- Kashefi, K.; Lovley, D.R. Extending the upper temperature limit for life. Science 2003, 301, 934. [Google Scholar]
- Cavicchioli, R.; Siddiqui, K.S.; Andrews, D.; Sowers, K.R. Low-temperature extremophiles and their applications. Curr. Opin. Biotechnol 2002, 13, 253–261. [Google Scholar]
- Feller, G.; Gerday, C. Psychrophylic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol 2003, 1, 200–208. [Google Scholar]
- Somero, G.N. Adaptation of enzymes to temperature: Searching for basic “strategies”. Comp. Biochem. Physiol. B 2004, 139, 321–333. [Google Scholar]
- Siddiqui, K.S.; Cavicchioli, R. Cold-Adapted Enzymes. Annu. Rev. Biochem 2006, 75, 403–433. [Google Scholar]
- Smalås, A.O.; Leiros, H.K.; Os, V.; Willassen, N.P. Cold adapted enzymes. Biotechnol. Annu. Rev 2000, 6, 1–57. [Google Scholar]
- D’Amico, S.; Gerday, C.; Feller, G. Structural determinants of cold adaptation and stability in a large protein. J. Biol. Chem 2001, 276, 25791–25796. [Google Scholar]
- D’Amico, S.; Marx, J.C.; Gerday, C.; Feller, G. Activity-stability relationships in extremophilic enzymes. J. Biol. Chem 2003, 278, 7891–7896. [Google Scholar]
- Michaux, C.; Massant, J.; Kerff, F.; Frère, J.M.; Docquier, J.D.; Vandenberghe, I.; Samyn, B.; Pierrard, A.; Feller, G.; Charlier, P.; et al. Crystal structure of a cold adapted class C beta-lactamase. FEBS J 2008, 275, 1687–1697. [Google Scholar]
- Auman, A.J.; Breezee, J.L.; Gosink, J.J.; Kämpfer, P.; Staley, J.T. Psychromonas ingrahamii sp. nov., a novel gas vacuolate, psychrophilic bacterium isolated from Arctic polar sea ice. Int. J. Syst. Evol. Microbiol 2006, 56, 1001–1007. [Google Scholar]
- Amadasi, A.; Bertoldi, M.; Contestabile, R.; Bettati, S.; Cellini, B.; di Salvo, M.L.; Borri-Voltattorni, C.; Bossa, F.; Mozzarelli, A. Pyridoxal 5′-phosphate enzymes as targets for therapeutic agents. Curr. Med. Chem 2007, 14, 1291–1324. [Google Scholar]
- Siglioccolo, A.; Bossa, F.; Pascarella, S. Structural adaptation of serine hydroxymethyltransferase to low temperatures. Int. J. Biol. Macromol 2010, 46, 37–46. [Google Scholar]
- Angelaccio, S.; Chiaraluce, R.; Consalvi, V.; Buchenau, B.; Giangiacomo, L.; Bossa, F.; Contestabile, R. Catalytic and thermodynamic properties of tetrahydromethanopterin-dependent serine hydroxymethyltransferase from Methanococcus jannaschii. J. Biol. Chem 2003, 278, 41789–41797. [Google Scholar]
- Paiardini, A.; Gianese, G.; Bossa, F.; Pascarella, S. Structural plasticity of thermophilic serine hydroxymethyltransferases. Proteins 2003, 50, 122–134. [Google Scholar]
- Birolo, L.; Tutino, M.L.; Fontanella, B.; Gerday, C.; Mainolfi, K.; Pascarella, S.; Sannia, G.; Vinci, F.; Marino, G. Aspartate aminotransferase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125 Cloning, expression, properties, and molecular modeling. Eur. J. Biochem 2000, 267, 2790–2802. [Google Scholar]
- Russell, N.J. Molecular adaptations in psychrophilic bacteria: Potential for biotechnological applications. Adv. Biochem. Eng. Biotechnol 1998, 61, 1–21. [Google Scholar]
- Schirch, V. Mechanism of Folate-Requiring Enzymes in One-Carbon Metabolism. In Comprehensive Biological Catalysis, 2nd ed; Sinnot, M.L., Ed.; Academic Press: New York, NY, USA, 1998; pp. 211–252. [Google Scholar]
- Strickland, E.H. Aromatic contributions to circular dichroism spectra of proteins. CRC Crit. Rev. Biochem 1974, 2, 113–175. [Google Scholar]
- Contestabile, R.; Paiardini, A.; Pascarella, S.; di Salvo, M.L.; D’Aguanno, S.; Bossa, F. l-Threonine aldolase, serine hydroxymethyltransferase and fungal alanine racemase, A subgroup of strictly related enzymes specialized for different functions. Eur. J. Biochem 2001, 268, 6508–6525. [Google Scholar]
- Schirch, V.; Hopkins, S.; Villar, E.; Angelaccio, S. Serine hydroxymethyltransferase from Escherichia coli: Purification and properties. J. Bacteriol 1985, 163, 1–7. [Google Scholar]
- Vivoli, M.; Angelucci, F.; Ilari, A.; Morea, V.; Angelaccio, S.; di Salvo, M.L.; Contestabile, R. Role of a conserved active site cation-π interaction in Escherichia coli serine hydroxymethyltransferase. Biochemistry 2009, 48, 12034–12046. [Google Scholar]
- Gerday, C.; Aittaleb, M.; Arpigny, J.L.; Baise, E.; Chessa, J.P.; Garsoux, G.; Petrescu, I.; Feller, G. Psychrophilic enzymes: A thermodynamic challenge. Biochim. Biophys. Acta 1997, 1342, 119–131. [Google Scholar]
