Identification and Characterization of the Actin-Binding Motif of Phostensin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sequence Analyses
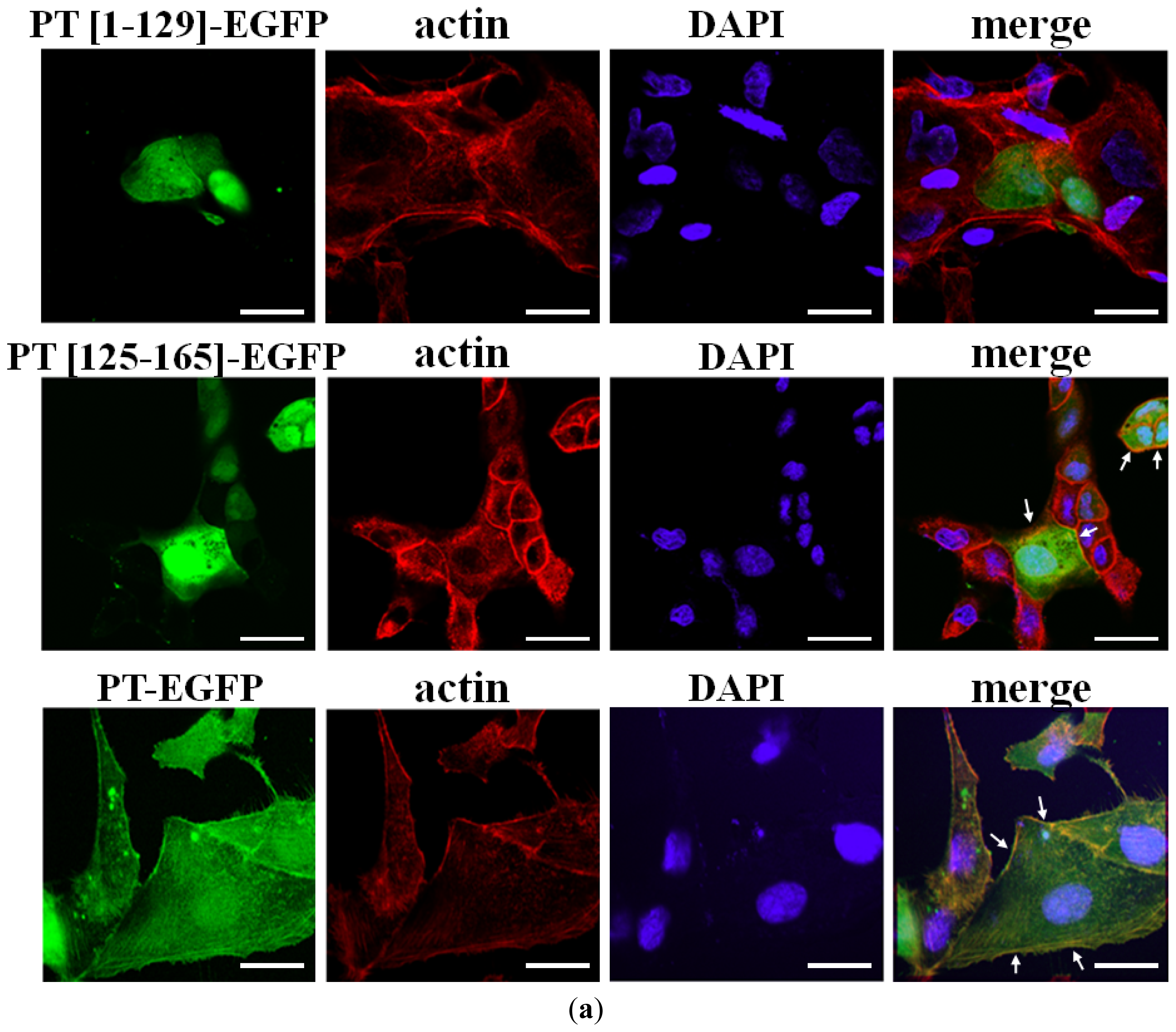
2.2. Cellular Colocalization and Actin Co-Sedimentation Assays
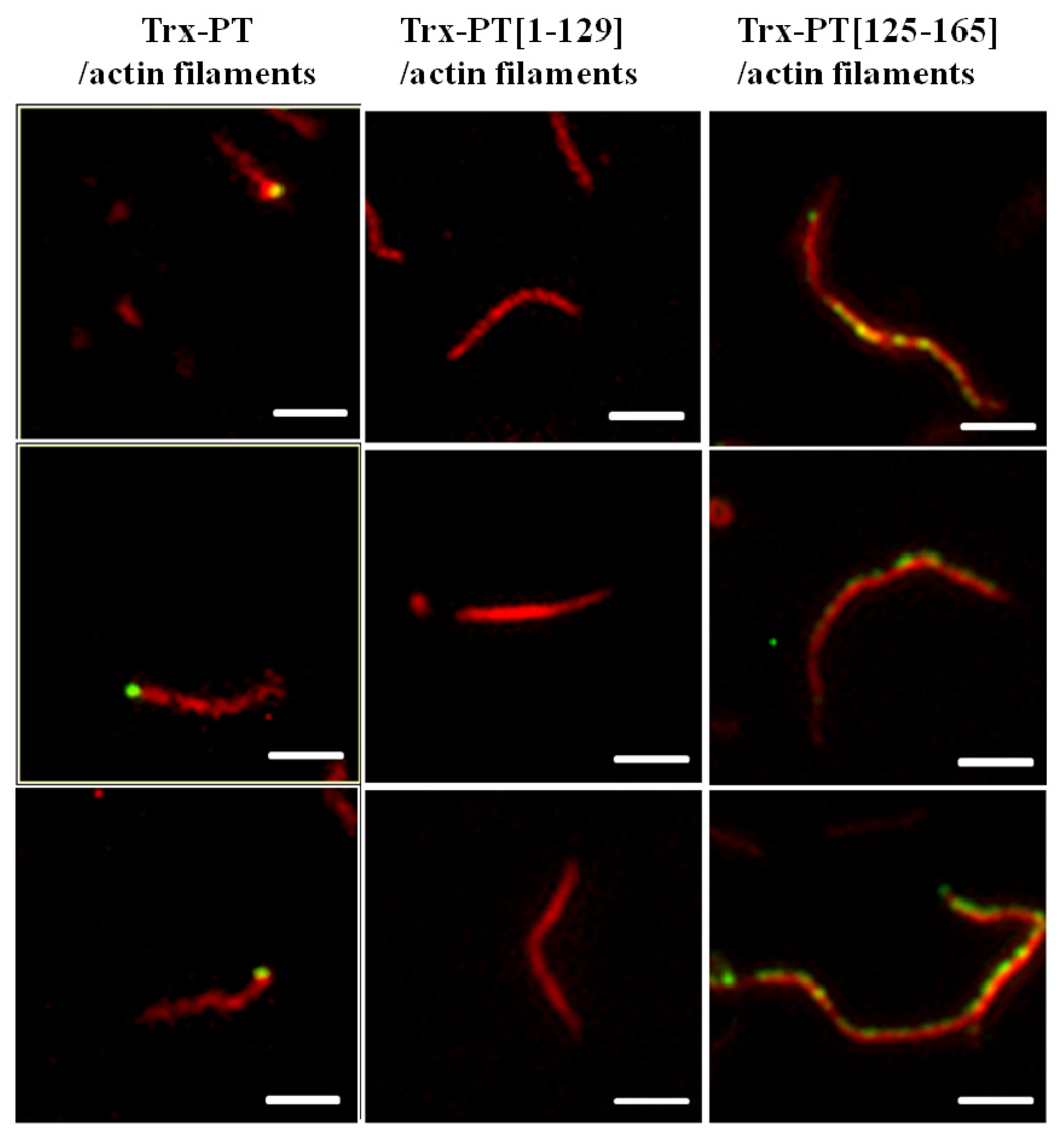
