Effect of Enzymatic Treatment of Different Starch Sources on the in Vitro Rate and Extent of Starch Digestion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Pulse 1H HR-MAS NMR Spectroscopy
2.2. In vitro Digestion
2.3. Relation Between Degree of Branching and in vitro Digestion Rate
3. Experimental Section
3.1. Preparation of Enzymatically Modified Starches
3.2. Single-Pulse 1H HR-MAS NMR Measurement
3.3. In vitro Starch Digestion
3.4. HPAEC-PAD Measurement
3.5. Calculation and Statistical Analysis
4. Conclusions
Acknowledgments
References
- Jobling, S. Improving starch for food and industrial applications. Curr. Opin. Plant Biol 2004, 7, 210–218. [Google Scholar]
- Zhang, G.Y.; Hamaker, B.R. Slowly digestible starch: Concept, mechanism, and proposed extended glycemic index. Crit. Rev. Food Sci. Nutr 2009, 49, 852–867. [Google Scholar]
- Mann, J.; Cummings, J.H.; Englyst, H.N.; Key, T.; Liu, S.; Riccardi, G.; Summerbell, C.; Uauy, R.; van Dam, R.M.; Venn, B.; et al. FAO/WHO Scientific Update on carbohydrates in human nutrition: conclusions. Eur. J. Clin. Nutr 2007, 61, S132–S137. [Google Scholar]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. J. Am. Med. Assoc 2002, 287, 2414–2423. [Google Scholar]
- Jenkins, D.J.A.; Wolever, T.M.S.; Taylor, R.H.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; et al. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr 1981, 34, 362–366. [Google Scholar]
- Akerberg, A.; Liljeberg, H.; Bjorck, I. Effects of amylose/amylopectin ratio and baking conditions on resistant starch formation and glycaemic indices. J. Cereal Sci 1998, 28, 71–80. [Google Scholar]
- Lee, C.K.; Le, Q.T.; Kim, Y.H.; Shim, J.H.; Lee, S.J.; Park, J.H.; Lee, K.P.; Song, S.H.; Auh, J.H.; Lee, S.J.; et al. Enzymatic synthesis and properties of highly branched rice starch amylose and amylopectin cluster. J. Agric. Food Chem 2008, 56, 126–131. [Google Scholar]
- Le, Q.T.; Lee, C.K.; Kim, Y.W.; Lee, S.J.; Zhang, R.; Withers, S.G.; Kim, Y.R.; Auh, J.H.; Park, K.H. Amylolytically-resistant tapioca starch modified by combined treatment of branching enzyme and maltogenic amylase. Carbohydr. Polym 2009, 75, 9–14. [Google Scholar]
- Takii, H.; Ishihara, K.; Kometani, T.; Okada, S.; Fushiki, T. Enhancement of swimming endurance in mice by highly branched cyclic dextrin. Biosci. Biotechnol. Biochem 1999, 63, 2045–2052. [Google Scholar]