- Bjelic, S.; Brandsdal, B.O.; Aqvist, J. Cold adaptation of enzyme reaction rates. Biochemistry 2008, 47, 10049–10057. [Google Scholar]
- Cai, K.; Schirch, D.; Schirch, V. The affinity of pyridoxal 5′-phosphate for folding intermediates of Escherichia coli serine hydroxymethyltransferase. J. Biol. Chem 1995, 270, 19294–19299. [Google Scholar]
- Malerba, F.; Bellelli, A.; Giorgi, A.; Bossa, F.; Contestabile, R. The mechanism of addition of pyridoxal 5′-phosphate to Escherichia coli apo-serine hydroxymethyltransferase. Biochem. J 2007, 404, 477–485. [Google Scholar]
- Zhao, G.H.; Li, H.; Liu, W.; Zhang, W.G.; Zhang, F.; Liu, Q.; Jiao, Q.C. Preparation of optically active β-hydroxy-α-amino acid by immobilized Escherichia coli cells with serine hydroxymethyl transferase activity. Amino Acids 2011, 40, 215–220. [Google Scholar]
- Hoyoux, A.; Blaise, V.; Collins, T.; D’Amico, S.; Gratia, E.; Huston, A.L.; Marx, J.C.; Sonan, G.; Zeng, Y.; Feller, G.; et al. Extreme catalysts from low-temperature environments. J. Biosci. Bioeng 2004, 98, 317–330. [Google Scholar]
- Merlino, A.; Krauss, I.R.; Castellano, I.; de Vendittis, E.; Rossi, B.; Conte, M.; Vergara, A.; Sica, F. Structure and flexibility in cold-adapted iron superoxide dismutases: The case of the enzyme isolated from Pseudoalteromonas haloplanktis. Struct. Biol 2010, 172, 343–352. [Google Scholar]
- Mereghetti, P.; Riccardi, L.; Brandsdal, B.O.; Fantucci, P.; de Gioia, L.; Papaleo, E. Near native-state conformational landscape of psychrophilic and mesophilic enzymes: Probing the folding funnel model. J. Phys. Chem. B 2010, 114, 7609–7619. [Google Scholar]
- Chiuri, R.; Maiorano, G.; Rizzello, A.; del Mercato, L.L.; Cingolani, R.; Rinaldi, R.; Maffia, M.; Pompa, P.P. Exploring local flexibility/rigidity in psychrophilic and mesophilic carbonic anhydrases. Biophys. J 2009, 96, 1586–1596. [Google Scholar]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem 1989, 182, 319–326. [Google Scholar]
- Schirch, L.; Peterson, D. Purification and properties of mitochondrial serine hydroxymethyltransferase. J. Biol. Chem 1980, 255, 7801–7806. [Google Scholar]
- Ulevitch, R.J.; Kallen, R.G. Purification and characterization of pyridoxal 50-phosphate-dependent serine hydroxymethyltransferase from lamb liver and its action upon β-phenylserines. Biochemistry 1977, 16, 5342–5350. [Google Scholar]
- GraphPad Prism, version 4.0; GraphPad Software Inc.: San Diego, CA, USA, 2003.
- OriginPro8, version 8.0988; OriginLab Corporation: Northampton, MA, USA, 2009.
- Bellapadrona, G.; Chiaraluce, R.; Consalvi, V.; Ilari, A.; Stefanini, S.; Chiancone, E. The mutations Lys 114 → Gln and Asp 126 → Asn disrupt an intersubunit salt bridge and convert Listeria innocua Dps into its natural mutant Listeria monocytogenes Dps. Effects on protein stability at low pH. Proteins 2007, 66, 975–983. [Google Scholar]
| Reactions | Psychromonas ingrahamii | Escherichia coli | ||||
|---|---|---|---|---|---|---|
| Substrate | KM (mM) | kcat (min−1) | kcat/KM (min−1 mM−1) | KM (mM) | kcat (min−1) | kcat/KM (min−1 mM−1) |
| l-threonine | 20.2 | 6.6 | 0.3 | 43 a | 4.3 a | 0.1 a |
| l-threo-phenylserine | 17.2 | 852 | 50 | 19 a | 167 a | 8.8 a |
| l-allo-threonine | 1.6 | 107 | 67 | 1.5 b | 30 b | 20 b |
| l-serine (H4PteGlu 30 μM) | 0.4 | 555 | 1388 | 0.3 b | 640 b | 2130 b |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Angelaccio, S.; Florio, R.; Consalvi, V.; Festa, G.; Pascarella, S. Serine Hydroxymethyltransferase from the Cold Adapted Microorganism Psychromonas ingrahamii: A Low Temperature Active Enzyme with Broad Substrate Specificity. Int. J. Mol. Sci. 2012, 13, 1314-1326. https://doi.org/10.3390/ijms13021314
Angelaccio S, Florio R, Consalvi V, Festa G, Pascarella S. Serine Hydroxymethyltransferase from the Cold Adapted Microorganism Psychromonas ingrahamii: A Low Temperature Active Enzyme with Broad Substrate Specificity. International Journal of Molecular Sciences. 2012; 13(2):1314-1326. https://doi.org/10.3390/ijms13021314
Chicago/Turabian StyleAngelaccio, Sebastiana, Rita Florio, Valerio Consalvi, Guido Festa, and Stefano Pascarella. 2012. "Serine Hydroxymethyltransferase from the Cold Adapted Microorganism Psychromonas ingrahamii: A Low Temperature Active Enzyme with Broad Substrate Specificity" International Journal of Molecular Sciences 13, no. 2: 1314-1326. https://doi.org/10.3390/ijms13021314