Production of (R)-3-Quinuclidinol by E. coli Biocatalysts Possessing NADH-Dependent 3-Quinuclidinone Reductase (QNR or bacC) from Microbacterium luteolum and Leifsonia Alcohol Dehydrogenase (LSADH)
Abstract
:1. Introduction
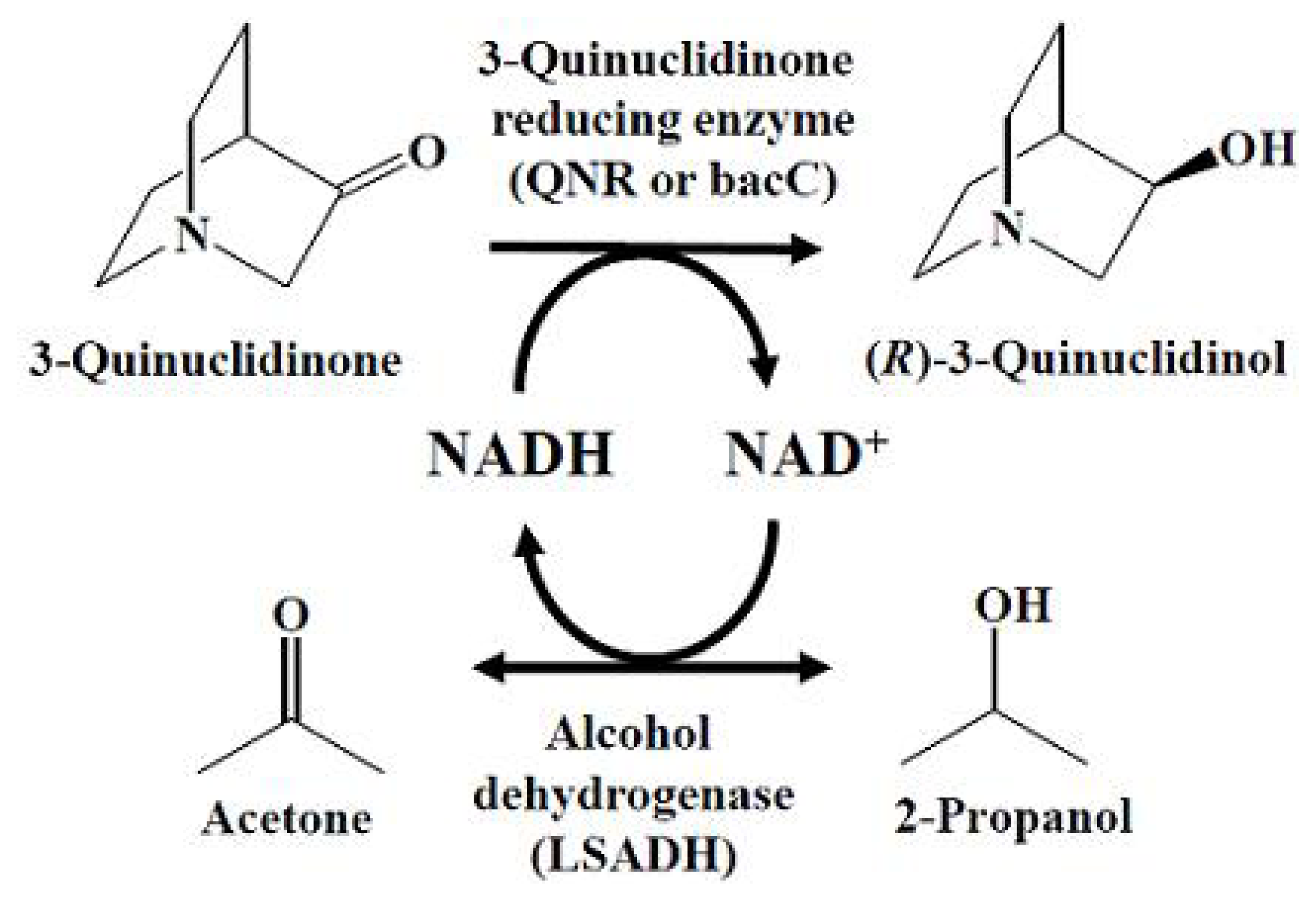
2. Results and Discussion
2.1. Construction of Expression Vector of QNR and LSADH
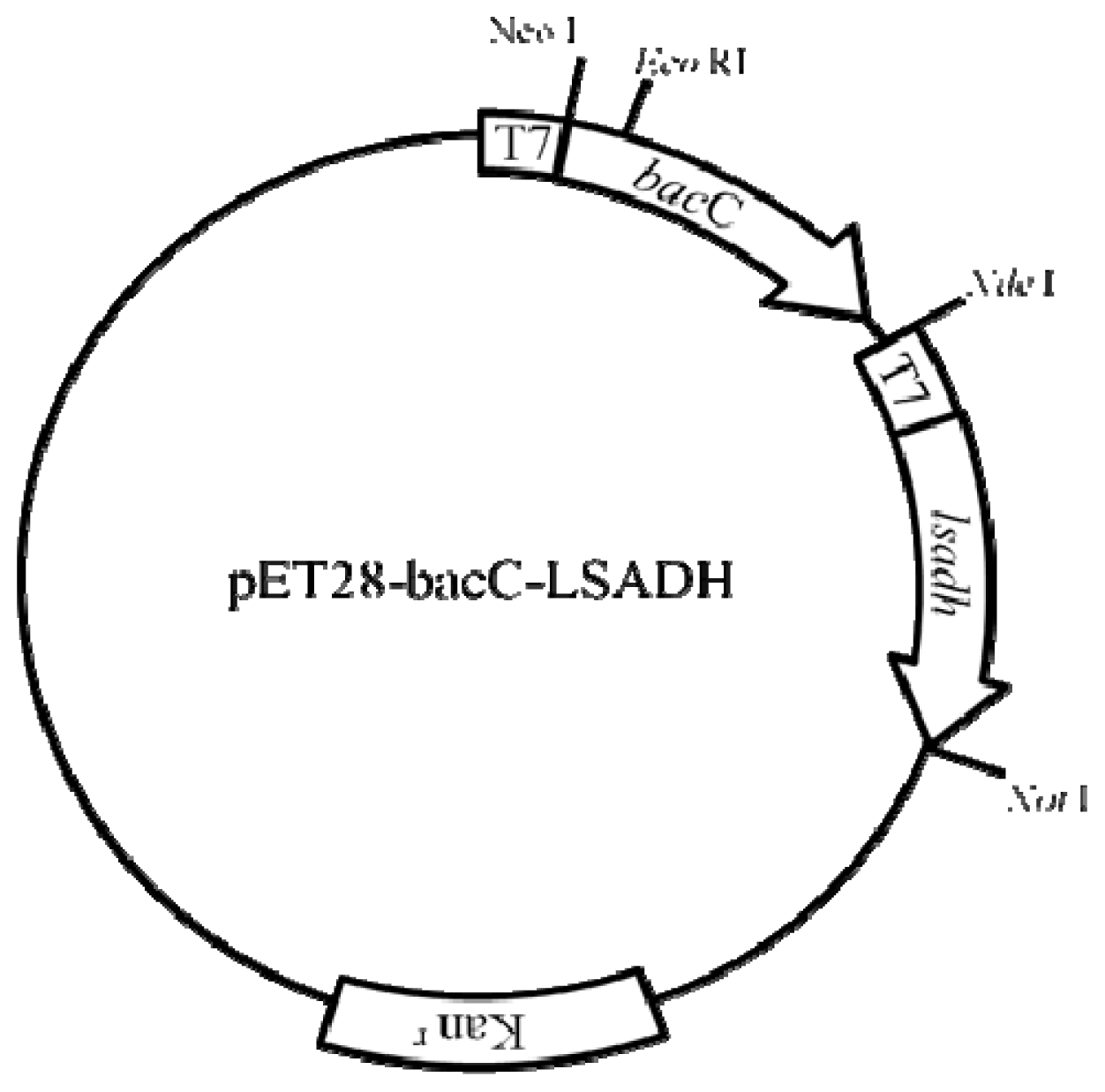
2.2. Construction of Expression Vector of bacC and LSADH
2.3. Enzymatic Activity of the E. coli Biocatalyst
2.4. Evaluation of E. coli Biocatalyst and Optimization of the Reaction
2.5. Evaluation of Immobilized E. coli Biocatalyst and the Conversion Time Course
3. Experimental Section
3.1. Chemicals
3.2. Methods
3.2.1. Preparation of E. coli Biocatalysts Overproducing Recombinant Enzymes
3.2.2. Immobilization Procedure of E. coli Cells
3.2.3. Enzyme Assay
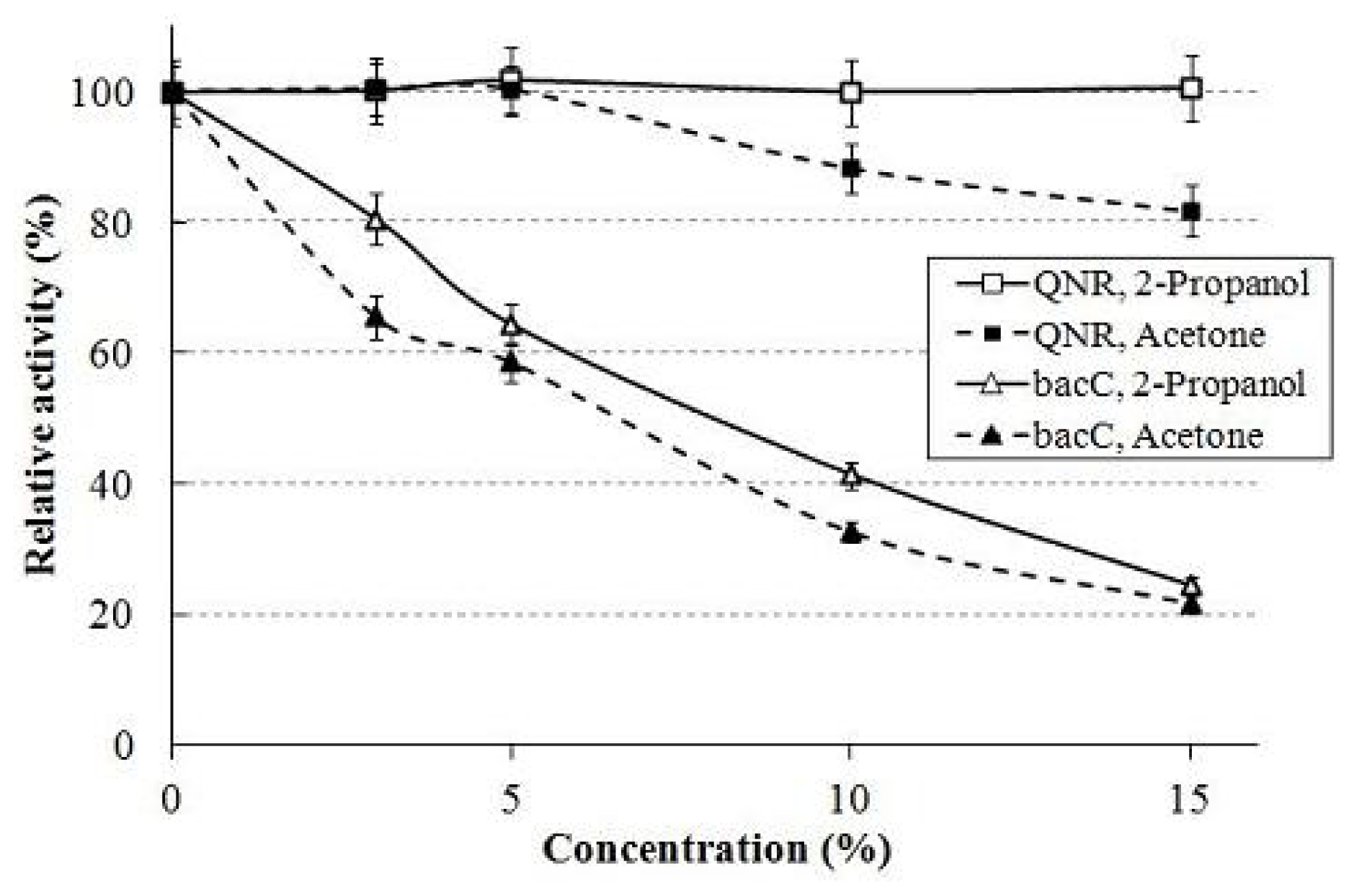
3.2.4. Effect of Organic Solvent for Enzymes
3.2.5. Biocatalytic Reaction to Reduce 3-Quinuclidinone to (R)-(−)-3-Quinuclidinol with a Coenzyme Regenerating System
3.2.6. Product Analysis by GC
4. Conclusions
References
- Noyori, R.; Ohkuma, T. Asymmetric catalysis by architectural and functional molecular engineering: Practical chemo- and stereoselective hydrogenation of ketones. Angew. Chem 2001, 40, 40–73. [Google Scholar]
- Tokunaga, M.; Larrow, J.F.; Kakiuchi, F.; Jacobsen, E.N. Asymmetric catalysis with water: Efficient kinetic resolution of terminal epoxides by means of catalytic hydrolysis. Science 1997, 277, 936–938. [Google Scholar]