2.3. Single Filament Binding Assay
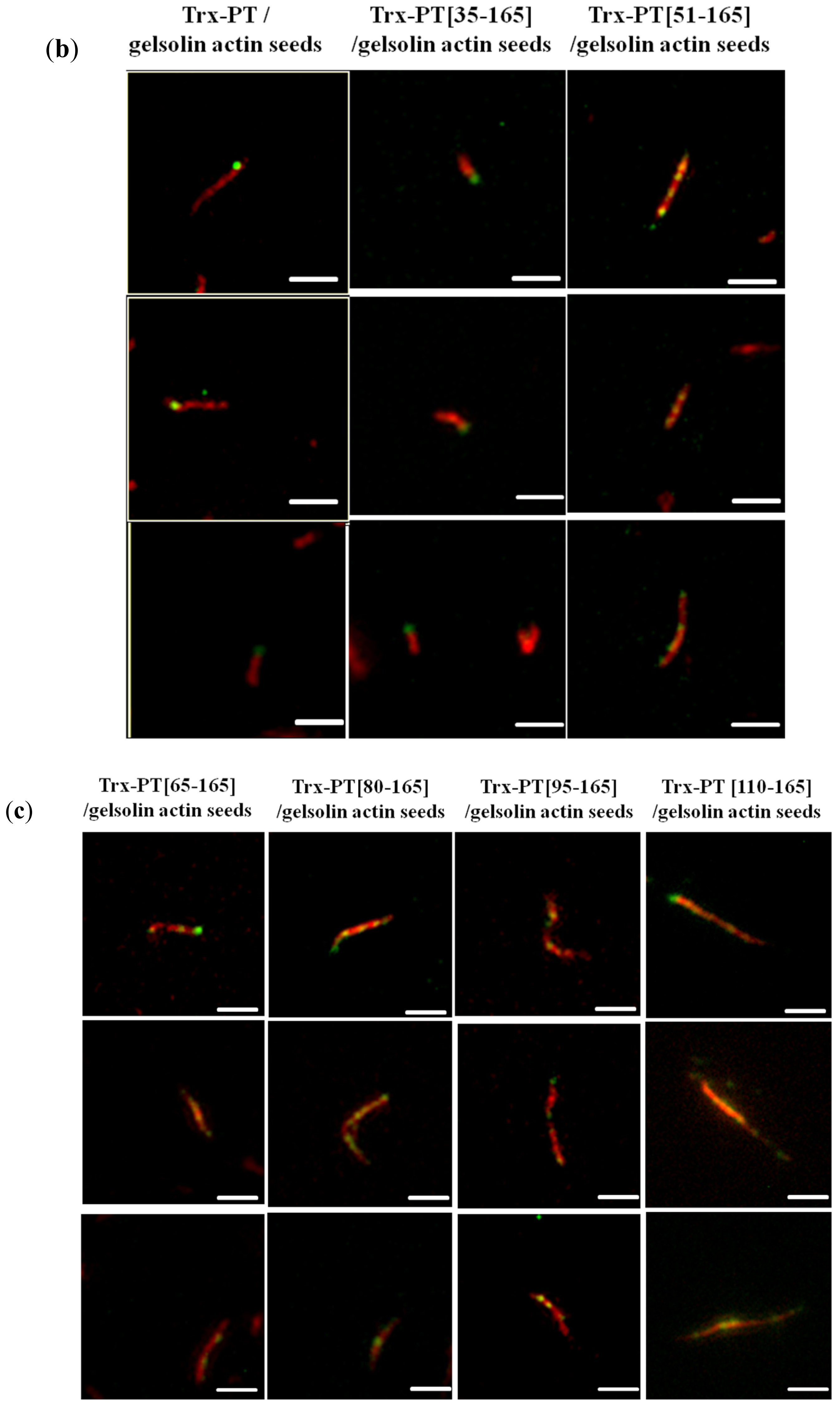
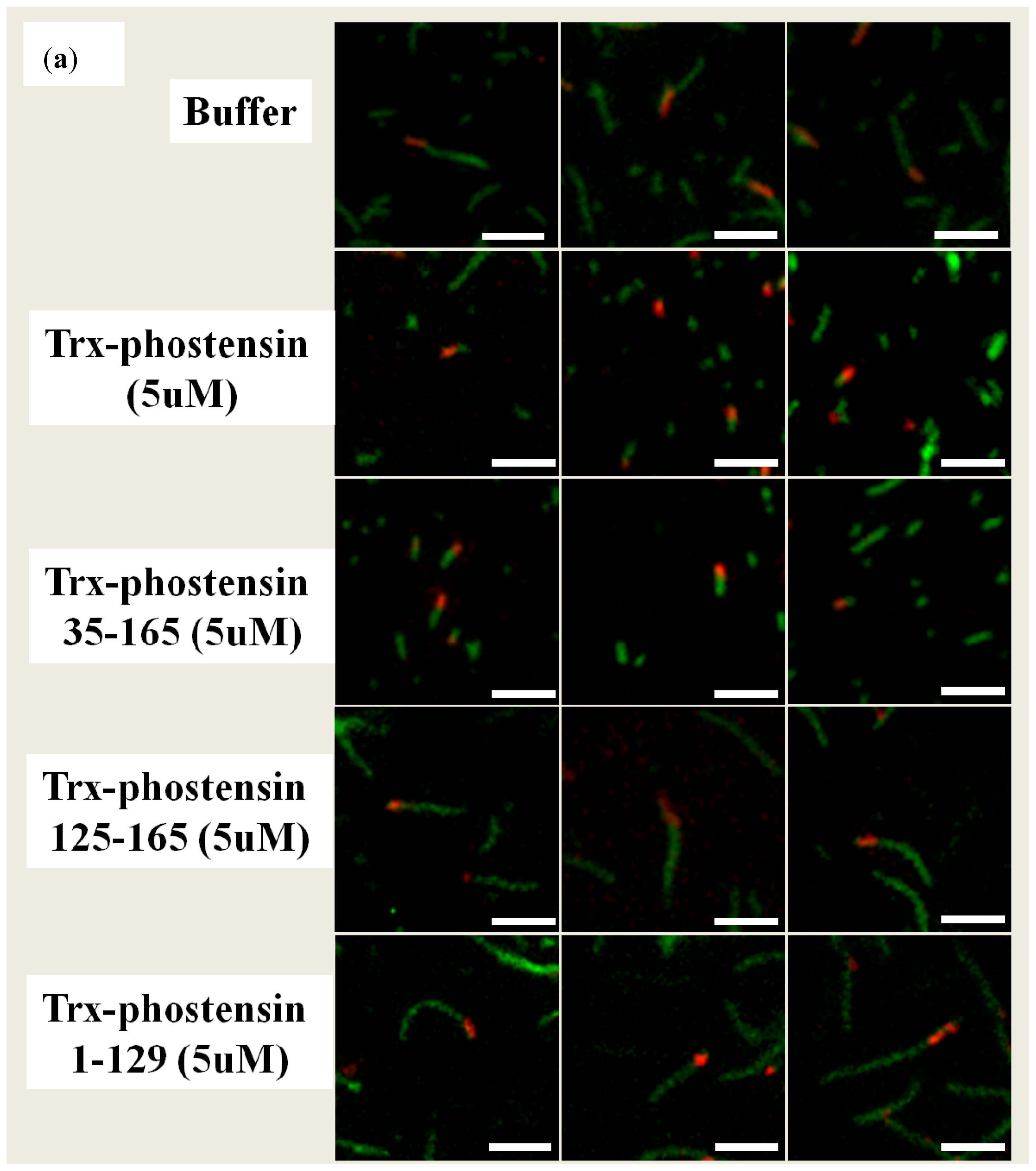
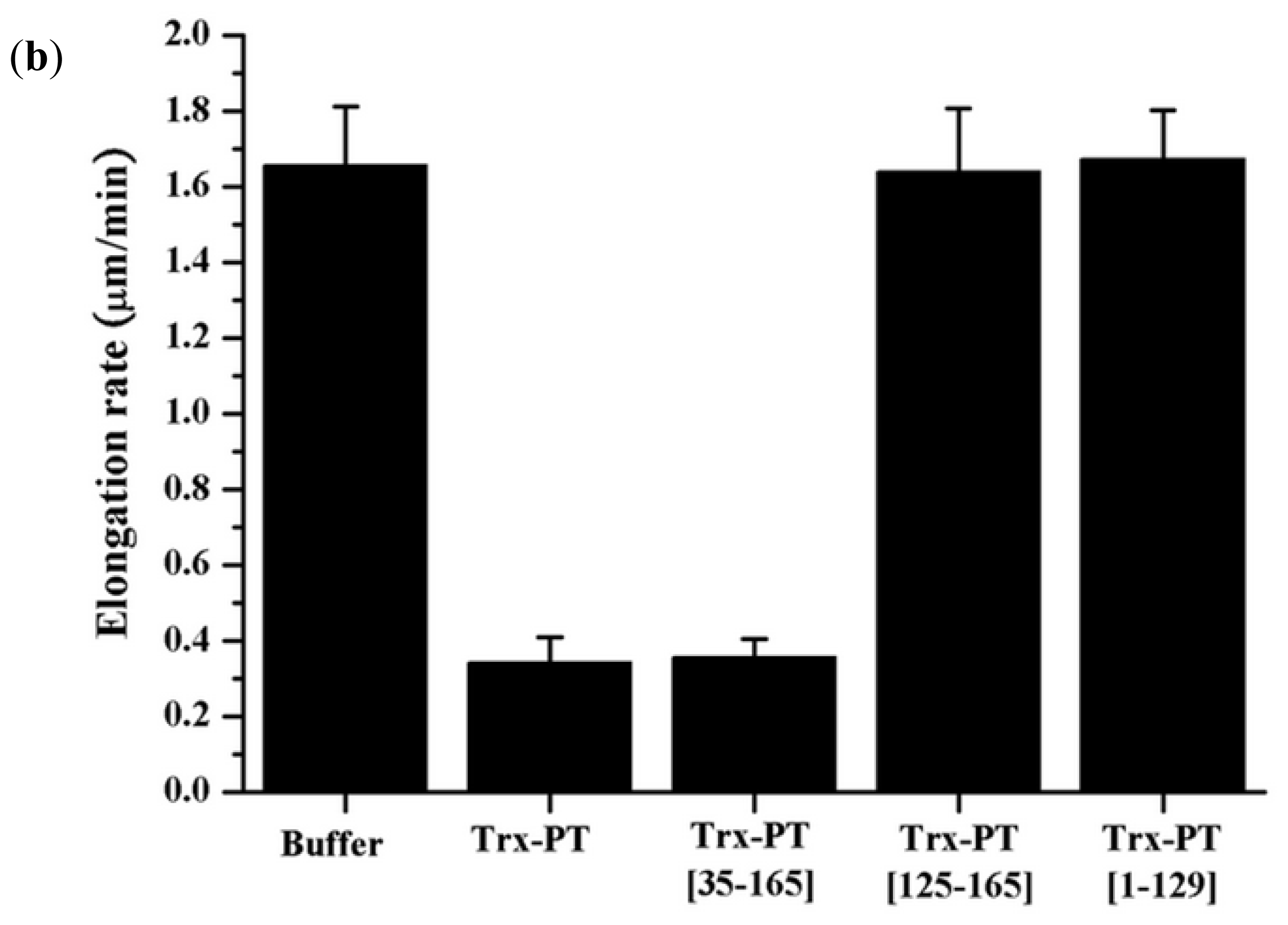
