Synthesis and Characterization of Mesoporous Silica Functionalized with Calix[4]arene Derivatives
Abstract
:1. Introduction
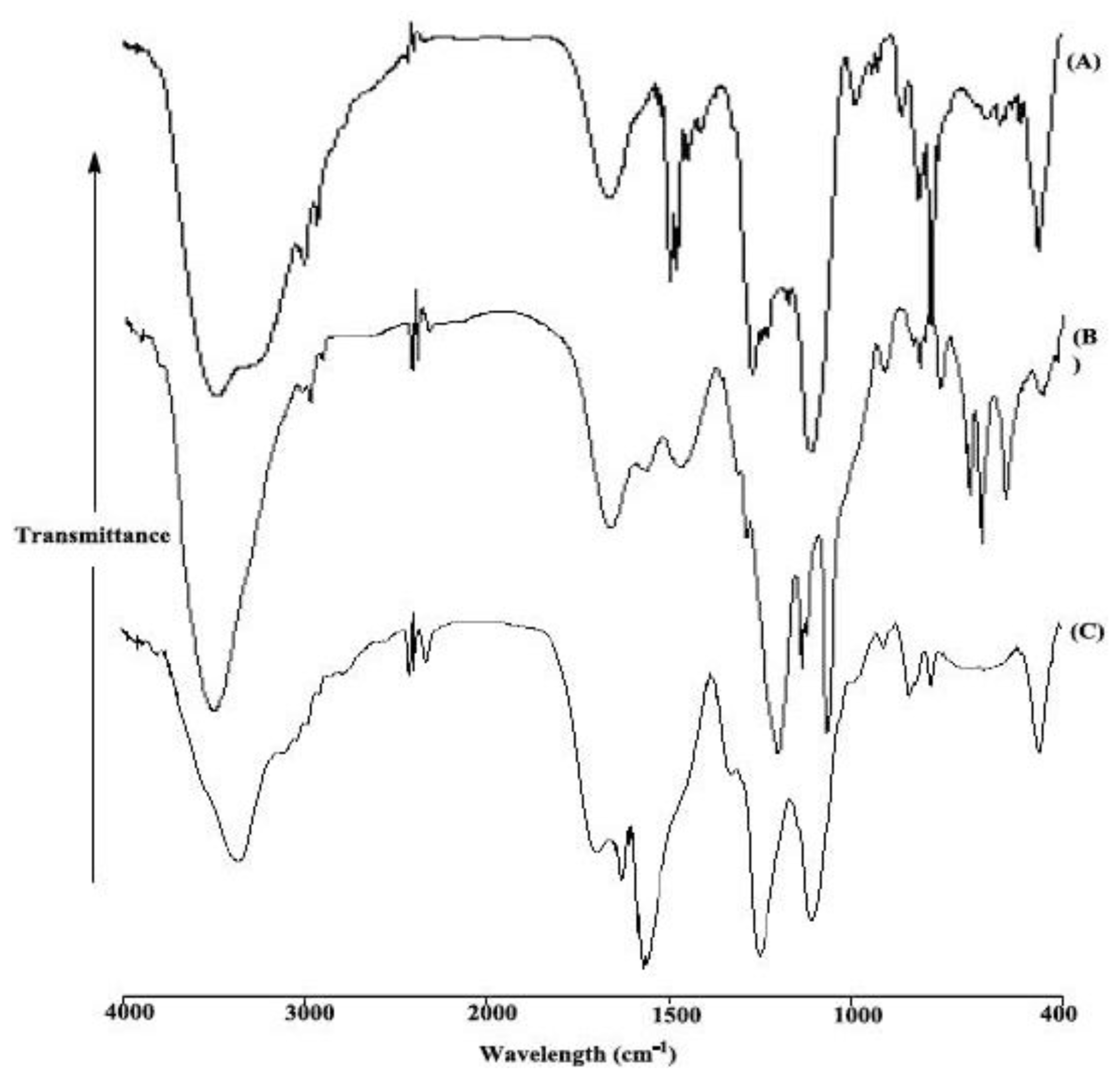
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Instrumentation
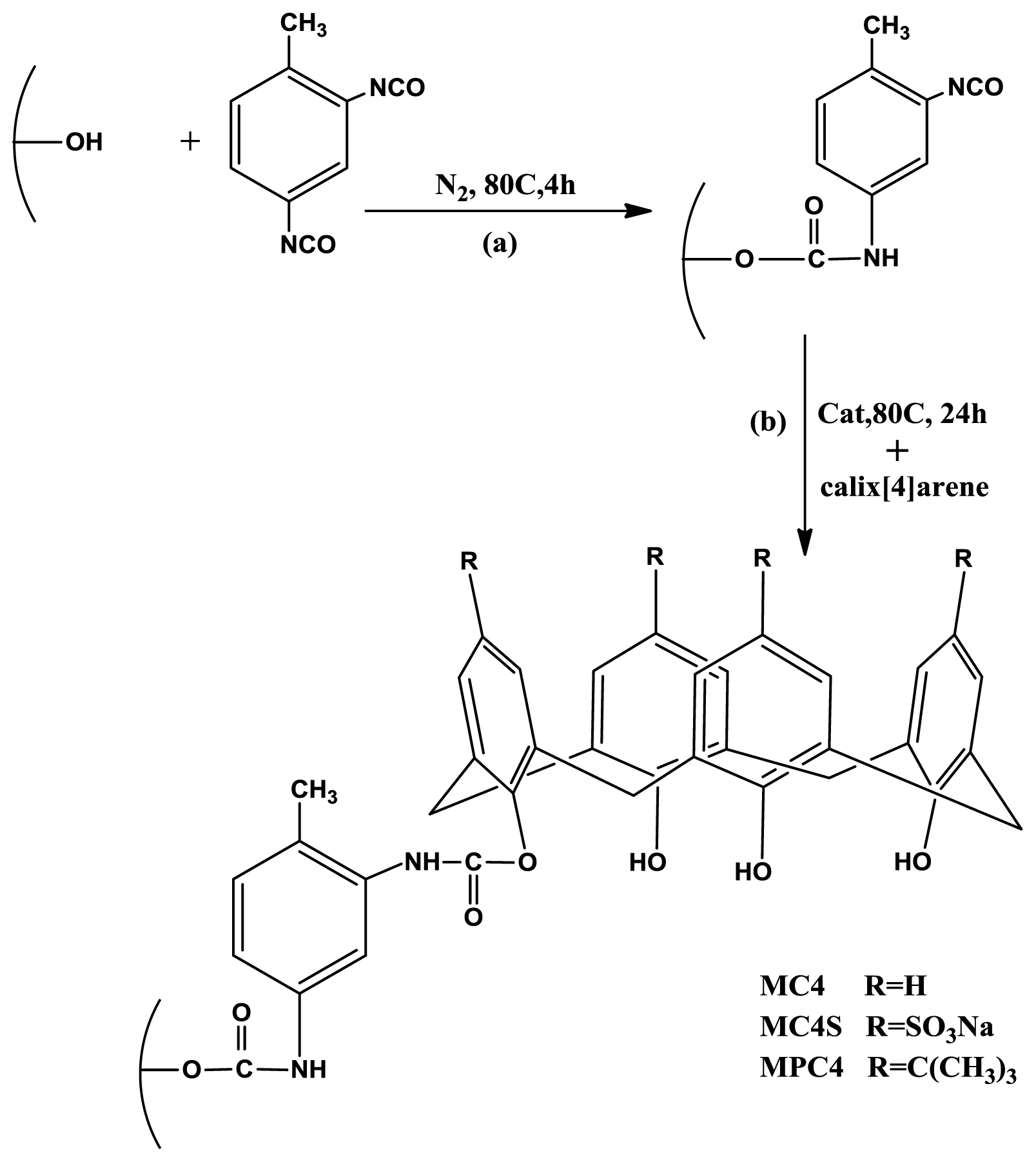
3.3. Synthesis Methods
3.3.1. Introduction of Diisocyanate Functional Groups into the Mesoporous Surfaces
3.3.2. Determination of Isocyanate Groups of the Reaction System
3.3.3. Grafting Modification of MCM-TDI with Calix[4]Arene Derivatives
4. Conclusions
Acknowledgments
References
- Koh, K.N.; Araki, K.; Ikeda, A.; Otsuka, H.; Shinkai, S. Reinvestigation of calixarene-based artificial-signaling acetylcholine receptors useful in neutral aqueous (water/methanol) solution. J. Am. Chem. Soc 1996, 118, 755–758. [Google Scholar]
- Wang, L.; Shi, X.F.; Jia, P.J.; Yang, Y. Preparation of calixarene-containing polymer with proton transport ability. J. Polym. Sci. A 2004, 42, 6259–6266. [Google Scholar]
- Beer, P.D.; Szemes, F.; Passaniti, P.; Maestri, M. Luminescent Ruthenium (II) Bipyridine-Calix[4]arene Complexes as Receptors for Lanthanide Cations. Inorg. Chem 2004, 43, 3965–3975. [Google Scholar]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin, Germany, 1994. [Google Scholar]
- Asfari, Z.; Bohmer, V.; Harrowfield, J.; Vicens, J. Calixarenes 2001; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Lai, X.H.; Li, L.; Wu, C.Y. Preparation and chromatographic characteristics of calix[4]arene polysiloxane as stationary phases for capillary gas chromatography. Chromatographia 1999, 50, 82–88. [Google Scholar]
