Preparation of Coaxial-Electrospun Poly[bis(p-methylphenoxy)]phosphazene Nanofiber Membrane for Enzyme Immobilization
Abstract
:1. Introduction
2. Results and Discussion
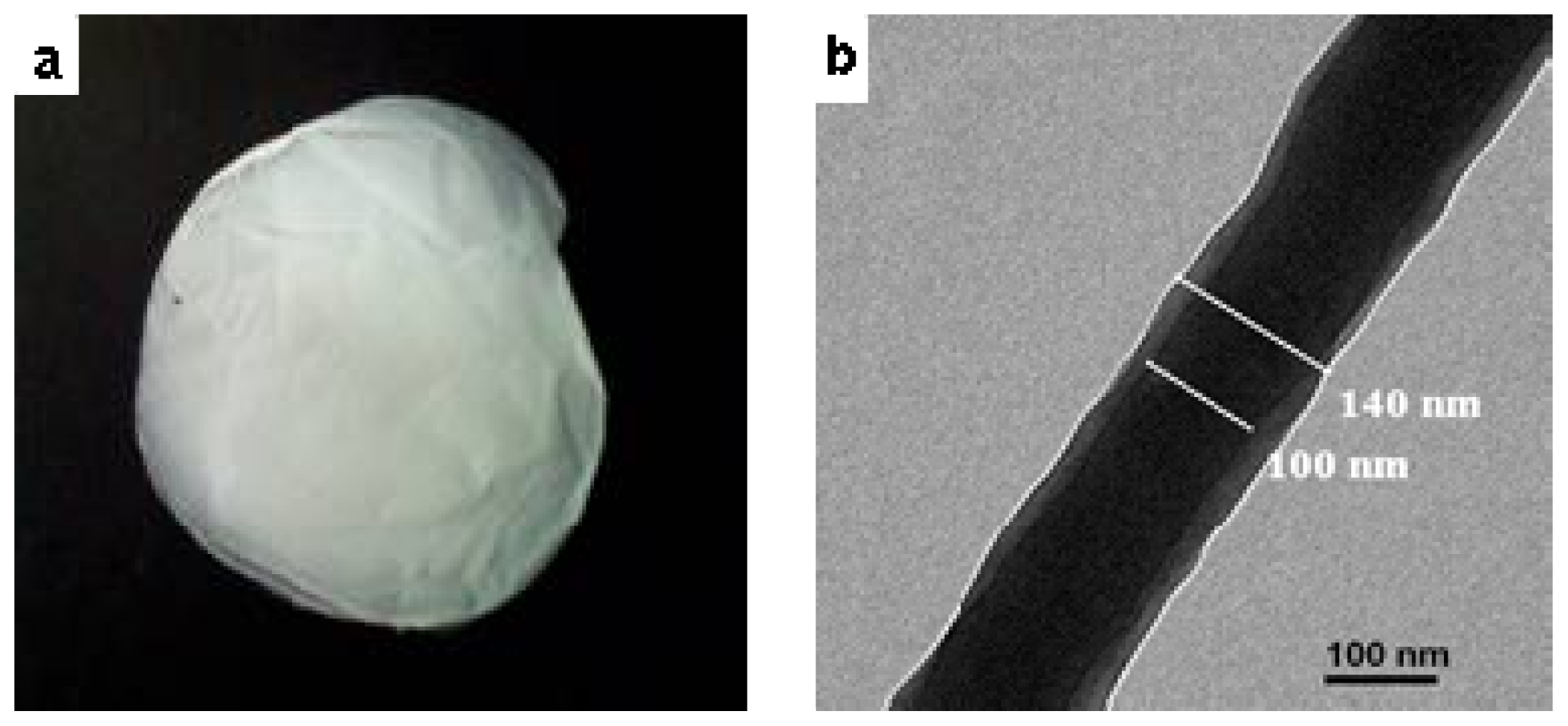
2.1. Characterization of PMPPh
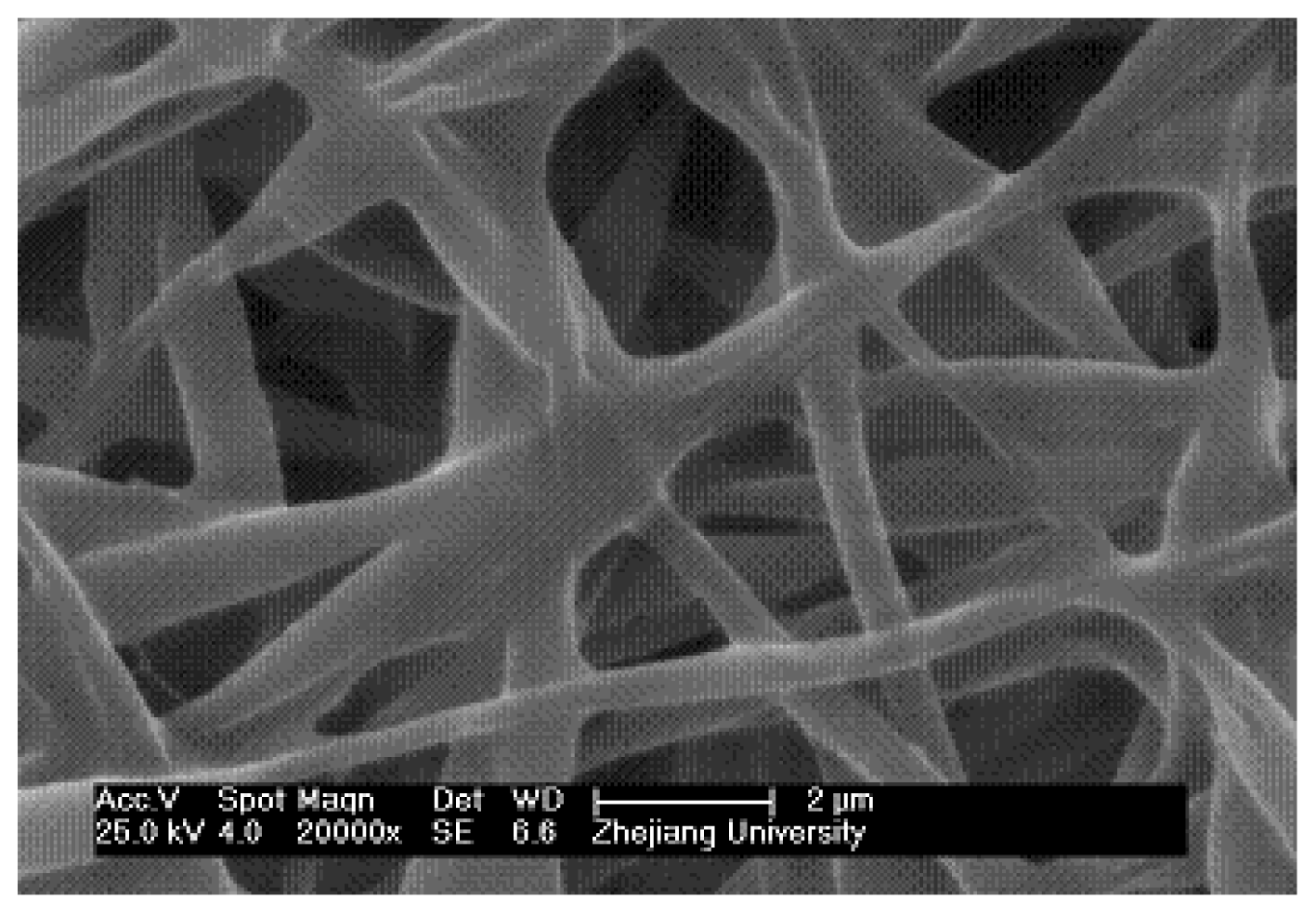
2.2. Traditional Electrospinning of PMPPh Nanofiber Membrane
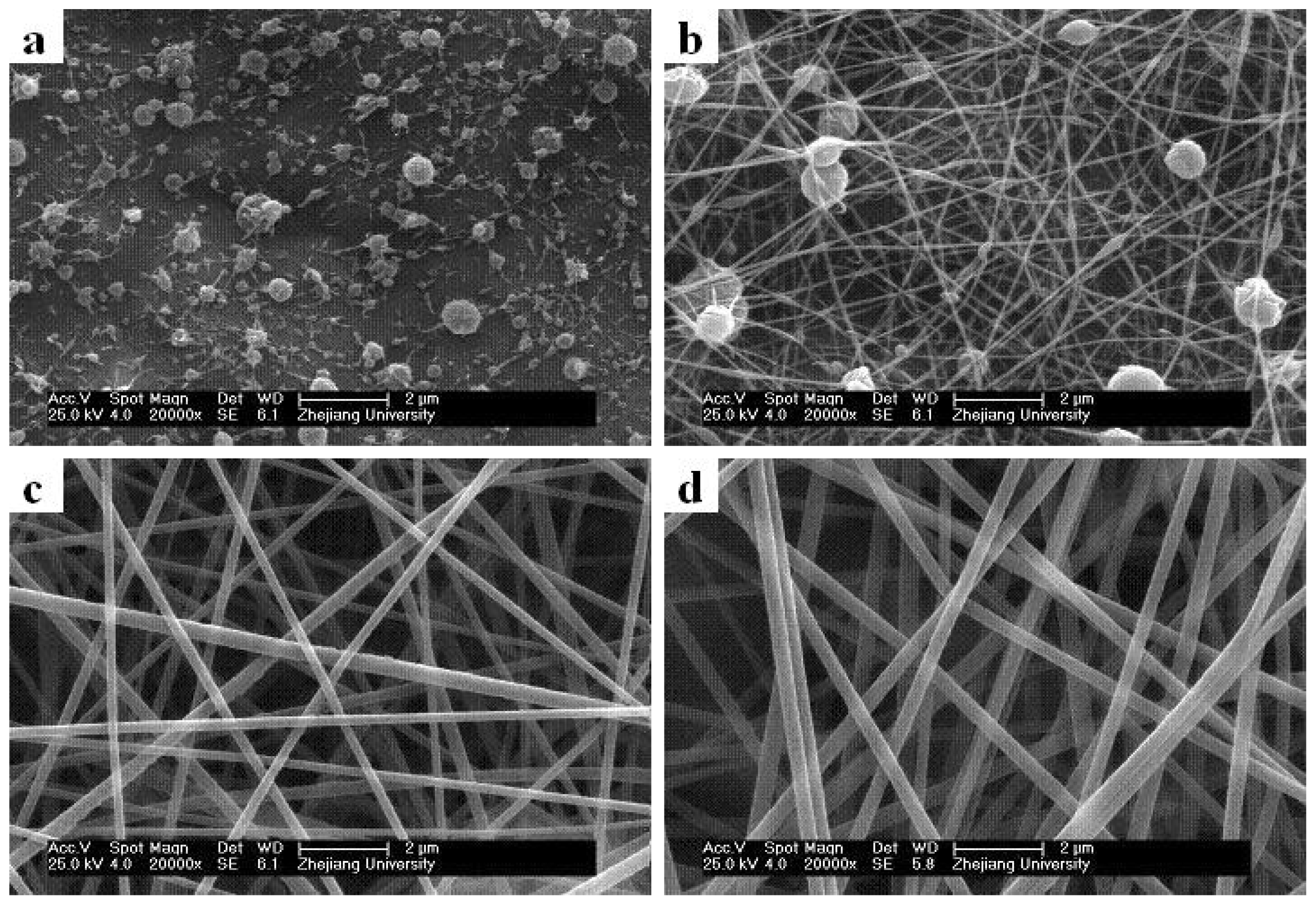
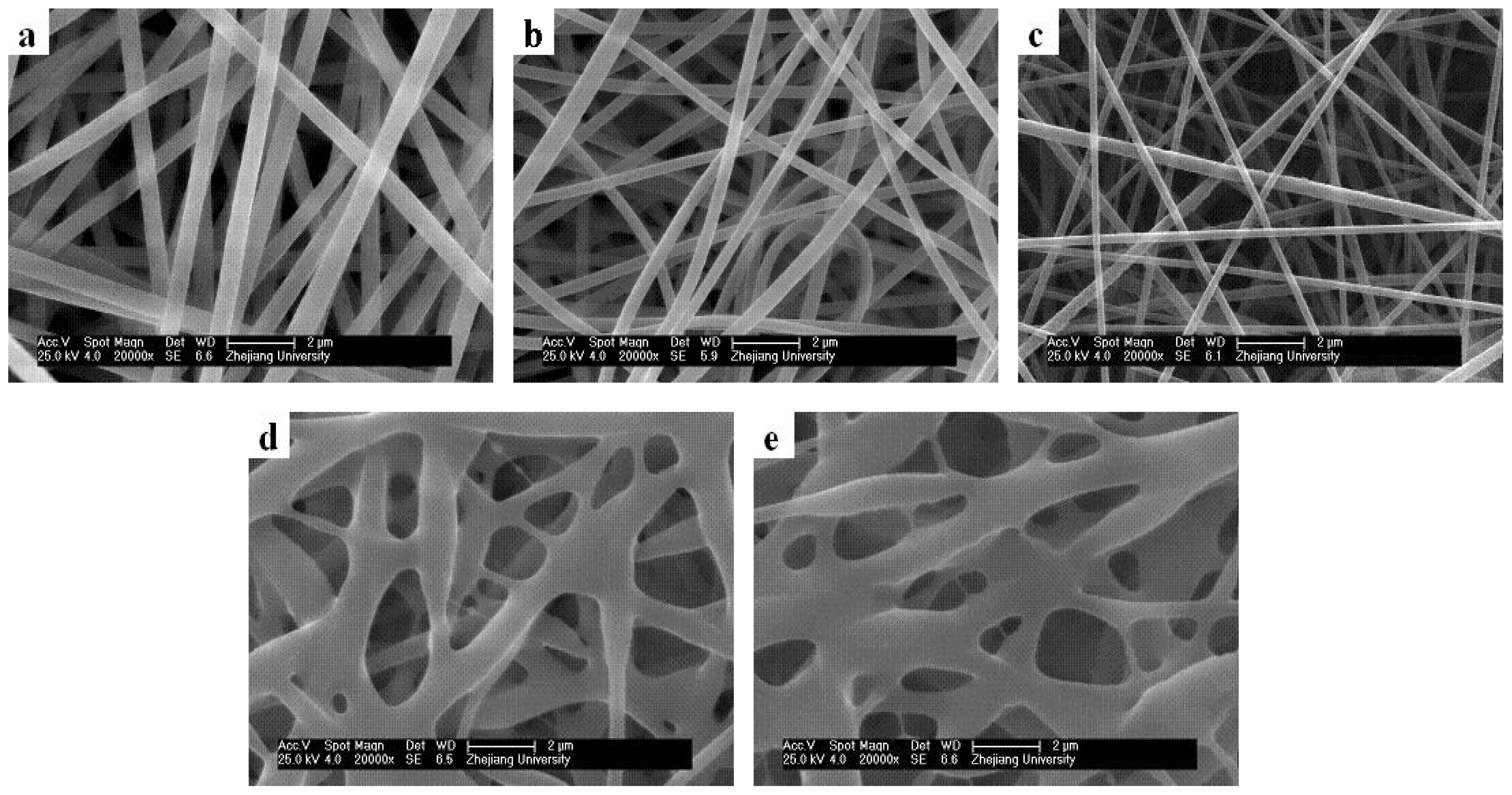
2.3. Effect of Solution Concentrations on Core/Sheath Nanofiber Morphology and Diameter
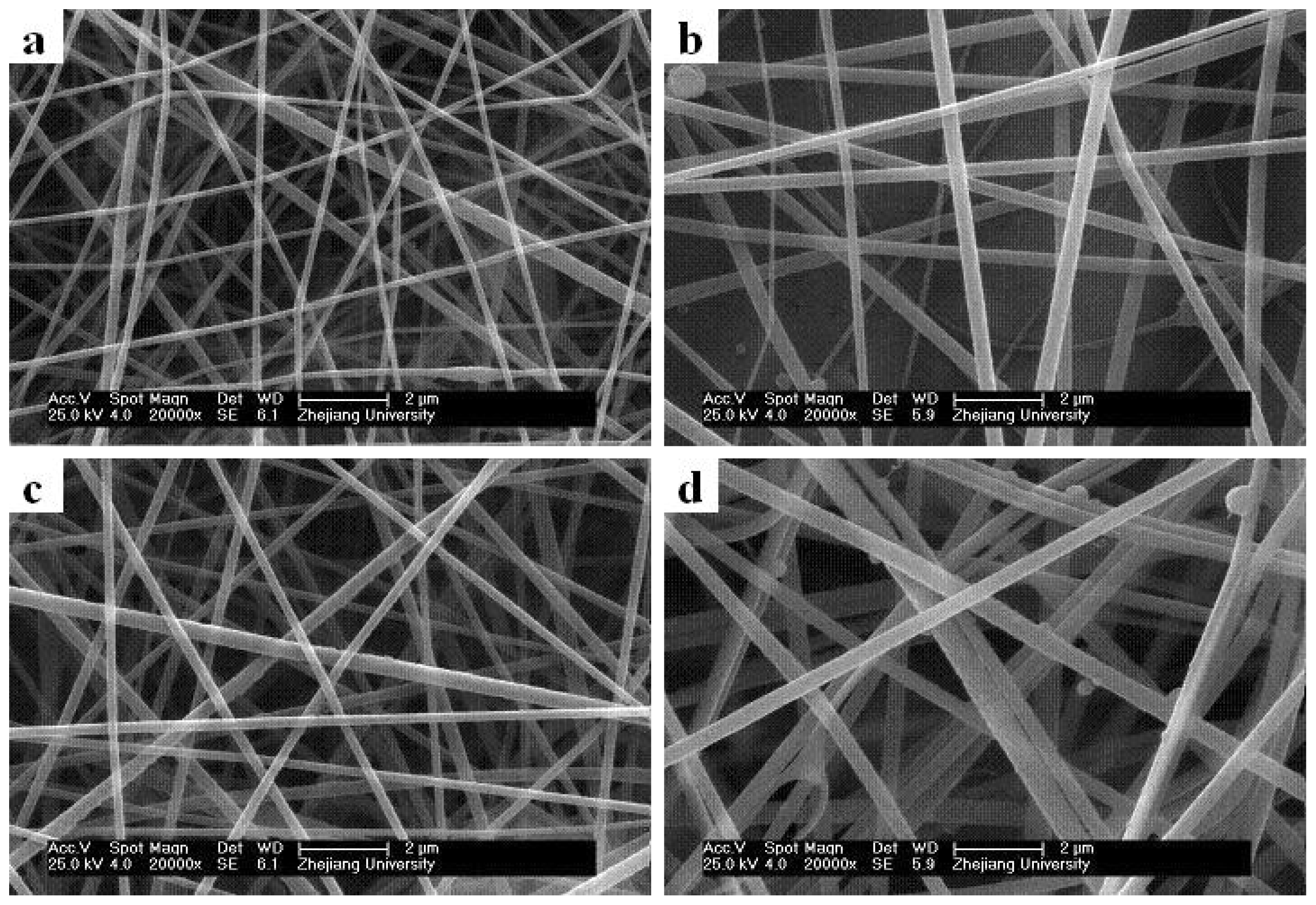
2.4. Effect of Core/Sheath Solution Flow Rates Ratio on Nanofiber Morphology and Diameter
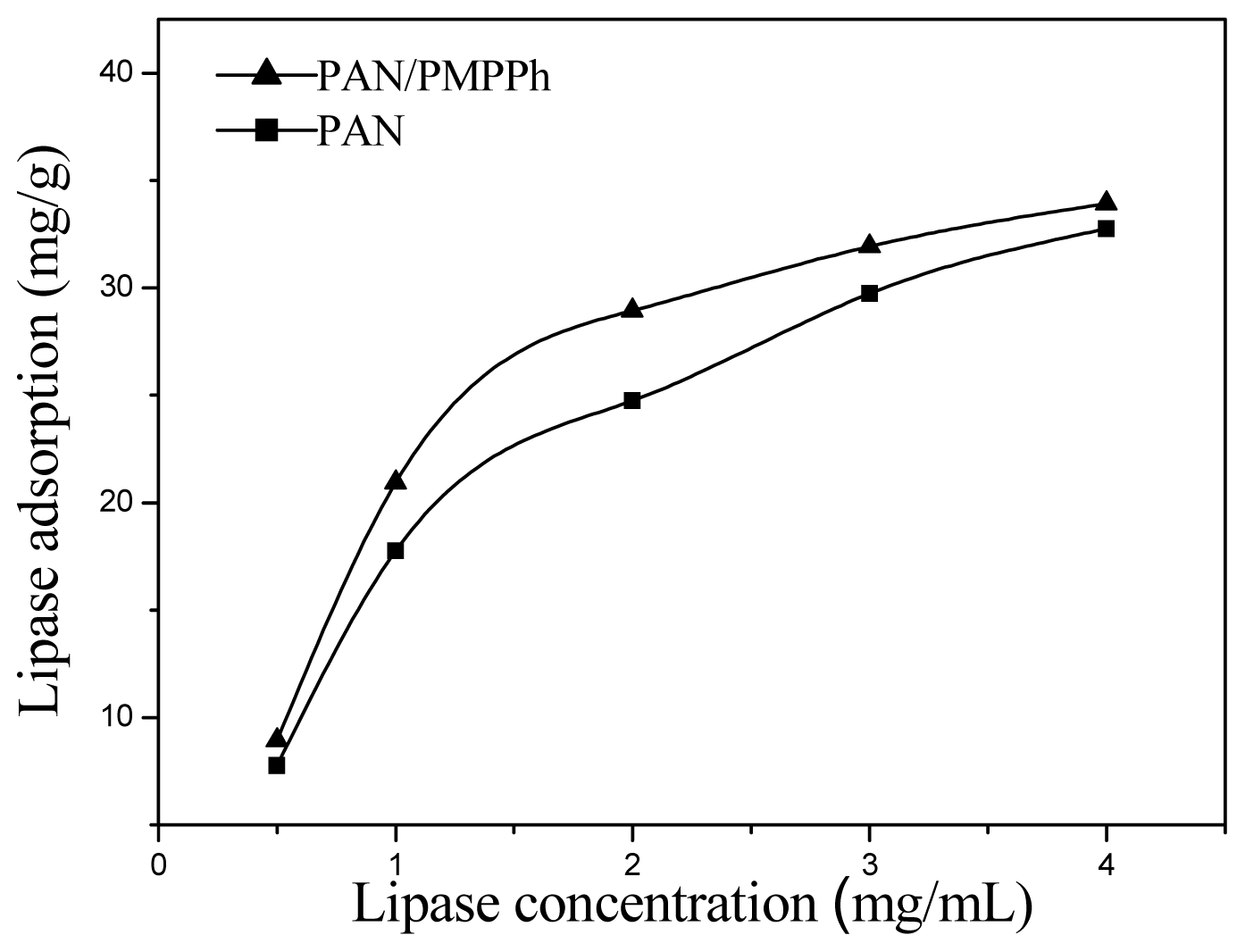
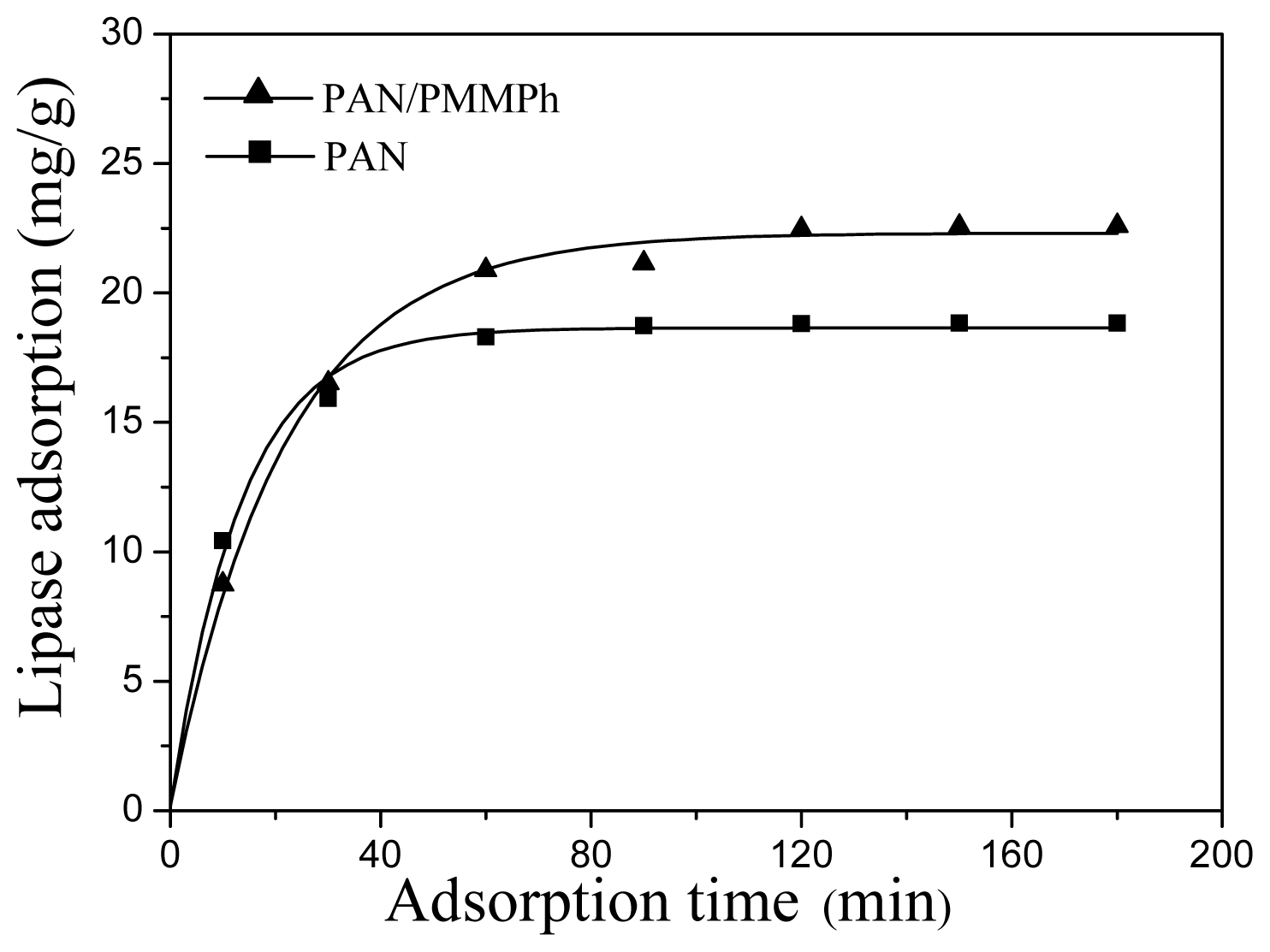
2.5. Enzyme Immobilization on PMPPh/PAN Nanofiber Membrane
2.6. Activities of Immobilized Lipases
3. Experimental Section
3.1. Materials
3.2. Synthesis and Analysis of PMPPh
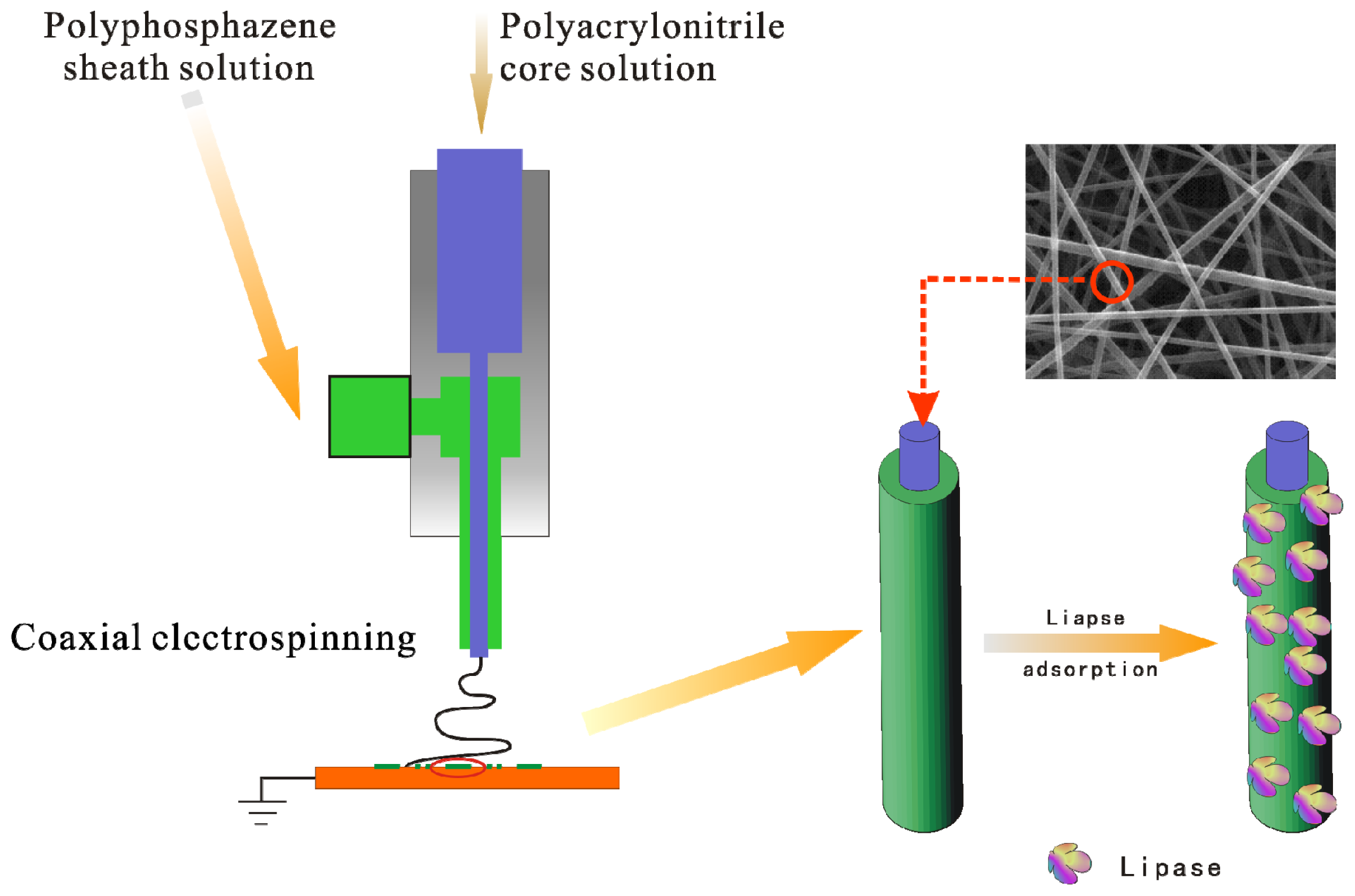
3.3. Preparation of Coaxial-Electrospun Nanofiber Membrane
3.4. Immobilization of Lipase on Nanofiber Membrane
3.5. Assay of Lipase Activity
4. Conclusions
Supplementary Information
ijms-13-14136-s001.pdfAcknowledgments
References
- Allcock, H.R. Recent developments in polyphosphazene materials science. Curr. Opin. Solid State Mater. Sci 2006, 10, 231–240. [Google Scholar]