- Larsen, F.H.; Blennow, A.; Engelsen, S.B. Starch granule hydration—A MAS NMR investigation. Food Biophys 2008, 3, 25–32. [Google Scholar]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr 1992, 46, S33–S50. [Google Scholar]
- Mcintyre, D.D.; Ho, C.; Vogel, H.J. One-dimensional nuclear-magnetic-resonance studies of starch and starch products. Starch-Starke 1990, 42, 260–267. [Google Scholar]
- Peng, Q.J.; Perlin, A.S. Observations on NMR-spectra of starches in dimethylsulfoxide, iodine-complexing, and solvation in water dimethylsulfoxide. Carbohydr. Res 1987, 160, 57–72. [Google Scholar]
- Byars, J.A. Jet cooking of waxy maize starch: Solution rheology and molecular weight degradation of amylopectin. Cereal Chem 2003, 80, 87–90. [Google Scholar]
- Atwell, W.A.; Hood, L.F.; Lineback, D.R.; Varrianomarston, E.; Zobel, H.F. The terminology and methodology associated with basic starch phenomena. Cereal Foods World 1988, 33, 306–311. [Google Scholar]
- Hernandez, J.M.; Gaborieau, M.; Castignolles, P.; Gidley, M.J.; Myers, A.M.; Gilbert, R.G. Mechanistic investigation of a starch-branching enzyme using hydrodynamic volume SEC analysis. Biomacromolecules 2008, 9, 954–965. [Google Scholar]
- Takata, H.; Kato, T.; Takagi, M.; Imanaka, T. Cyclization reaction catalyzed by Bacillus cereus branching enzyme, and the structure of cyclic glucan produced by the enzyme from amylose. J. Appl. Glycosci 2005, 52, 359–365. [Google Scholar]
- Ao, Z.H.; Simsek, S.; Zhang, G.Y.; Venkatachalam, M.; Reuhs, B.L.; Hamaker, B.R. Starch with a slow digestion property produced by altering its chain length, branch density, and crystalline structure. J. Agric. Food Chem 2007, 55, 4540–4547. [Google Scholar]
- Cai, L.M.; Shi, Y.C.; Rong, L.X.; Hsiao, B.S. Debranching and crystallization of waxy maize starch in relation to enzyme digestibility. Carbohydr. Polym 2010, 81, 385–393. [Google Scholar]
- Hanashiro, I.; Abe, J.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr. Res 1996, 283, 151–159. [Google Scholar]
- Zhang, G.Y.; Ao, Z.H.; Hamaker, B.R. Slow digestion property of native cereal starches. Biomacromolecules 2006, 7, 3252–3258. [Google Scholar]
- Kerr, R.W.; Cleveland, F.C.; Katzbeck, W.J. The action of amylo-glucosidase on amylose and amylopectin. J. Am. Chem. Soc 1951, 73, 3916–3921. [Google Scholar]