- Jacobsen, E.N.; Kakiuchi, F.; Konsler, R.G.; Larrow, J.F.; Tokunaga, M. Enantioselective catalytic ring opening of epoxides with carboxylic acids. Tetrahedron Lett 1997, 38, 773–776. [Google Scholar]
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Kesseler, M.; Stürmer, R.; Zelinski, T. Industrial methods for the production of optically active intermediates. Angew. Chem 2004, 43, 788–824. [Google Scholar]
- Shimizu, S.; Kataoka, M.; Kita, K. Chiral alcohol synthesis with microbial carbonyl reductases in a water-organic solvent two-phase system. Ann. N. Y. Acad. Sci 1998, 864, 87–95. [Google Scholar]
- Matsuyama, A.; Yamamoto, H.; Kobayashi, Y. Practical application of recombinant whole-cell biocatalysts for the manufacturing of pharmaceutical such as chiral alcohols. Org. Process Res. Dev 2002, 6, 558–561. [Google Scholar]
- Nakamura, K.; Yamanaka, R.; Matsuda, T.; Harada, T. Recent development in asymmetric reduction of ketones with biocatalysts. Tetrahedron Asymmetry 2003, 14, 2659–2681. [Google Scholar]
- Kataoka, M.; Kita, K.; Wada, M.; Yasohara, Y.; Hasegawa, J.; Shimizu, S. Novel bioreduction system for the production of chiral alcohols. Appl. Microbiol. Biotechnol 2003, 62, 437–445. [Google Scholar]
- Goldberg, K.; Schroer, K.; Lutz, S.; Liese, A. Biocatalytic ketone reduction-a powerful tool for the production of chiral alcohols-part I: Processes with isolated enzymes. Appl. Microbiol. Biotechnol 2007, 76, 237–248. [Google Scholar]
