Development of an Innovative Nutraceutical Fermented Beverage from Herbal Mate (Ilex paraguariensis A.St.-Hil.) Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of the Formulation and LAB Strain
2.2. Probiotic Fermentation of the HME
2.3. Caffeine Content
2.4. Antioxidant Activity
2.5. Sensorial Analysis of the Beverage
3. Experimental Section
3.1. Preparation of the HME
3.2. HME Formulation and LAB Strains
3.3. pH and Acidity
3.4. Sugars and Organic Acids Concentration
3.5. LAB Count and Fermentation
3.6. Caffeine Content
3.7. Antioxidant Activity
3.8. Sensorial Analysis
4. Conclusions
Acknowledgments
References
- Berté, K.A.; Beux, M.R.; Spada, P.K.; Salvador, M.; Hoffmann-Ribani, R. Chemical composition and antioxidant activity of yerba-mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) extract as obtained by spray drying. J. Agric. Food Chem 2011, 59, 5523–5527. [Google Scholar]
- Filip, R.; Lopez, P.; Giberti, G.; Coussio, J.; Ferraro, G. Phenolic compounds in seven South American Ilex species. Fitoterapia 2003, 72, 774–778. [Google Scholar]
- Bixby, M.; Spieler, L.; Menini, T.; Gugliucci, A. Ilex paraguariensis extracts are potent inhibitors of nitrosative stress: A comparative study with green tea and wines using nitration model and mammalian cell cytotoxicity. Life Sci 2005, 77, 345–358. [Google Scholar]
- Lunceford, N.; Gugliucci, A. Ilex paraguariensis extracts inhibit AGE formation more efficiently than green tea. Fitoterapia 2005, 76, 419–427. [Google Scholar]
- Bravo, L.; Goya, L.; Lecumberri, L. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res. Int 2007, 40, 393–405. [Google Scholar]
- Heck, C.I.; Demejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci 2007, 72, 138–151. [Google Scholar]
- Lanzetti, M.; Bezerra, F.S.; Romana-Souza, B.; Brando-Lima, A.C.; Koatz, V.L.; Porto, L.C.; Valenca, S.S. Mate tea reduced acute lung inflammation in mice exposed to cigarette smoke. Nutrition 2008, 24, 375–381. [Google Scholar]
- Athayde, M.; Coelho, G.C.; Schenkel, E.P. Caffeine and theobromine in epicuticular wax of Ilex paraguariensis. Phytochemistry 2000, 55, 853–857. [Google Scholar]
- Chandra, S.; DeMejia Gonzalez, E. Polyphenolic compounds, antioxidant capacity, and quinone reductase activity of an aqueous extract of Ardisia compressa in comparison to mate (Ilex paraguariensis) and green (Camellia sinensis) teas. J. Agric. Food Chem 2004, 52, 3583–3589. [Google Scholar]
- Lee, Y.K.; Nomoto, K.; Salminen, S.; Gorbach, S.L. Safety of Probiotic Bacteria. In Handbook of Probiotics; John Wiley & Sons: New York, NY, USA, 1999; pp. 17–21. [Google Scholar]
- Stiles, M.E. Biopreservation by lactic acid bacteria. Antonie Van Leeuwenhoek 1996, 70, 331–345. [Google Scholar]
- Naaber, P.; Mikelsaar, M. Interactions between Lactobacilli and antibiotic-associated diarrhea. Adv. Appl. Microbiol 2004, 54, 231–260. [Google Scholar]
- Kaizu, H.; Sasaki, M.; Nakajima, H.; Suzuki, Y. Effect of antioxidative lactic acid bacteria on rats fed a diet deficient in vitamin E. J. Dairy Sci 1993, 76, 2493–2499. [Google Scholar]
- Associação Brasileira das Indústrias da Alimentação (ABIA). Mercado Brasileiro dos alimentos industrializados. 2009. Available online: http://www.abia.org.br/ecopublall17.asp accessed on 3 March 2009.
- Nielsen Company. Relatórios Executivos de Notícias. Os Produtos Mais Quentes do Mundo: Informações sobre Categorias de Alimentos & Bebidas. 2009. Available online: http://br.nielsen.com/reports/reportesejecutivosglobales.shtml accessed on 15 March 2009.