- Kalchenko, O.I.; Lipkowski, J.; Kalchenko, V.I.; Vysotzky, M.A.; Markovsky, L.N. Effect of octakis(diethoxyphosphoryloxy)-tert-butyl-calix[8]arene in mobile phase on the reversed-phase retention behavior of aromatic compounds: Host-guest complex formation and stability constants determination. J. Chromatogr. Sci 1998, 36, 269–273. [Google Scholar]
- Sokoliess, T.; Menyes, U.; Roth, U.; Jira, T. Separation of cis- and trans-isomers of thioxanthene and dibenz[b,e]oxepin derivatives on calixarene- and resorcinarene-bonded high-performance liquid chromatography stationary phases. J. Chromatogr. A 2002, 948, 309–319. [Google Scholar]
- Tanaka, Y.; Kishimoto, Y.; Otsuka, K.; Terabe, S. Strategy for selecting separation solutions in capillary electrophoresis-mass spectrometry. J. Chromatogr. A 1998, 817, 49–57. [Google Scholar]
- Mibradt, R.; Böhmer, V. Calixarenes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; p. 663. [Google Scholar]
- Zhao, T.; Hu, X.; Cheng, J.; Lu, X. Use of calix[4]arene to separate positional isomers in capillary electrophoresis. Anal. Chim. Acta 1998, 358, 263–268. [Google Scholar]
- Glennon, J.D.; O’Connor, K.; Srijaranai, S.; Manley, K.; Harris, S.J.; McKervey, M.A. Enhanced Chromatographic Selectivity for Na+ Ions on a Calixarene-Bonded Silica Phase. Anal. Lett 1993, 26, 153–162. [Google Scholar]
- Glennon, J.D.; Horne, E.; O’Connor, K.; Kearney, G.; Harris, S.J.; McKervey, M.A. Chromatographic selectivity for amino acid esters and alkali metal ions on a silica bonded calix[4]arene tetraester stationary phase. Anal. Proc 1994, 31, 33–35. [Google Scholar]