- Prat, M.; Buil, M.A.; Fernandez, M.D.; Castro, J.; Monleon, J.M.; Tort, L.; Casals, G.; Ferrer, M.; Huerta, J.M.; Espinosa, S.; et al. Discovery of novel quaternary ammonium derivatives of (3R)-quinuclidinyl carbamates as potent and long acting muscarinic antagonists. Bioorg. Med. Chem. Lett 2011, 21, 3457–3461. [Google Scholar]
- Nomoto, F.; Hirayama, Y.; Ikunaka, M.; Inoue, T.; Otsuka, K. A practical chemoenzymatic process to access (R)-quinuclidin-3-ol on scale. Tetrahedron Asymmetry 2003, 14, 1871–1877. [Google Scholar]
- Hashimoto, T.; Nakajima, K.; Ongena, G.; Yamada, Y. Two tropinone reductases with distinct stereospecificities from cultured roots of Hyoscyamus niger. Plant. Physiol 1992, 100, 836–845. [Google Scholar]
- Uzura, A.; Nomoto, F.; Sakoda, A.; Nishimoto, Y.; Kataoka, M.; Shimizu, S. Stereoselective synthesis of (R)-3-quinuclidinol through asymmetric reduction of 3-quinuclidinone with 3-quinuclidinone reductase of Rhodotorula rubra. Appl. Microbiol. Biotechnol 2009, 83, 617–626. [Google Scholar]
- Yamamoto, H.; Ueda, M.; Pan, R.; Hamatani, T. Methods for producing optically active alcohols. U.S. Patent 2003/0143700, 2003. [Google Scholar]
- Yamamoto, H.; Mitsuhashi, K.; Kimoto, N.; Kobayashi, Y.; Esaki, N. Robust NADH-regenerator: improved alpha-haloketone-resistant formate dehydrogenase. Appl. Microbiol. Biotechnol 2005, 67, 33–39. [Google Scholar]
- Kataoka, M.; Yamamoto, K.; Kawabata, H.; Wada, K.; Kita, H.; Yanase, H.; Shimizu, S. Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by Eschericha coli transformant cells coexpressiong the aldehyde reductase and glucose dehydrogenase genes. Appl. Microbiol. Biotechnol 1999, 51, 486–490. [Google Scholar]