- Miao, M.; Jiang, B.; Zhang, T. Effect of pullulanase debranching and recrystallization on structure and digestibility of waxy maize starch. Carbohydr. Polym 2009, 76, 214–221. [Google Scholar]
- Cai, L.M.; Shi, Y.C. Structure and digestibility of crystalline short-chain amylose from debranched waxy wheat, wazy maize and waxy potato starches. Carbohydr. Polym 2010, 79, 1117–1123. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed; Iowa State University Press: Ames, IA, USA, 1973. [Google Scholar]
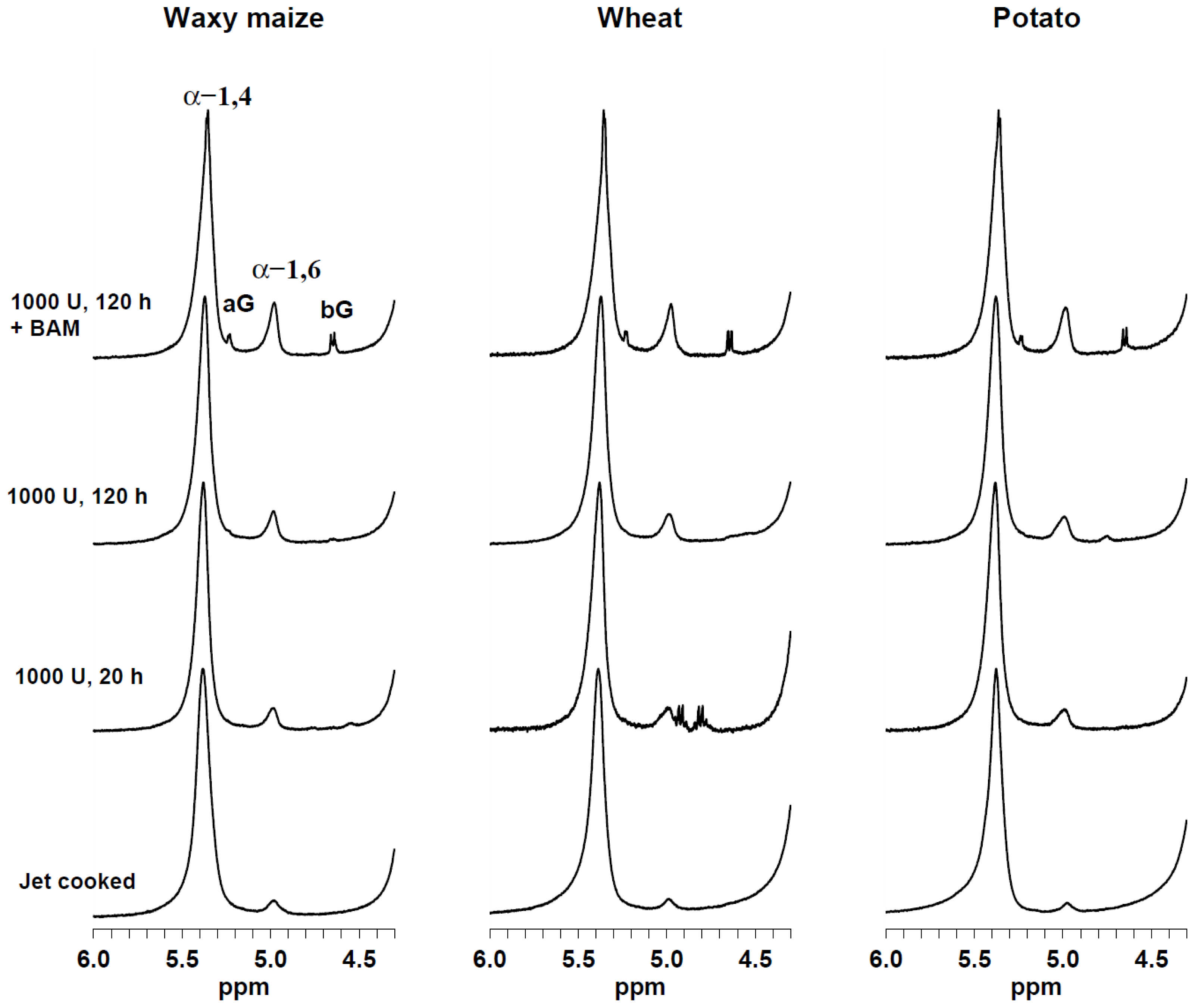
| Source | α-glucose | β-glucose | α-1,6 | α-1,4 | α-1,6/(α-1,6 + α-1,4) |
|---|---|---|---|---|---|
| Waxy maize | |||||
| Jet cooked | 3.6 | 96.4 | 3.6 | ||
| 20 U 20 h | 4.9 | 95.1 | 4.9 | ||
| 1000 U 20 h | 6.2 | 93.8 | 6.2 | ||
| 1000 U 120 h | 7.9 | 91.9 | 7.9 | ||
| 1000 U 120 h + BAM | 0.4 | 1.9 | 13.1 | 84.6 | 13.4 |
| 1000 U 120 h + AMG | 0.5 | 2 | 11.2 | 86.4 | 11.5 |
| 1000 U 120 h + AGLU | 0.5 | 1.6 | 14.9 | 83 | 15.3 |
| Wheat | |||||
| Jet cooked | 2.6 | 97.4 | 2.6 | ||
| 1000 U 20 h | 7.6 | 92.4 | 7.6 | ||
| 1000 U 120 h | 7.3 | 92.7 | 7.3 | ||
| 1000 U 120 h + BAM | 0.3 | 2 | 12.9 | 84.8 | 13.2 |
| Potato | |||||
| Jet cooked | 2.1 | 97.9 | 2.1 | ||
| 1000 U 20 h | 5.1 | 94.9 | 5.1 | ||
| 1000 U 120 h | 7.5 | 92.5 | 7.5 | ||
| 1000 U 120 + BAM | 0.5 | 1.7 | 12.2 | 85.6 | 12.5 |
| Glucidex 2 | 0.6 | 1.8 | 4.6 | 93 | 4.7 |
| Glucidex 6 | 1.1 | 2.4 | 3.2 | 93.3 | 3.3 |
| TS | RDS/TS | SDS/TS | RS/TS | |||||
|---|---|---|---|---|---|---|---|---|
| % of d.m. * | % of TS (total starch) | |||||||
| Jet cooked | 98 | a | 74 | bc | 25 | b | 2 | ab |
| 20 U 20 h | 96 | bc | 72 | cd | 29 | a | −1 | b |
| 1000 U 20 h | 95 | c | 76 | a | 25 | b | −1 | b |
| 1000 U 120 h | 95 | bc | 69 | d | 27 | ab | 4 | a |
| Glucidex 2 | 95 | bc | 70 | d | 29 | a | 1 | ab |
| Glucidex 6 | 97 | ab | 74 | ab | 25 | b | 0 | b |
| SE | 0.6 | 0.8 | 0.9 | 0.9 | ||||
| p value | 0.0065 | 0.0003 | 0.0217 | 0.0268 | ||||
| TS | RDS | SDS | RS | |||||
|---|---|---|---|---|---|---|---|---|
| % of d.m.* | % of TS | |||||||
| Waxy maize | ||||||||
| Jetcooked | 98 | a | 74 | cd | 25 | cd | 2 | cd |
| 1000 U 20 h | 95 | bcd | 76 | bc | 25 | cd | −1 | d |
| 1000 U 120 h | 95 | bc | 69 | ef | 27 | c | 4 | bc |
| 1000 U 120 h + BAM | 90 | fg | 69 | efg | 31 | b | 1 | cd |
| Wheat | ||||||||
| Jetcooked | 97 | ab | 70 | ef | 22 | d | 8 | a |