- Brindle, R.; Albert, K.; Harrisb, S.J.; Triiltzsch, C.; Horned, E.; Glennon, J.D. Silica-bonded calixarenes in chromatography: I. Synthesis and characterization by solid-state NMR spectroscopy. J. Chromatogr. A 1996, 731, 41–46. [Google Scholar]
- Huayu, H.; Chuande, Z.; Yongsheng, J.; Rong, N.; Pan, Z.; Haixia, Z. Preparation, characterization and application of p-tert-butyl-calix[4]arene-SBA-15 mesoporous silica molecular sieves. J. Hazard. Mater 2010, 178, 680–685. [Google Scholar]
- Su, B.-L.; Ma, X.-C.; Xu, F.; Chen, L.-H.; Fu, Z.-Y.; Moniotte, N.; Maamar, S.B.; Lamartine, R.; Vocanson, F. SBA-15 mesoporous silica coated with macrocyclic calix[4]arene derivatives: Solid extraction phases for heavy transition metal ions. J. Colloid. Interface Sci 2011, 360, 86–92. [Google Scholar]
- Remi, M.; Isabelle, L.; Benedicte, L.; Bernard, V. A mesoporous silica functionalized by a covalently bound calixarene-based fluoroionophore for selective optical sensing of mercury (II) in water. J. Mater. Chem 2005, 15, 2965–2973. [Google Scholar]
- Li, Y.-J.; Yan, B.; Wang, L. Rare earth (Eu3+, Tb3+) mesoporous hybrids with calix[4]arene derivative covalently linkingMCM-41: Physical characterization and photoluminescence property. J. Solid State Chem 2011, 184, 2571–2579. [Google Scholar]
- Xia, H.; Song, M. Preparation and characterisation of polyurethane grafted single-walled carbon nanotubes and derived polyurethane nanocomposites. J. Mater. Chem 2006, 16, 1843–1851. [Google Scholar]
