The Role of Neurotrophins in Multiple Sclerosis—Pathological and Clinical Implications
Abstract
:1. Introduction
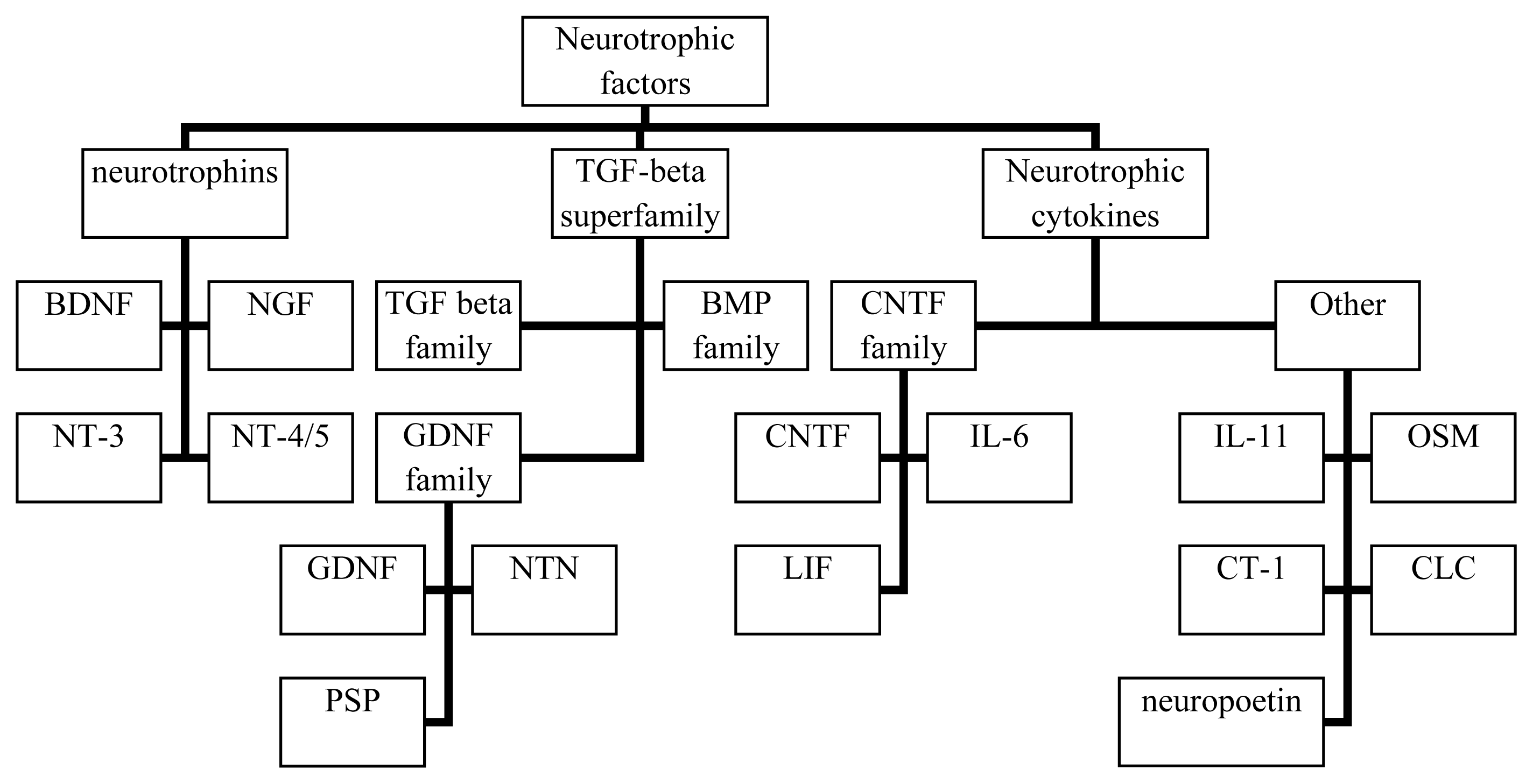
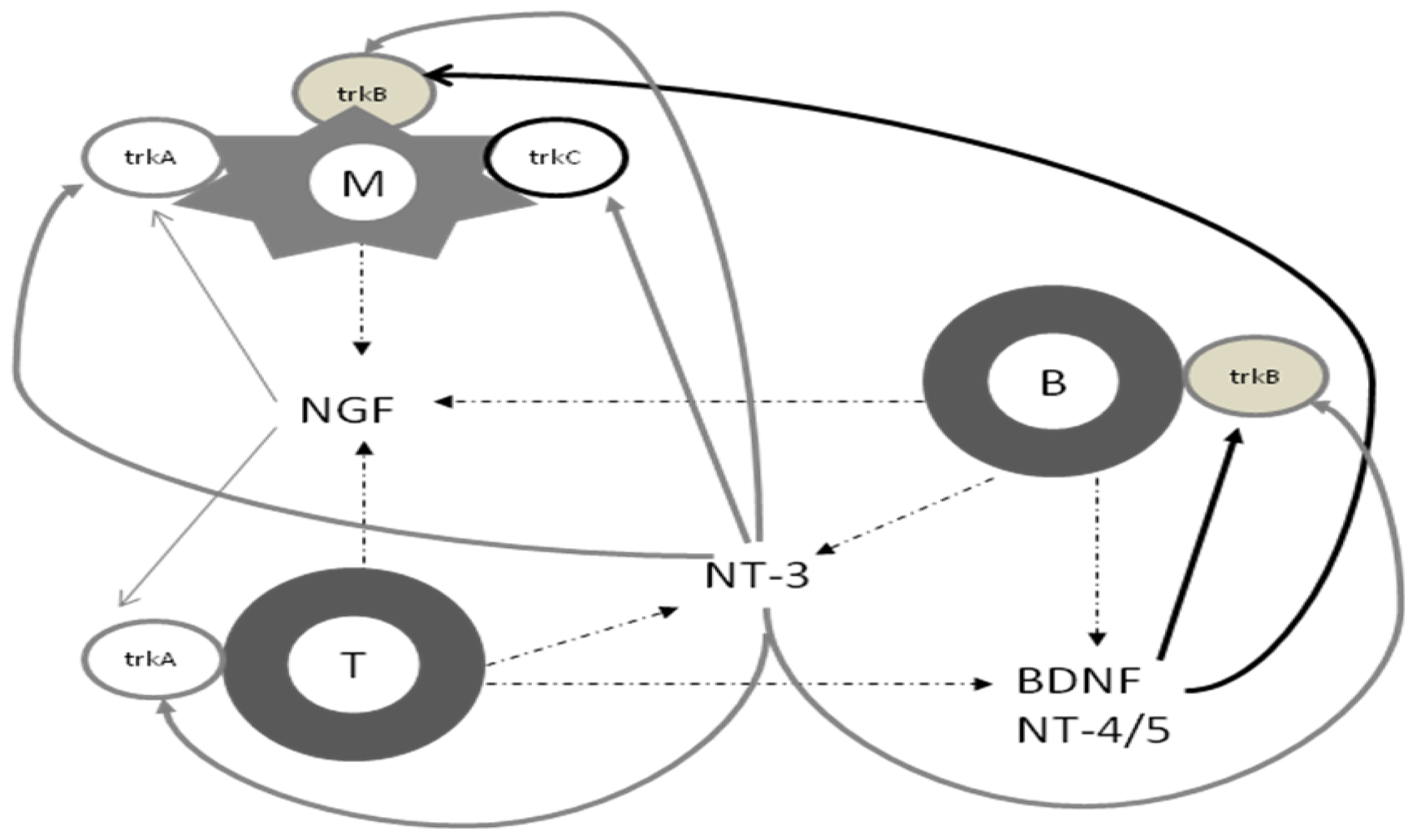
2. Biology of Neurotrophins
3. Neurotrophins and the Immune System
4. Neuroinflammation, Neurodegeneration and Neuroprotective Autoimmunity in MS
5. Neurotrophins and MS Pathology
6. Implications for Therapy
7. Concluding Remarks
References
- Confavreux, C.; Aimard, G.; Devic, M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain 1980, 103, 281–300. [Google Scholar]
- Confavreux, C.; Vukusic, S.; Moreau, T.; Adeleine, P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med 2000, 343, 1430–1438. [Google Scholar]
- Amato, M.P.; Ponziani, G. A prospective study on the prognosis of multiple sclerosis. Neurol. Sci 2000, 21 4 Suppl 2, S831–S838. [Google Scholar]
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple sclerosis. N. Engl. J. Med 2000, 343, 938–952. [Google Scholar]
- Connor, B.; Dragunow, M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res. Rev 1998, 27, 1–39. [Google Scholar]
- Altman, L. Programmed cell death: The paths to suicide. Trends Neurosci 1992, 15, 278–280. [Google Scholar]
- Johnson, E.M.; Chang, J.Y.; Koike, T.; Martin, D.P. Why do neurons die when deprived of trophic factors? Neurobiol. Aging 1989, 10, 549–552. [Google Scholar]
- Thoenen, H. Neurotrophins and activity-dependent plasticity. Prog. Brain Res 2000, 128, 183–191. [Google Scholar]
- Raff, M.C.; Whitmore, A.V.; Finn, J.T. Axonal self-destruction and neurodegeneration. Science 2002, 296, 868–871. [Google Scholar]
- Levi-Montalcini, R. Effects of mouse tumor transplantation on the nervous system. Ann. N. Y. Acad. Sci 1952, 55, 330–3440. [Google Scholar]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci 2001, 24, 677–736. [Google Scholar]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol 2000, 10, 381–391. [Google Scholar]
- Chao, M.V. The p75 neurotrophin receptor. J. Neurobiol 1994, 25, 1373–1385. [Google Scholar]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci 2003, 4, 299–309. [Google Scholar]
- Harrington, A.W.; Kim, J.Y.; Yoon, S.O. Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J. Neurosci 2002, 22, 156–166. [Google Scholar]
- Khursigara, G.; Bertin, J.; Yano, H.; Moffett, H.; DiStefano, P.S.; Chao, M.V. A prosurvival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor-interacting protein 2. J. Neurosci 2001, 21, 5854–5863. [Google Scholar]
- Faustman, D.; Davis, M. TNF receptor 2 pathway: Drug target for autoimmune diseases. Nat. Rev. Drug Discov 2010, 9, 482–493. [Google Scholar]