- Allcock, H.R.; Kugel, R.L.; Valan, K.J. Phosphonitrilic compounds. VI. High molecular weight poly(a1koxy- and aryloxyphosphazenes). Inorg. Chem 1966, 5, 1709–1715. [Google Scholar]
- Krogman, N.R.; Weikel, A.L.; Kristhart, K.A.; Nukavarapu, S.P.; Deng, M.; Nair, L.S.; Laurencin, C.T. The influence of side group modification in polyphosphazenes on hydrolysis and cell adhesion of blends with PLGA. Biomaterials 2009, 30, 3035–3041. [Google Scholar]
- Sethuraman, S.; Nair, L.S.; Amin, S.E.; Nguyen, M.T.; Singh, A.; Krogman, N.; Greish, Y.E.; Allcock, H.R.; Brown, P.W.; Laurencin, C.T. Mechanical properties and osteocompatibility of novel biodegradable alanine based polyphosphazenes: Side group effects. Acta Biomater 2010, 6, 1931–1937. [Google Scholar]
- Paulsdorf, J.; Kaskhedikar, N.; Burjanadze, M.; Obeidi, S.; Stolwilk, N.A.; Wilmer, D.; Wiemhöfer, H.D. Synthesis and ionic conductivity of polymer electrolytes based on a polyphosphazene with short side groups. Chem. Mater 2006, 18, 1281–1288. [Google Scholar]
- Allcock, H.R.; Phelps, M.V.; Barrett, E.W. Ultraviolet photolithographic development of polyphosphazene hydrogel microstructures for potential use in microarray biosensors. Chem. Mater 2006, 18, 609–613. [Google Scholar]
- Allcock, H.R.; Pucher, S.R.; Scopelianos, A.G. Poly[(amino acid ester)phosphazenes] as substrates for the controlledrelease of small molecules. Biomaterials 1994, 15, 563–569. [Google Scholar]