2.4. Single Filament Elongation Assay
3. Experimental Section
3.1. Materials
3.2. Fluorescence Microscopy
3.3. Actin Co-Sedimentation Assay
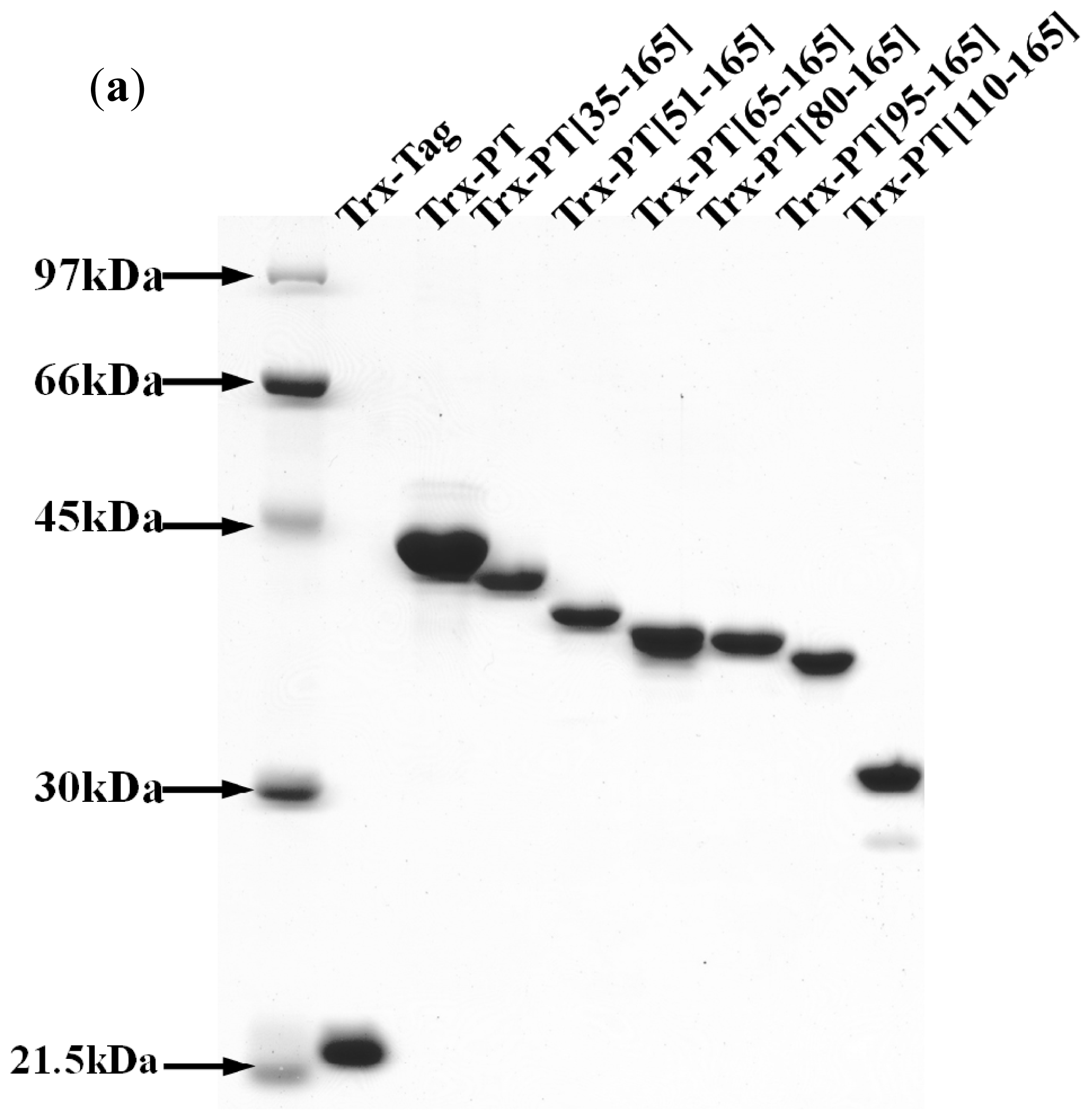
3.4. Preparation of Recombinant Thioredoxin-Phostensin (trx-PT) and Phostensin Mutant Proteins
3.5. Alexa-488 Conjugation
3.6. Single Filament Binding Assay
4. Conclusions
Acknowledgements
- Conflict of InterestThe authors declare no conflict of interest.