- Beattie, M.S.; Harrington, A.W.; Lee, R.; Kim, J.Y.; Boyce, S.L.; Longo, F.M.; Bresnahan, J.C.; Hempstead, B.L.; Yoon, S.O. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron 2002, 36, 375–386. [Google Scholar]
- Lin, S.Y.; Wu, K.; Levine, E.S.; Mount, H.T.; Suen, P.C.; Black, I.B. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res. Mol. Brain Res 1998, 55, 20–27. [Google Scholar]
- Tucker, K.; Fadool, D.A. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J. Physiol 2002, 542, 413–429. [Google Scholar]
- Wong, S.T.; Henley, J.R.; Kanning, K.C.; Huang, K.H.; Bothwell, M.; Poo, M.M. A p75 (NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci 2002, 5, 1302–1308. [Google Scholar]
- Wang, K.C.; Kim, J.A.; Sivasankaran, R.; Segal, R.; He, Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 2002, 420, 74–78. [Google Scholar]
- Linker, R.A.; Gold, R.; Lühder, F. Function of neurotrophic factors beyond the nervous system: Inflammation and autoimmune demyelination. Crit. Rev. Immunol 2009, 29, 43–68. [Google Scholar]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci 2006, 361, 1545–1564. [Google Scholar]
- Vega, J.A.; García-Suárez, O.; Hannestad, J.; Pérez-Pérez, M.; Germanà, A. Neurotrophins and the immune system. J. Anat 2003, 203, 1–19. [Google Scholar]
- Hohlfeld, R. Neurotrophic cross-talk between the nervous and immune systems: Relevance for repair strategies in multiple sclerosis? J. Neurol. Sci 2008, 265, 93–96. [Google Scholar]
- Kerschensteiner, M.; Stadelmann, C.; Dechant, G.; Wekerle, H.; Hohlfeld, R. Neurotrophic cross-talk between the nervous and immune systems: Implications for neurological diseases. Ann. Neurol 2003, 53, 292–304. [Google Scholar]
- Tabakman, R.; Lecht, S.; Sephanova, S.; Arien-Zakay, H.; Lazarovici, P. Interactions between the cells of the immune and nervous system: Neurotrophins as neuroprotection mediators in CNS injury. Prog. Brain Res 2004, 146, 387–401. [Google Scholar]
- Linker, R.; Lee, D.-H.; Siglienti, I.; Gold, R. Is there a role for neurotrophins in the pathology of multiple sclerosis? J. Neurol 2007, 254 Suppl 1, I/33–I/40. [Google Scholar]
- Wekerle, H.; Lassman, H. The immunology of inflammatory demyelinating disease. In McAlpine’s Multiple Sclerosis, 4th ed; Compston, A., Confavreux, C., Lassmann, H., McDonald, I., Miller, D., Noseworthy, J., Smith, K., Werkerle, H., Eds.; Churchill Livingstone Elsevier: Linn, MO, USA, 2006; pp. 491–555. [Google Scholar]
- Lassman, H. What drives disease in multiple sclerosis: Inflammation or neurodegeneration? Clin. Exp. Neuroimmunol 2010, 1, 2–11. [Google Scholar]