- Lakshmia, S.; Kattia, D.S.; Laurencin, C.T. Biodegradable polyphosphazenes for drugdelivery applications. Adv. Drug Delivery Rev 2003, 55, 467–482. [Google Scholar]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Delivery Rev 2007, 59, 1392–1402. [Google Scholar]
- Cuetos, A.; Valenzuela, M.L.; Gotor, V.; Carriedo, G.A. Polyphosphazenes as tunable and recyclable supports to immobilize alcohol dehydrogenases and lipases: Synthesis, catalytic activity, and recycling efficiency. Biomacromolecules 2010, 11, 1291–1297. [Google Scholar]
- Allcock, H.R.; Kwon, S. An ionically cross-linkable polyphosphazene: poly[bis(carboxylatophenoxy)phosphazene] and its hydrogels and membranes. Macromolecules 1989, 22, 75–79. [Google Scholar]
- Golemme, G.; Drioli, E. Polyphosphazene membrane separations—Review. J. Inorg. Organomet. Polym 1996, 6, 341–365. [Google Scholar]
- Chang, Y.K.; Lee, S.C.; Kim, K.T.; Kim, C.; Reeves, S.D.; Allcock, H.R. Synthesis and micellar characterization of an amphiphilic diblock copolyphosphazene. Macromolecules 2001, 34, 269–274. [Google Scholar]
- Wang, Z.G.; Wan, L.S.; Liu, Z.M.; Huang, X.J.; Xu, Z.K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B 2009, 56, 189–195. [Google Scholar]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Comp. Sci. Technol 2003, 63, 2223–2253. [Google Scholar]