References
- Kao, S.C.; Chen, C.Y.; Wang, S.L.; Yang, J.J.; Hung, W.C.; Chen, Y.C.; Lai, N.S.; Liu, H.T.; Huang, H.L.; Chen, H.C.; et al. Identification of phostensin, a PP1 F-actin cytoskeleton targeting subunit. Biochem. Biophy. Res. Commun 2007, 356, 594–598. [Google Scholar]
- Nagase, T.; Kikuno, R.; Ohara, O. Prediction of the coding sequences of unidentified human genes. XXII. The complete sequences of 50 new cDNA clones which code for large proteins. DNA Res 2001, 8, 319–327. [Google Scholar]
- Shigenari, A.; Ando, A.; Renard, C.; Chardon, P.; Shiina, T.; Kulski, J.K.; Yasue, H.; Inoko, H. Nucleotide sequencing analysis of the swine 433-kb genomic segment located between the non-classical and classical SLA class I gene clusters. Immunogenetics 2004, 55, 695–705. [Google Scholar]
- Su, Y.A.; Yang, J.; Tao, L.; Nguyen, H.; He, P. Undetectable and decreased expression of KIAA1949 (phostensin) encoded on chromosome 6p21.33 in human breast cancers reversed by transcriptome analysis. J. Cancer 2011, 1, 38–50. [Google Scholar]
- Lin, Y.S.; Huang, K.Y.; Wang, T.F.; Huang, H.L.; Yu, H.C.; Yen, J.Y.; Hung, S.H.; Liu, S.Q.; Lai, N.S.; Huang, H.B. Immunolocalization of phostensin in lymphatic cells and tissues. J. Histochem. Cytochem 2011, 59, 741–749. [Google Scholar]
- Lai, N.S.; Wang, T.F.; Wang, S.L.; Chen, C.Y.; Yen, J.Y.; Huang, H.L.; Li, C.; Huang, K.Y.; Liu, S.Q.; Lin, T.H.; et al. Phostensin caps to the pointed end of actin filaments and modulates actin dynamics. Biochem. Biophys. Res. Commum 2009, 387, 676–681. [Google Scholar]
- Pollard, T.D.; Cooper, J.A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Ann. Rev. Biochem 1986, 55, 987–1035. [Google Scholar]
- Fowler, V.M. Regulation of actin filament length in erythrocytes and striated muscle. Curr. Opin. Cell. Biol 1996, 8, 86–96. [Google Scholar]
- Cooper, J.A.; Schafer, D.A. Control of actin assembly and disassembly at filament ends. Curr. Opin. Cell Biol 2000, 12, 97–103. [Google Scholar]
- Barron-Casella, E.A.; Torres, M.A.; Scherer, S.W.; Heng, H.Q.; Tsui, L.C.; Casella, J.F. Sequence analysis and chromosomal localization of human Cap Z. Conserved residues within the actin-binding domain may link Cap Z to gelsolin/severin and profilin protein families. J. Biol. Chem 1995, 270, 21472–21479. [Google Scholar]
- Ampe, C.; Vanderkerckhove, J. The F-actin capping proteins of Physarum polycephalum: Cap42(a) is very similar, if not identical, to fragmin and is structurally and functionally very homologous to gelsolin; cap42(b) is Physarum actin. EMBO J 1987, 6, 4149–4157. [Google Scholar]
- Tellam, R.L.; Morton, D.J.; Clarke, F.M. A common theme in the amino acid sequences of actin and many actin-binding proteins. Trends Biochem. Sci 1989, 14, 130–133. [Google Scholar]