| 1000 U 20 h | 92 | ef | 76 | b | 22 | d | 2 | cd |
| 1000 U 120 h | 94 | bcd | 69 | efg | 25 | cd | 6 | ab |
| 1000 U 120 h + BAM | 85 | h | 67 | g | 35 | a | −2 | d |
| Potato | ||||||||
| Jet cooked | 93 | cde | 67 | fg | 25 | cd | 8 | a |
| 1000 U20 h | 90 | f | 79 | a | 22 | d | −1 | d |
| 1000 U 120 h | 93 | de | 73 | d | 24 | d | 4 | bc |
| 1000 U 120 h + BAM | 88 | g | 70 | e | 30 | b | 0 | d |
| SE | 0.8 | 0.9 | 1.1 | 1.2 | ||||
| Source | <0001 | 0.0169 | 0.1164 | 0.0372 | ||||
| Treatment | <0001 | <0001 | <0001 | <0001 | ||||
| Source × Treatment | 0.0435 | 0.0002 | 0.0344 | 0.0151 | ||||
| TS | RDS | SDS | RS | ||||
|---|---|---|---|---|---|---|---|
| % of d.m.* | % of TS | ||||||
| Jet cooked | 98 | a | 74 | a | 25 | c | 2 |
| 1000 U 120 h | 95 | b | 69 | b | 27 | bc | 4 |
| 1000 U 120 h + BAM | 90 | d | 69 | b | 31 | ab | 1 |
| 1000 U 120 h + AMG | 93 | c | 69 | b | 29 | abc | 2 |
| 1000 U 120 h + AGLU | 93 | c | 64 | c | 33 | a | 3 |
| SE | 0.5 | 0.7 | 1.5 | 1.1 | |||
| p value | <0.0001 | <0.0001 | 0.0201 | 0.3588 | |||
| Waxy maize | Wheat | Potato | |
|---|---|---|---|
| No enzyme | x | x | x |
| 20 U BE, 20 h | x | ||
| 1000 U BE, 20 h | x | x | x |
| 1000 U BE, 120 h | x | x | x |
| 1000 U BE, 120 h + BAM, 24 h | x | x | x |
| 1000 U BE, 120 h + AGLU, 23 h | x | ||
| 1000 U BE, 120 h + AMG, 2 h | x |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kasprzak, M.M.; Lærke, H.N.; Hofmann Larsen, F.; Bach Knudsen, K.E.; Pedersen, S.; Jørgensen, A.S. Effect of Enzymatic Treatment of Different Starch Sources on the in Vitro Rate and Extent of Starch Digestion. Int. J. Mol. Sci. 2012, 13, 929-942. https://doi.org/10.3390/ijms13010929
Kasprzak MM, Lærke HN, Hofmann Larsen F, Bach Knudsen KE, Pedersen S, Jørgensen AS. Effect of Enzymatic Treatment of Different Starch Sources on the in Vitro Rate and Extent of Starch Digestion. International Journal of Molecular Sciences. 2012; 13(1):929-942. https://doi.org/10.3390/ijms13010929
Chicago/Turabian StyleKasprzak, Mirosław Marek, Helle Nygaard Lærke, Flemming Hofmann Larsen, Knud Erik Bach Knudsen, Sven Pedersen, and Anne Skov Jørgensen. 2012. "Effect of Enzymatic Treatment of Different Starch Sources on the in Vitro Rate and Extent of Starch Digestion" International Journal of Molecular Sciences 13, no. 1: 929-942. https://doi.org/10.3390/ijms13010929