- Li, D.; Xia, Y. Electrospinning of nanofiber: Reinventing the wheel. Adv. Mater 2004, 16, 1151–1170. [Google Scholar]
- Huang, X.J.; Yu, A.G.; Xu, Z.K. Covalent immobilization of lipase from Candida rugosa onto poly(acrylonitrile-co-2-hydroxyethyl methacrylate) electrospun fibrous membranes for potential bioreactor application. Bioresour. Technol 2008, 99, 5459–5455. [Google Scholar]
- Singh, A.; Steely, L.; Allcock, H.R. Poly[bis(2,2,2-trifluoroethoxy)phosphazene] superhydrophobic nanofibers. Langmuir 2005, 21, 11604–11607. [Google Scholar]
- Laurencin, C.T.; Ibim, S.A.; Willoughby, D.A.; Allcock, H.R.; Ambrosio, A.A. A highly porous 3-dimensional polyphosphazene polymer matrix for skeletal tissue regeneration. J. Biomed. Mater. Rev 1996, 30, 133–138. [Google Scholar]
- Deng, M.; Kumbar, S.G.; Nair, L.S.; Weikel, A.L.; Allcock, H.R.; Laurencin, C.T. Biomimetic structures: Biological implications of dipeptide-substituted polyphosphazene—polyester blend nanofiber matrices for load-bearing bone regeneration. Adv. Funct. Mater 2011, 21, 2641–2651. [Google Scholar]
- Sun, Z.C.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound core-shell polymer nanofiber by co-electrospinnig. Adv. Mater 2003, 15, 1927–1932. [Google Scholar]