- Bradshaw, C.W.; Fu, H.; Shen, G.J.; Wong, C.H. A Pseudomonas sp. alcohol dehydrogenase with broad substrate specificity and unusual stereospecificity for organic synthesis. J. Org. Chem 1992, 57, 1526–1532. [Google Scholar]
- Stampfer, W.; Kosjek, B.; Faber, K.; Kroutil, W. Biocatalytic asymmetric hydrogen transfer employing Rhodococcus ruber DSM 44541. J. Org. Chem 2003, 68, 402–406. [Google Scholar]
- Inoue, K.; Makino, Y.; Itoh, N. Production of (R)-chiral alcohols by a hydrogen-transfer bioreduction with NADH-dependent Leifsonia alcohol dehydrogenase (LSADH). Tetrahedron Asymmetry 2005, 16, 2539–2549. [Google Scholar]
- Inoue, K.; Makino, Y.; Dairi, T.; Itoh, N. Gene cloning and expression of Leifsonia alcohol dehydrogenase (LSADH) involved in asymmetric hydrogen-transfer bioreduction to produce (R)-form chiral alcohols. Biosci. Biotechnol. Biochem 2006, 70, 418–426. [Google Scholar]
- Itoh, N.; Isotani, K.; Nakamura, M.; Inoue, K.; Isogai, Y.; Makino, Y. Efficient synthesis of optically pure alcohols by asymmetric hydrogen-transfer biocatalysis: application of engineered enzymes in a 2-propanol-water medium. Appl. Microbiol. Biotechnol 2012, 93, 1075–1085. [Google Scholar]
- Jakoblinnert, A.; Madenov, R.; Paul, A.; Sibilla, F.; Schwaneberg, U.; Ansorqe-Schumacher, M.B.; de Maria, P.D. Asymmetric reduction of ketones with recombinant E. coli whole cells in neat substrates. Chem. Commun 2011, 47, 12230–12232. [Google Scholar]