- Barnett, M.H.; Prineas, J.W. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann. Neurol 2004, 55, 458–468. [Google Scholar]
- Stys, P.K. Multiple sclerosis: Autoimmune disease or autoimmune reaction? Can. J. Neurol. Sci 2010, 37 Suppl 2, S16–S23. [Google Scholar]
- Lucchinetti, C.; Brück, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol 2000, 47, 707–717. [Google Scholar]
- Bjartmar, C.; Kidd, G.; Mörk, S.; Rudick, R.; Trapp, B.D. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann. Neurol 2000, 48, 893–901. [Google Scholar]
- Fisher, E.; Rudick, R.A.; Simon, J.H.; Cutter, G.; Baier, M.; Lee, J.C.; Miller, D.; Weinstock-Guttman, B.; Mass, M.K.; Dougherty, D.S.; et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology 2002, 59, 1412–1420. [Google Scholar]
- Nair, A.; Frederick, T.J.; Miller, S.D. Astrocytes in multiple sclerosis: A product of their environment. Cell Mol. Life Sci 2008, 65, 2702–2720. [Google Scholar]
- Bsibsi, M.; Persoon-Deen, C.; Verwer, R.W.; Meeuwsen, S.; Ravid, R.; van Noort, J.M. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 2006, 53, 688–695. [Google Scholar]
- Standelmann, C.; Kerschensteiner, M.; Misgeld, T.; Brück, W.; Hohlfeld, R.; Lassmann, H. BDNF and gp145trkB in multiple sclerosis brain lesions: Neuroprotective interactions between immune and neuronal cells? Brain 2002, 125 Pt 1, 75–85. [Google Scholar]
- Butzkueven, H.; Zhang, J.G.; Soilu-Hanninen, M.; Hochrein, H.; Chionh, F.; Shipham, K.A.; Emery, B.; Turnley, A.M.; Petratos, S.; Ernst, M.; et al. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat. Med 2002, 8, 613–619. [Google Scholar]
- Yan, Q.; Elliot, J.; Snider, W.D. Brain-derived nerotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature 1992, 360, 753–755. [Google Scholar]
- Mo, L.; Yang, Z.; Zhang, A.; Li, X. The repair of the injured adult rat hippocampus with NT-3-chitosan carriers. Biomaterials 2010, 31, 2184–2192. [Google Scholar]
- Sarchielli, P.; Greco, L.; Stipa, A.; Floridi, A.; Gallai, V. Brain-derived neurotrophic factor in patients with multiple sclerosis. J. Neuroimmunol 2002, 132, 180–188. [Google Scholar]
- Azoulay, D.; Urshansky, A.; Karni, A. Low and dysregulated BDNF secretion from immune cells of MS patients is related to reduced neuroprotection. J. Neuroimmunol 2008, 195, 186–193. [Google Scholar]
- Lalive, P.H.; Kantengwa, S.; Benkhoucha, M.; Juillard, C.; Chofflon, M. Interferon-β induces brain-derived neurotrophic factor in peripheral blood mononuclear cells of multiple sclerosis patients. J. Neuroimmunol 2008, 197, 147–151. [Google Scholar]