- Zhang, H.; Zhao, C.G.; Zhao, Y.H.; Tang, G.W.; Yuan, X.Y. Electrospinning of ultrafine core/shell fibers for biomedical applications. Sci. China Ser. B 2010, 53, 1246–1254. [Google Scholar]
- Han, D.W.; Steckl, A.J. Superhydrophobic and oleophobic fibers by coaxial electrospinning. Langmuir 2009, 25, 9454–9462. [Google Scholar]
- Li, S.; Sun, B.; Li, X.R.; Yuan, X.Y. Characterization of electrospun core/shell poly(vinylpyrrolidone)/poly(l-lactide-co-ɛ-caprolactone) fibrousmembranes and their cytocompatibility in vitro. J. Biomater. Sci. Polym. Ed 2008, 19, 245–258. [Google Scholar]
- Li, D.; Babel, A.; Jenekhe, S.A.; Xia, Y. Nanofiber of conjugated polymers prepared by electrospinning with a two-capillary spinneret. Adv. Mater 2004, 16, 2062–2066. [Google Scholar]
- Huang, X.J.; Yu, A.G.; Jiang, J.; Pan, C.; Qian, J.W. Surface modification of nanofibrous poly(acrylonitrile-co-acrylic acid) membrane with biomacromolecules for lipase immobilization. J. Mol. Catal. B 2010, 70, 95–100. [Google Scholar]
- Huang, X.J.; Chen, P.C.; Huang, Fu.; Ou, Y.; Chen, M.R.; Xu, Z.K. Immobilization of Candida rugosa lipase on electrospun cellulose nanofiber membrane. J. Mol. Catal. B 2011, 70, 95–100. [Google Scholar]