- Itoh, N. Quinuclidinone reductase and method for producing optically active 3-quinuclidinol. Jpn. Patent JP2010-051207, 2010. [Google Scholar]
- Itoh, N. Quinuclidinone reductase and method for producing optically active 3-quinuclidinol. Jpn. Patent JP2011-147349, 2011. [Google Scholar]
- Itoh, N.; Nakamura, M.; Inoue, K.; Makino, Y. Continuous production of chiral 1,3-butanediol using immobilized biocatalysts in a packed bed reactor: Promising biocatalysis method with an asymmetric hydrogen-transfer bioreduction. Appl. Microbiol. Biotechnol 2007, 75, 1249–1256. [Google Scholar]
- Bahulekar, R.; Ayyangar, N.R.; Ponrathnam, S. Polyethyleneimine in immobilization of biocatalysts. Enzym. Microb. Technol 1991, 13, 858–868. [Google Scholar]


| Plasmid for expression | 3-Quinuclidionone reduction (U/mL culture broth) | 2-Propanol oxidation (U/mL culture broth) |
|---|---|---|
| pET28-QNR * | 8.4 | 0 |
| pET28-bacC * | 0.3 | 0 |
| pETDuet-QNR/pRSFDuet-LSADH | 0.3 | 0.12 |
| pET28-bacC-LSADH | 0.1 | 0.09 |
| pKELA | 0 | 1.0 |
| Biocatalyst | Amount of cells a | Cells-mixing ratio | Production level b, molar conversion | |
|---|---|---|---|---|
| Resting cells | ||||
| (a) | pET28-QNR, pKELA | 40.6 mg | 4:1 | 100 mg/mL ± 1, 100% |
| 42.3 mg | 1:1 | 100 mg/mL ± 1, 100% | ||
| 43.9 mg | 1:4 | 96 mg/mL ± 2, 96% | ||
| 42.3 mg | 1:1 | 51 mg/mL ± 3, 34% c | ||
| 84.5 mg | 1:1 | 63 mg/mL ± 3, 42%c | ||
| (b) | pET28-bacC, pKELA | 40.2 mg | 4:1 | 98 mg/mL ± 2, 98% |
| 42.0 mg | 1:1 | 99 mg/mL ± 1, 99% | ||