- Chun, Y.S.; Ha, K.; Lee, Y.J.; Lee, J.S.; Kim, H.S.; Park, Y.S.; Yoon, K.B. Diisocyanates as novel molecular binders for monolayer assembly of zeolite crystals on glass. Chem. Commun 2002, 2002, 1846–1847. [Google Scholar]
- Arnold, R.G.; Nelson, J.A.; Verbanc, J.J. Recent advances in isocyanate chemistry. Chem. Rev 1957, 57, 47–76. [Google Scholar]
- Simons, D.M.; Arnold, R.G. Relative reactivity of the isocyanate groups in toluene-2,4-diisocyanate. J. Am. Chem. Soc 1956, 78, 1658–1659. [Google Scholar]
- Che, J.; Xiao, Y.; Wang, X.; Pan, A.; Yuan, W.; Wu, X. Grafting polymerization of polyacetal onto nano-silica surface via bridging isocyanate. Surf. Coat. Technol 2007, 201, 4578–4584. [Google Scholar]
- Boven, G.; Oosterling, M.L.C.M.; Challa, G.; Schonten, A.J. Grafting kinetics of poly(methyl methacrylate) on microparticulate silica. Polymer 1990, 31, 2377–2383. [Google Scholar]
- Furer, V.L.; Borisoglebskaya, E.I.; Zverev, V.V.; Kovalenko, V.I. DFT and IR spectroscopic analysis of p-tert-butylthiacalix[4]arene. Spectrochim. Acta Part A 2006, 63, 207–212. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.L.; Roth, W.J.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar]
- Lim, M.H.; Stein, A. Comparative studies of grafting and direct syntheses of inorganic—Organic hybrid mesoporous materials. Chem. Mater 1999, 11, 3285–3295. [Google Scholar]
- Marler, B.; Oberhagemann, U.; Vortmann, S.; Gies, H. Influence of the sorbate type on the XRD peak intensities of loaded MCM-4. Micropor. Matter 1996, 6, 375–383. [Google Scholar]