- Huang, X.J.; Yu, A.G.; Ge, D.; Xu, Z.K. Immobilization and properties of lipase from Candida rugosa on electrospun nanofibrous membranes with biomimetic phospholipid moieties. Chem. Res. Chin. Univ 2008, 24, 31–37. [Google Scholar]
- Noureddini, H.; Gao, X.; Philkana, R.S. Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour. Technol 2005, 96, 769–777. [Google Scholar]
- Compton, D.L.; Laszlo, J.A.; Berhow, M.A. Identification and quantification of feruloylated mono-, di-, and triacylglycerols from vegetable oils. J. Am. Oil Chem. Soc 2006, 83, 753–758. [Google Scholar]
- Ragupathy, L.; Pluhar, B.; Ziener, U.; Keller, H.; Dyllick-Brenzinger, R.; Landfester, K. Enzymatic aminolysis of lactones in aqueous miniemulsion: Catalysis through a novel pathway. J. Mol. Catal. B 2010, 62, 270–276. [Google Scholar]
- Ye, P.; Xu, Z.K.; Wu, J.; Innocent, C.; Seta, P. Nanofibrous membranes containing reactive groups: Electrospinning from poly(acrylonitrile-co-maleic acid) for lipase immobilization. Macromolecules 2006, 39, 1041–1045. [Google Scholar]
- Huang, X.J.; Xu, Z.K.; Wan, L.S.; Innocent, C.; Seta, P. Electrospun nanofibers modified with phospholipid moieties for enzyme immobilization. Macromol. Rapid Commun 2006, 27, 1341–1345. [Google Scholar]
- Ye, P.; Xu, Z.K.; Wu, J.; Innocent, C.; Seta, P. Nanofibrous poly(acrylonitrile-co-maleic acid) membranes functionalized with gelatin and chitosan for lipase immobilization. Biomaterials 2006, 27, 4169–4176. [Google Scholar]