- Virtanen, T.; Pihlanto, A.; Akkanen, S.; Korhonen, H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J. Appl. Microbiol 2007, 102, 106–115. [Google Scholar]
- Chen, X.; Xu, J.; Shuai, J.; Chen, J.; Zhang, Z.; Fang, W. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int. J. Food Microbiol 2007, 115, 307–312. [Google Scholar]
- Klaenhammer, T.R.; Altermann, E.; Pfeiler, E.; Buck, B.L.; Goh, Y.J.; O’Flaherty, S.; Barrangou, R.; Duong, T. Functional genomics of probiotic lactobacilli. J. Clin. Gastroenterol 2008, 42, S160–S162. [Google Scholar]
- Barrangou, R.; Altermann, E.; Hutkins, R. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2003, 100, 8957–8962. [Google Scholar]
- Gilliland, S.E.; Walker, D.K. Factors to consider when selecting a culture of Lactobacillus acidophilus as a dietary adjunct to produce a hypocholesterolemic effect in humans. J. Dairy Sci 1999, 73, 905–911. [Google Scholar]
- Percival, M. Choosing a probiotic supplement. Clin. Nutr. Insights 1997, 6, 1–4. [Google Scholar]
- De Dea Lindner, J.; Canchaya, C.; Zhang, Z.; Neviani, E.; Fitzgerlad, G.F.; Sinderen, D.V.; Ventura, M. Exploiting Bifidobacterium genomes: The molecular basis of stress response. Int. J. Food Microbiol 2007, 120, 13–24. [Google Scholar]
- Reid, G.; Beuerman, D.; Heinemann, C.; Bruce, A.W. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol. Med. Microbiol 2001, 32, 37–41. [Google Scholar]
- Lin, M.; Chang, F. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig. Dis. Sci 2000, 45, 1617–1622. [Google Scholar]