| 43.8 mg | 1:4 | 65 mg/mL ± 3, 65% | ||
| 42.0 mg | 1:1 | 34 mg/mL ± 3, 22% c | ||
| 84.0 mg | 1:1 | 58 mg/mL ± 4, 39%c | ||
| (c) | pETDuet-QNR/pRSFDuet-LSADH | 35.5 mg | - | 100 mg/mL ± 1, 100% |
| (d) | pET28-bacC-LSADH | 36.5 mg | - | 98 mg/mL ± 2, 98% |
| Immobilized cells | ||||
| (e) | pET28-QNR, pKELA | 84.5 mg | 1:1 | 150 mg/mL ± 2, 100% c |
| (f) | pET28-bacC, pKELA | 84.0 mg | 1:1 | 24 mg/mL ± 3, 16% b |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Isotani, K.; Kurokawa, J.; Itoh, N. Production of (R)-3-Quinuclidinol by E. coli Biocatalysts Possessing NADH-Dependent 3-Quinuclidinone Reductase (QNR or bacC) from Microbacterium luteolum and Leifsonia Alcohol Dehydrogenase (LSADH). Int. J. Mol. Sci. 2012, 13, 13542-13553. https://doi.org/10.3390/ijms131013542
Isotani K, Kurokawa J, Itoh N. Production of (R)-3-Quinuclidinol by E. coli Biocatalysts Possessing NADH-Dependent 3-Quinuclidinone Reductase (QNR or bacC) from Microbacterium luteolum and Leifsonia Alcohol Dehydrogenase (LSADH). International Journal of Molecular Sciences. 2012; 13(10):13542-13553. https://doi.org/10.3390/ijms131013542
Chicago/Turabian StyleIsotani, Kentaro, Junji Kurokawa, and Nobuya Itoh. 2012. "Production of (R)-3-Quinuclidinol by E. coli Biocatalysts Possessing NADH-Dependent 3-Quinuclidinone Reductase (QNR or bacC) from Microbacterium luteolum and Leifsonia Alcohol Dehydrogenase (LSADH)" International Journal of Molecular Sciences 13, no. 10: 13542-13553. https://doi.org/10.3390/ijms131013542