- Li, S.F.; Chen, J.P.; Wu, W.T. Electrospun polyacrylonitrile nanofibrous membranes for lipase immobilization. J. Mol. Catal. B 2007, 47, 117–124. [Google Scholar]
- Cygler, M.; Schrag, J.D. Structure and conformational flexibility of Candida rugosa lipase. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1999, 1441, 205–214. [Google Scholar]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol 2007, 40, 1451–1462. [Google Scholar]
- Pizarro, C.; Branes, M.C.; Markovits, A.; Fernandez-Lorente, G.; Guisan, J.M.; Chamy, R.; Wilson, L. Influence of different immobilization techniques for Candida cylindracea lipase on its stability and fish oil hydrolysis. J. Mol. Catal. B 2010, 78, 111–118. [Google Scholar]
- Palla, C.A.; Pacheco, C.; Carrin, M.E. Preparation and modification of chitosan particles for Rhizomucor miehei lipase immobilization. Biochem. Eng. J 2011, 55, 199–207. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–301. [Google Scholar]
| Support | Immobilization method | Fiber diameter (nm) | Enzyme loading (mg/g fiber) | Activity retention (%) | References |
|---|---|---|---|---|---|
| PAN NF a | Physical | 150 ± 20 | 18.8 ± 2.4 | 46.4 | This work |
| PMPPh/PAN NF b | Physical | 150 ± 20 | 20.4 ± 2.7 | 63.7 | This work |