- Mazzaferra, P. Mate drinking: Caffeine and phenolic acid intake. Food Chem 1997, 60, 67–71. [Google Scholar]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr 2006, 46, 101–123. [Google Scholar]
- Satel, S. Is caffeine addictive?—A review of the literature. Am. J. Drug Alcohol Abuse 2006, 32, 493–502. [Google Scholar]
- Carrillo, J.A.; Benitez, J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin. Pharmacokinet 2000, 39, 127–153. [Google Scholar]
- Newell, A.M.B.; Chandra, S.; Gonzalez de Mejia, E. Ethnic Teas and Their Bioactive Components. In Hispanic Foods: Chemistry and Flavor; Tunick, M.H., de Mejia, E.G., Eds.; American Chemistry Society: Washington, DC, USA, 2007; pp. 127–131. [Google Scholar]
- Schinella, G.R.; Troiani, G.; Davila, V.; de Buschiazzo, P.M.; Tournier, H.A. Antioxidant effects of an aqueous extract of Ilex paraguariensis. Biochem. Biophys. Res. Commun 2000, 269, 357–360. [Google Scholar]
- Anesini, C.; Ferraro, G.; Filip, R. Peroxidase-like activity of Ilex paraguariensis. Food Chem 2006, 97, 459–464. [Google Scholar]
- Ramadan-Hassanien, M.F. Total antioxidant potential of juices, beverages and hot drinks consumed in Egypt screened by DPPH in vitro assay. Grasas y Aceites 2008, 59, 254–259. [Google Scholar]
- Dutcosky, S.D. Análise Sensorial de Alimentos; Champagnat: Curitiba, Brazil, 1996. [Google Scholar]
- Ramadan, M.F.; Kroh, L.W.; Moersel, J.T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J. Agric. Food Chem 2003, 51, 6961–6969. [Google Scholar]
- Monteiro, C.L.B. Técnicas de Avaliação Sensorial, 2nd ed; UFPR/CEPPA: Curitiba, Brazil, 1984; p. 101. [Google Scholar]