- Zhang, W.H.; Lu, X.B.; Xiu, J.H.; Hua, Z.L.; Zhang, L.X.; Robertson, M.; Shi, J.L.; Yan, D.S.; Holmes, J.D. Synthesis and characterization of bifunctionalized ordered mesoporous materials. Adv. Funct. Mater 2004, 14, 544–552. [Google Scholar]
- Yang, H.; Zhang, G.; Hong, X.; Zhu, Y. Dicyano-functionalized MCM-41 anchored-palladium complexes as recoverable catalysts for Heck reaction. J. Mol. Catal. A 2004, 210, 143–148. [Google Scholar]
- Sakthivel, A.; Hijazi, A.K.; Hanzlik, M.; Chiang, A.S.T.; Kühn, F.E. Heterogenization of [Cu(NCCH3)6][B(C6F5)4]2 and its application in catalytic olefin aziridination. Appl. Catal. A 2005, 294, 161–167. [Google Scholar]
- Caps, V.; Tsang, S.C. Heterogenisation of Os species on MCM-41 structure for epoxidation of trans-stilbene. Appl. Catal. A 2003, 248, 19–31. [Google Scholar]
- Makha, M.; Raston, C.L. Direct synthesis of calixarenes with extended arms: p-Phenylcalix[4,5,6,8]arenes and their water-soluble sulfonated derivatives. Tetrahedron Lett 2001, 42, 6215–6217. [Google Scholar]
- Yang, M.; Gao, Y.; He, J.P.; Li, H.M. Preparation of polyamide 6/silica nanocomposites from silica surface initiated ring-opening anionic polymerization. eXPRESS Polym. Lett 2007, 1, 433–442. [Google Scholar]



| Sample | %C | %H | %N | %S |
|---|---|---|---|---|
| M-TDI | 18.64 | 3.04 | 5.93 | - |
| MC4 | 42.64 | 3.05 | 1.06 | - |
| MC4S | 33.31 | 4.16 | 3.97 | 4.04 |
| MPC4 | 40.53 | 4.84 | 4.30 | - |
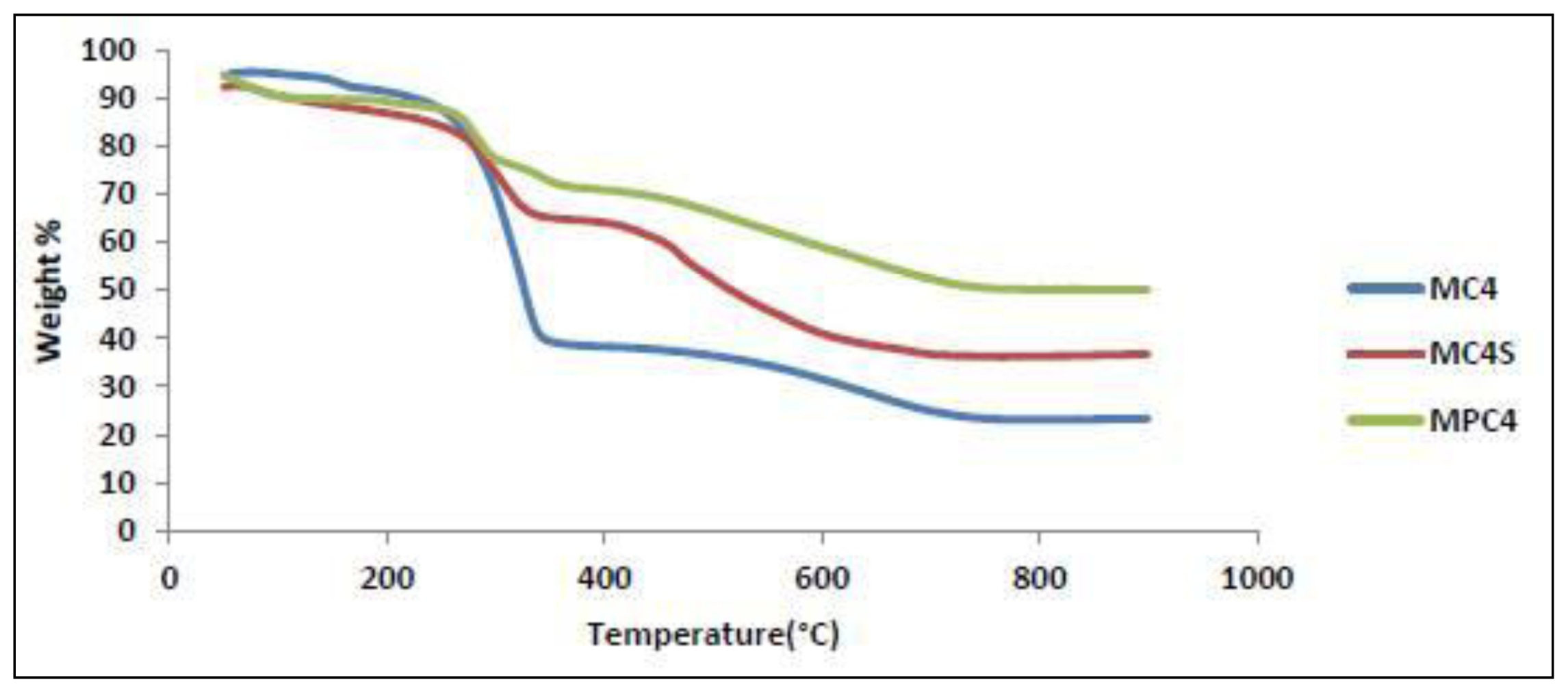
| Sample | Region °C | Weight-loss % | Assignment |
|---|---|---|---|
| MC4 | 45–150 | 4.9 | Moisture |
| 150–280 | 8.4 | calix[4]arene | |
| 280–380 | 21.4 | Carbamate group and calix[4]arene | |