| PANCMPC NF c | Physical | 90 ± 20 | 22.2 ± 1.7 | 72.9 ± 0.8 | [33] |
| PANCHEMA d | Chemical | 80–50 | 16.2 ± 1.1 | 40.6 ± 0.6 | [17] |
| PANCMA NF e | Chemical | 100 | 21.2 ± 0.71 | 37.6 ± 1.8 | [32] |
| Chitosan-tethered PANCMA NF | Chemical | NA f | 22.5 ± 0.75 | 45.6 ± 1.8 | [34] |
| Gelatin-tethered PANCMA NF | Chemical | NA | 20.7 ± 0.75 | 49.7 ± 1.8 | [34] |
| PAN NF | Chemical | 150–300 | 21.2 ± 1.3 | 81.3 ± 1.1 | [35] |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, S.-G.; Jiang, X.; Chen, P.-C.; Yu, A.-G.; Huang, X.-J. Preparation of Coaxial-Electrospun Poly[bis(p-methylphenoxy)]phosphazene Nanofiber Membrane for Enzyme Immobilization. Int. J. Mol. Sci. 2012, 13, 14136-14148. https://doi.org/10.3390/ijms131114136
Wang S-G, Jiang X, Chen P-C, Yu A-G, Huang X-J. Preparation of Coaxial-Electrospun Poly[bis(p-methylphenoxy)]phosphazene Nanofiber Membrane for Enzyme Immobilization. International Journal of Molecular Sciences. 2012; 13(11):14136-14148. https://doi.org/10.3390/ijms131114136
Chicago/Turabian StyleWang, Shu-Gen, Xin Jiang, Peng-Cheng Chen, An-Guo Yu, and Xiao-Jun Huang. 2012. "Preparation of Coaxial-Electrospun Poly[bis(p-methylphenoxy)]phosphazene Nanofiber Membrane for Enzyme Immobilization" International Journal of Molecular Sciences 13, no. 11: 14136-14148. https://doi.org/10.3390/ijms131114136