- Azoulay, D.; Vachapova, V.; Shihman, B.; Miler, A.; Karni, A. Lower brain-derived neurotrophic factor in serum of relapsing-remitting MS: Reversal by glatiramer acetate. J. Neuroimmunol 2005, 167, 215–218. [Google Scholar]
- Gielen, A.; Khademi, M.; Muhallab, S.; Olsson, T.; Piehl, F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing–remitting multiple sclerosis patients. Scand. J. Immunol 2003, 57, 493–497. [Google Scholar]
- Petereit, H.F.; Lindemann, H.; Schoppe, S. Effect of immunomodulatory drugs on in vitro production of brain-derived neurotrophic factor. Mult. Scler 2003, 9, 16–20. [Google Scholar]
- Liguori, M.; Fera, F.; Patitucci, A.; Manna, I.; Condino, F.; Valentino, P.; Telarico, P.; Cerasa, A.; Gioia, M.C.; di Palma, G.; et al. A longitudinal observation of brain derived neurotrophic factor mRNA levels in patients with relapsing–remitting multiple sclerosis. Brain Res 2009, 1256, 123–128. [Google Scholar]
- Sarchielli, P.; Zaffaroni, M.; Floridi, A.; Greco, L.; Candeliere, A.; Mattioni, A.; Tenaglia, S.; di Filippo, M.; Calabresi, P. Production of brain derived neurotrophic factor by mononuclear cells of patients with multiple sclerosis treated with glatiramer acetate, interferon-beta 1a, and high doses of immunoglobulins. Mult. Scler 2007, 13, 313–331. [Google Scholar]
- Azoulay, D.; Mausner-Fainberg, K.; Urshansky, N.; Fahoum, F.; Karni, A. Interferon-beta therapy upregulates BDNF secretion from PBMCs of MS patients through a CD40-dependent mechanism. J. Neuroimmunol 2009, 211, 114–119. [Google Scholar]
- Weinstock-Guttman, B.; Zivadinov, R.; Tamano-Blanco, M.; Abdelrahman, N.; Badgett, D.; Durfee, J.; Hussein, S.; Feichter, J.; Patrick, K.; Benedict, R.; et al. Immune cell BDNF secretion is associated with white matter voulume in multiple sclerosis. J. Neuroimmunol 2007, 188, 167–174. [Google Scholar]
- Linker, R.A.; Lee, D.H.; Demir, S.; Wiese, S.; Kuse, N.; Siglienti, I.; Gerhardt, E.; Neumann, H.; Sendtner, M.; Luehder, F.; et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: Therapeutic implications in a model of multiple sclerosis. Brain 2010, 133, 2248–2263. [Google Scholar]
- Kalinowska-Łyszczarz, A.; Pawlak, M.A.; Michalak, S.; Paprzycki, W.; Losy, J. Immune cell NT-3 expression is associated with brain atrophy in multiple sclerosis patients. J. Neuroimmunol 2011, 240–241, 109–113. [Google Scholar]
- Kalinowska-Łyszczarz, A.; Pawlak, M.A.; Michalak, S.; Losy, J. Cognitive deficit is related to immune-cell beta-NGF in multiple sclerosis patients. J. Neurol. Sci 2012, 321, 43–48. [Google Scholar]
- Patanella, A.K.; Zinno, M.; Quaranta, D.; Nociti, V.; Frisullo, G.; Gainotti, G.; Tonali, P.A.; Batocchi, A.P.; Marra, C. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J. Neurosci. Res 2010, 88, 1106–1112. [Google Scholar]