| 380–800 | 32.49 | calix[4]arene | |
| MC4S | 45–150 | 4.7 | Moisture |
| 150–280 | 5.9 | Carbamate group | |
| 350–800 | 37.9 | calix[4]arene sulfonate | |
| MPC4 | 45–150 | 2.4 | Moisture |
| 200–350 | 44.9 | Carbamate group and para tert butyl calix[4]arene | |
| 400–800 | 15.7 | para tert butyl calix[4]arene |
| Sample | SBET (m2/g) | V (cm3/g) | D (nm) |
|---|---|---|---|
| MCM-41 | 993 | 0.86 | 2.9 |
| MC4 | 733 | 0.67 | 3.6 |
| MC4S | 452 | 0.43 | 3.8 |
| MPC4 | 339 | 0.32 | 3.9 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alahmadi, S.M.; Mohamad, S.; Maah, M.J. Synthesis and Characterization of Mesoporous Silica Functionalized with Calix[4]arene Derivatives. Int. J. Mol. Sci. 2012, 13, 13726-13736. https://doi.org/10.3390/ijms131013726
Alahmadi SM, Mohamad S, Maah MJ. Synthesis and Characterization of Mesoporous Silica Functionalized with Calix[4]arene Derivatives. International Journal of Molecular Sciences. 2012; 13(10):13726-13736. https://doi.org/10.3390/ijms131013726
Chicago/Turabian StyleAlahmadi, Sana M., Sharifah Mohamad, and Mohd Jamil Maah. 2012. "Synthesis and Characterization of Mesoporous Silica Functionalized with Calix[4]arene Derivatives" International Journal of Molecular Sciences 13, no. 10: 13726-13736. https://doi.org/10.3390/ijms131013726
APA StyleAlahmadi, S. M., Mohamad, S., & Maah, M. J. (2012). Synthesis and Characterization of Mesoporous Silica Functionalized with Calix[4]arene Derivatives. International Journal of Molecular Sciences, 13(10), 13726-13736. https://doi.org/10.3390/ijms131013726