- Andre, E.; Lottspeich, F.; Schleicher, M.; Noegel, A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J. Biol. Chem 1988, 263, 722–727. [Google Scholar]
- McLaughlin, P.J.; Gooch, J.T.; Mannherz, H.G.; Weeds, A.G. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature 1993, 364, 685–692. [Google Scholar]
- Burtnick, L.D.; Koepf, E.D.K.; Grimes, J.; Jones, E.Y.; Stuart, D.I.; Mclaughlin, P.J.; Robinson, R.C. The crystal structure of plasma gelsonlin: Implication for actin severing, capping, and nucleation. Cell 1997, 90, 661–670. [Google Scholar]
- Weeds, A.G.; Gooch, J.; McLaughlin, P.; Pope, B.; Bengtsodotter, M.; Karlsson, R. Identification of the trapped calcium in the gelsolin segment 1-actin complex: Implications for the role of calcium in the control of gelsolin activity. FEBS Lett 1995, 360, 227–230. [Google Scholar]
- Kabsch, W.; Mannherz, H.G.; Suck, D.; Pai, E.P.; Holmes, K.C. Atomic structure of actin: DNase I complex. Nature 1990, 247, 37–44. [Google Scholar]
- Holmes, K.C.; Popp, D.; Gebhard, W.; Kabsch, W. Atomic model of the actin filament. Nature 1990, 247, 44–49. [Google Scholar]
- Troys, M.V.; Dewitte, D.; Goethals, M.; Carlier, M.F.; Vandekerckhove, J.; Ampe, C. The actin binding site of thymosin b4 mapped by mutational analysis. EMBO J 1996, 15, 201–210. [Google Scholar]
- Safer, D.; Sosnick, T.R.; Elzinga, M. Thymosin 4 binds actin in an extended conformation and contacts both the barbed and pointed ends. Biochemistry 1997, 36, 5806–5816. [Google Scholar]
- Fowler, V.M.; Greenfield, N.J.; Moyer, J. Tropomodulin contains two actin filament pointed end-capping domains. J. Biol. Chem 2003, 278, 40000–40009. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, T.-F.; Lai, N.-S.; Huang, K.-Y.; Huang, H.-L.; Lu, M.-C.; Lin, Y.-S.; Chen, C.-Y.; Liu, S.-Q.; Lin, T.-H.; Huang, H.-B. Identification and Characterization of the Actin-Binding Motif of Phostensin. Int. J. Mol. Sci. 2012, 13, 15967-15982. https://doi.org/10.3390/ijms131215967
Wang T-F, Lai N-S, Huang K-Y, Huang H-L, Lu M-C, Lin Y-S, Chen C-Y, Liu S-Q, Lin T-H, Huang H-B. Identification and Characterization of the Actin-Binding Motif of Phostensin. International Journal of Molecular Sciences. 2012; 13(12):15967-15982. https://doi.org/10.3390/ijms131215967
Chicago/Turabian StyleWang, Tzu-Fan, Ning-Sheng Lai, Kuang-Yung Huang, Hsien-Lu Huang, Ming-Chi Lu, Yu-Shan Lin, Chun-Yu Chen, Su-Qin Liu, Ta-Hsien Lin, and Hsien-Bin Huang. 2012. "Identification and Characterization of the Actin-Binding Motif of Phostensin" International Journal of Molecular Sciences 13, no. 12: 15967-15982. https://doi.org/10.3390/ijms131215967