- Weinstock-Guttman, B.; Benedict, R.H.; Tamaño-Blanco, M.; Ramasamy, D.P.; Stosic, M.; Polito, J.; Zivadinov, R.; Ramanathan, M. The rs2030324 SNP of brain-derived neurotrophic factor (BDNF) is associated with visual cognitive processing in multiple sclerosis. Pathophysiology 2011, 18, 43–52. [Google Scholar]
- Pradat, P.F.; Kennel, P.; Naimi-Sadaoui, S.; Finiels, F.; Orsini, C.; Revah, F.; Delaere, P.; Mallet, J. Continuous delivery of neurotrophin 3 by gene therapy has a neuroprotective effect in experimental models of diabetic and acrylamide neuropathies. Hum. Gene. Ther 2001, 12, 2237–2249. [Google Scholar]
- Pradat, P.F.; Kennel, P.; Naimi-Sadaoui, S.; Finiels, F.; Scherman, D.; Orsinim, C.; Delaere, P.; Mallet, J.; Revah, F. Viral and non-viral gene therapy partially prevents experimental cisplatin-induced neuropathy. Gene. Ther 2002, 19, 1333–1337. [Google Scholar]
- Pardridge, W.M. Neurotrophins, neuroprotection and the blood-brain barrier. Curr. Opin. Investig. Drugs 2002, 3, 1753–1757. [Google Scholar]
- Blesch, A. Neurotrophin gene therapy for Alzheimer’s disease. Future Neurol 2006, 1, 179–187. [Google Scholar]
- Dawbarn, D.; Allen, S.J. Neurotrophins and neurodegeneration. Neuropathol. Appl. Neurobiol 2003, 29, 211–230. [Google Scholar]
- Tuszynski, M.H.; Thal, L.; Pay, M.; Salmon, D.P.; U, H.S.; Bakay, R.; Patel, P.; Blesch, A.; Vahlsing, H.L.; Ho, G.; et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med 2005, 11, 551–555. [Google Scholar]
- Drinkut, A.; Tereshchenko, Y.; Schulz, J.B.; Bähr, M.; Kügler, S. Efficient gene therapy for Parkinson’s disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol. Ther 2012, 20, 534–543. [Google Scholar]
- Calvo, A.C.; Moreno-Igoa, M.; Mancuso, R.; Manzano, R.; Oliván, S.; Muñoz, M.J.; Penas, C.; Zaragoza, P.; Navarro, X.; Osta, R. Lack of a synergistic effect of a non-viral ALS gene therapy based on BDNF and a TTC fusion molecule. Orphanet. J. Rare Dis 2011, 6, 10. [Google Scholar]
- Giralt, A.; Carretón, O.; Lao-Peregrin, C.; Martín, E.D.; Alberch, J. Conditional BDNF release under pathological conditions improves Huntington’s disease pathology by delaying neuronal dysfunction. Mol. Neurodegener 2011, 6, 71. [Google Scholar]
- Jones, J.L.; Anderson, J.M.; Phuah, C.L.; Fox, E.J.; Selmaj, K.; Margolin, D.; Lake, S.L.; Palmer, J.; Thompson, S.J.; Wilkins, A.; et al. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain 2010, 133, 2232–2247. [Google Scholar]
- Ziemssen, T.; Kuempfel, T.; Klinkert, W.E.F.; Neuhaus, O.; Hohlfeld, R. Glatiramer acetate-specific T-helper 1- and 2-type cell lines produce BDNF: Implications for multiple sclerosis therapy. Brain 2002, 125, 2381–2391. [Google Scholar]
- Aharoni, R.; Eilam, R.; Domev, H.; Labunskay, G.; Sela, M.; Arnon, R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc. Natl. Acad. Sci. USA 2005, 102, 19045–19050. [Google Scholar]
- Biernacki, K.; Antel, J.P.; Blain, M.; Narayanan, S.; Arnold, D.L.; Prat, A. Interferon Beta Promotes Nerve Growth Factor Secretion Early in the Course of Multiple Sclerosis. Arch. Neurol 2005, 62, 563–568. [Google Scholar]
- Aharoni, R.; Saada, R.; Eilam, R.; Hayardeny, L.; Sela, M.; Arnon, R. Oral treatment with laquinimod augments regulatory T-cells and brain-derived neurotrophic factor expression and reduces injury in the CNS of mice with experimental autoimmune encephalomyelitis. J. Neuroimmunol 2012, 251, 14–24. [Google Scholar]
- Thöne, J.; Ellrichmann, G.; Seubert, S.; Peruga, I.; Lee, D.H.; Conrad, R; Hayardeny, L.; Comi, G.; Wiese, S.; Linker, R.A.; et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am. J. Pathol 2012, 180, 267–274. [Google Scholar]
- Shibata, A.; Zelivyanskaya, M.; Limoges, J.; Carlson, K.A.; Gorantla, S.; Branecki, C.; Bishu, S.; Xiong, H.; Gendelman, H.E. Peripheral nerve induces macrophage neurotrophic activities: Regulation of neuronal process outgrowth, intracellular signaling and synaptic function. J. Neuroimmunol 2003, 142, 112–129. [Google Scholar]
- Mach, F.; Schönbeck, U.; Sukhova, G.K.; Bourcier, T.; Bonnefoy, J.Y.; Pober, J.S.; Libby, P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40-CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA 1997, 94, 1931–1936. [Google Scholar]
- Calingasan, N.Y.; Erdely, H.A.; Altar, C.A. Identification of CD40 ligand in Alzheimer’s disease and in animal models of Alzheimer’s disease and brain injury. Neurobiol. Aging 2002, 23, 31–39. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kalinowska-Lyszczarz, A.; Losy, J. The Role of Neurotrophins in Multiple Sclerosis—Pathological and Clinical Implications. Int. J. Mol. Sci. 2012, 13, 13713-13725. https://doi.org/10.3390/ijms131013713
Kalinowska-Lyszczarz A, Losy J. The Role of Neurotrophins in Multiple Sclerosis—Pathological and Clinical Implications. International Journal of Molecular Sciences. 2012; 13(10):13713-13725. https://doi.org/10.3390/ijms131013713
Chicago/Turabian StyleKalinowska-Lyszczarz, Alicja, and Jacek Losy. 2012. "The Role of Neurotrophins in Multiple Sclerosis—Pathological and Clinical Implications" International Journal of Molecular Sciences 13, no. 10: 13713-13725. https://doi.org/10.3390/ijms131013713