| Formulations | Carbohydrate and nitrogen substrates (w/v) a | |||
|---|---|---|---|---|
| Yeast extract | Honey | Malt extract | Sugar cane molasse | |
| A | 0.5 | 4 | - | - |
| B | 0.5 | 6 | - | - |
| C | 0.5 | 8 | - | - |
| D | 0.5 | 10 | - | - |
| E | 0.5 | 4 | - | - |
| F | 1.5 | 4 | - | - |
| G | - | - | 4 | - |
| H | - | - | 6 | - |
| I | - | - | 8 | - |
| J | - | - | - | 4 |
| K | - | - | - | 6 |
| L | - | - | - | 8 |
| M | - | 10 | - | - |
| N | - | 14 | - | - |
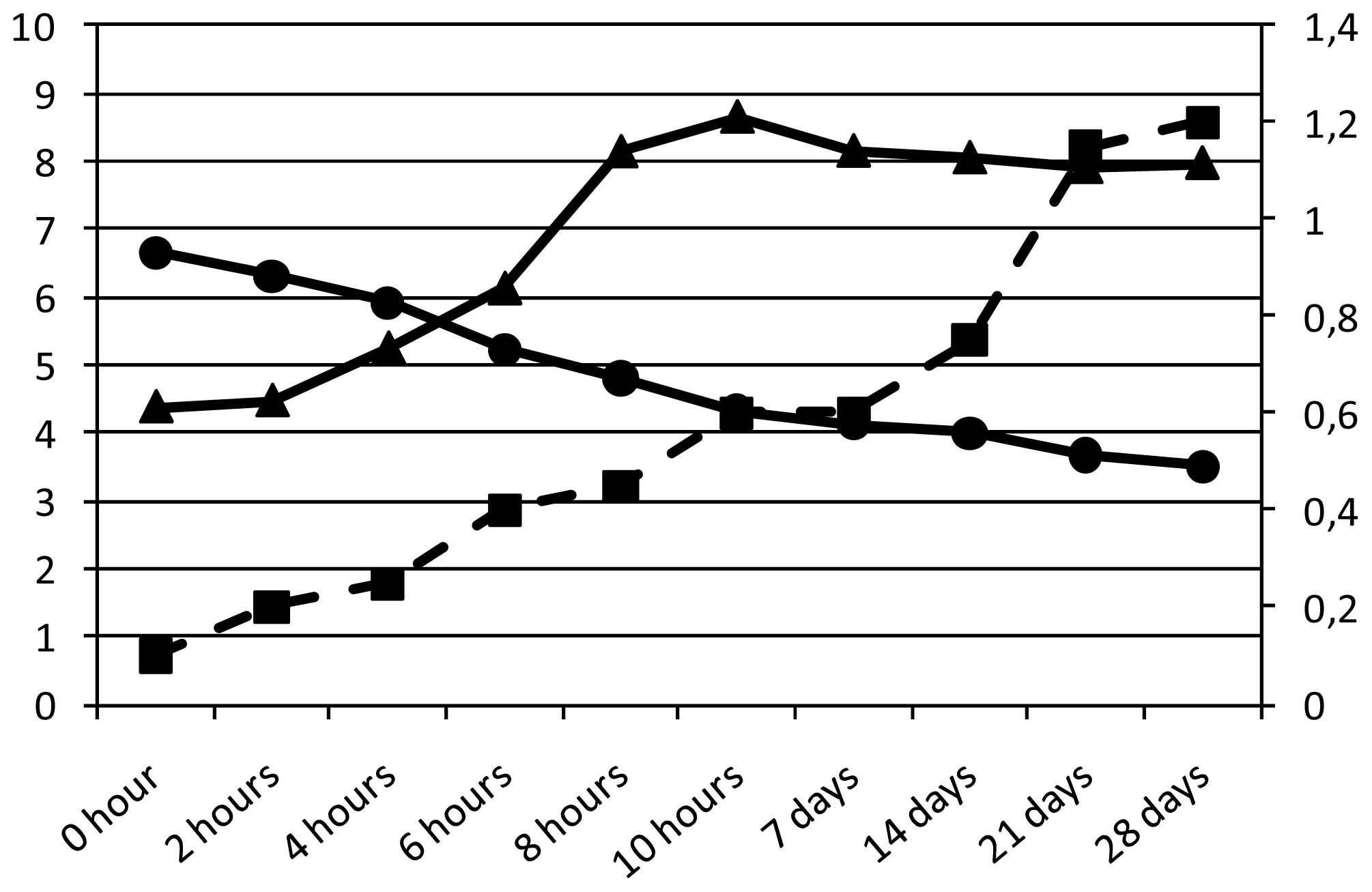
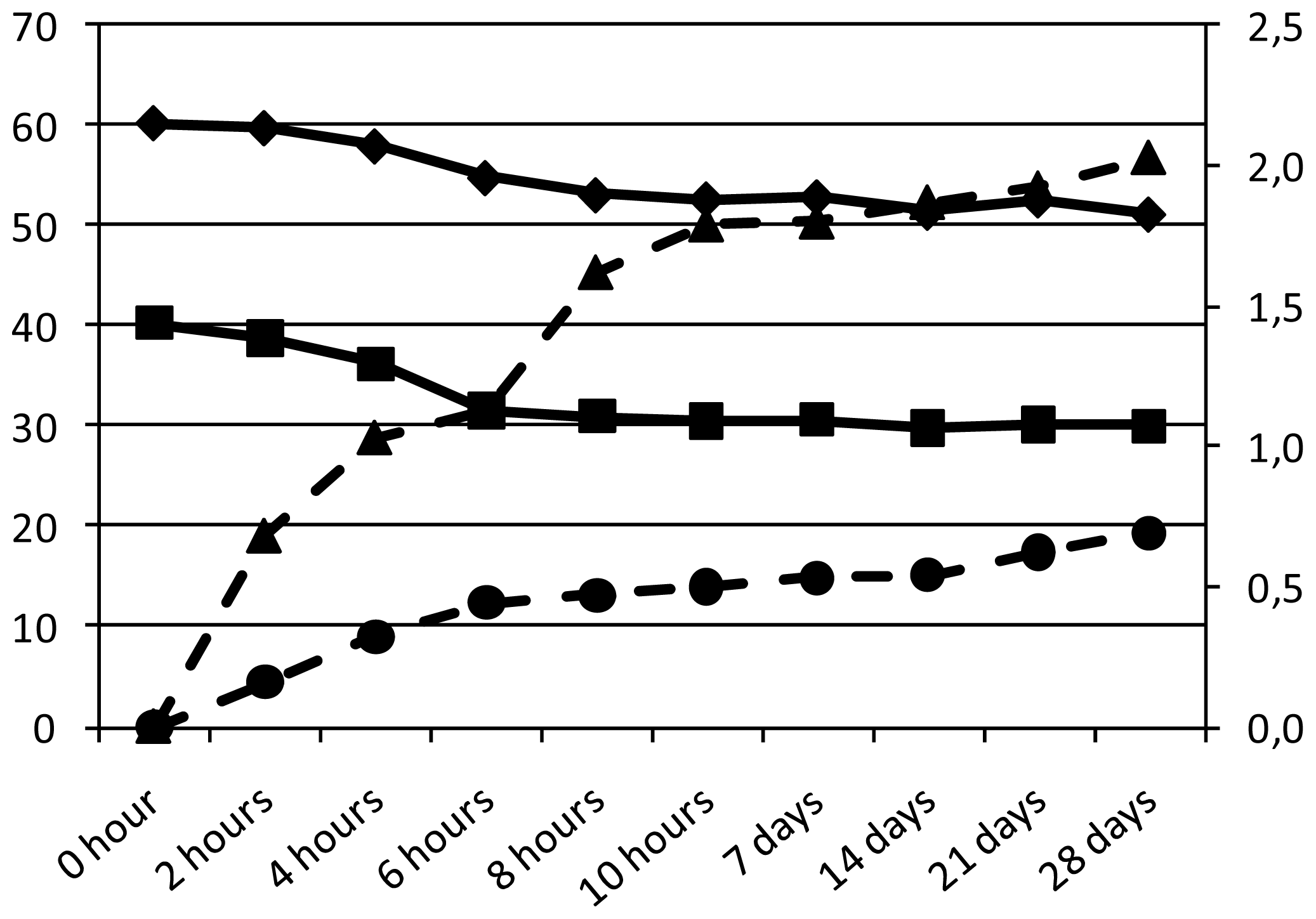
| pH | Acidity a | Bacterial count b | Sugar c | Organic acid c | Caffeine d | Antioxidant activity e | |||
|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Lactic | Acetic | ||||||
| 0 h | 6.65 | 0.10 | 4.37 ± 3.12 | 40.2 | 60.2 | 0.00 | 0.00 | nd | nd |
| 2 h | 6.30 | 0.20 | 4.46 ± 2.15 | 38.7 | 59.7 | 0.68 | 0.16 | nd | nd |
| 4 h | 5.92 | 0.25 | 5.23 ± 3.94 | 36.2 | 57.9 | 1.03 | 0.32 | nd | nd |
| 6 h | 5.23 | 0.40 | 6.11 ± 4.18 | 31.5 | 54.8 | 1.13 | 0.44 | nd | nd |
| 8 h | 4.80 | 0.45 | 8.13 ± 3.24 | 30.9 | 53.1 | 1.62 | 0.47 | nd | nd |
| 10 h | 4.30 | 0.60 | 8.64 ± 5.14 | 30.5 | 52.7 | 1.79 | 0.50 | 6.56 | 58 ± 2 |
| 12 h | 3.67 | 0.70 | 7.69 ± 4.47 | 30.1 | 52.6 | 1.94 | 0.53 | nd | nd |
| 7 days | 4.12 | 0.60 | 8.14 ± 4.56 | 30.6 | 52.9 | 1.80 | 0.53 | 6.70 | 56 ± 3 |
| 14 days | 4,00 | 0.75 | 8.04 ± 2.98 | 29.8 | 51.4 | 1.87 | 0.54 | 6.82 | 55 ± 2 |
| 21 days | 3.67 | 1.15 | 7.90 ± 3.35 | 30.1 | 52.6 | 1.92 | 0.62 | 6.68 | 59 ± 2 |
| 28 days | 3.50 | 1.20 | 7.96 ± 4.16 | 30.0 | 51.2 | 2.03 | 0.69 | 6.70 | 58 ± 1 |
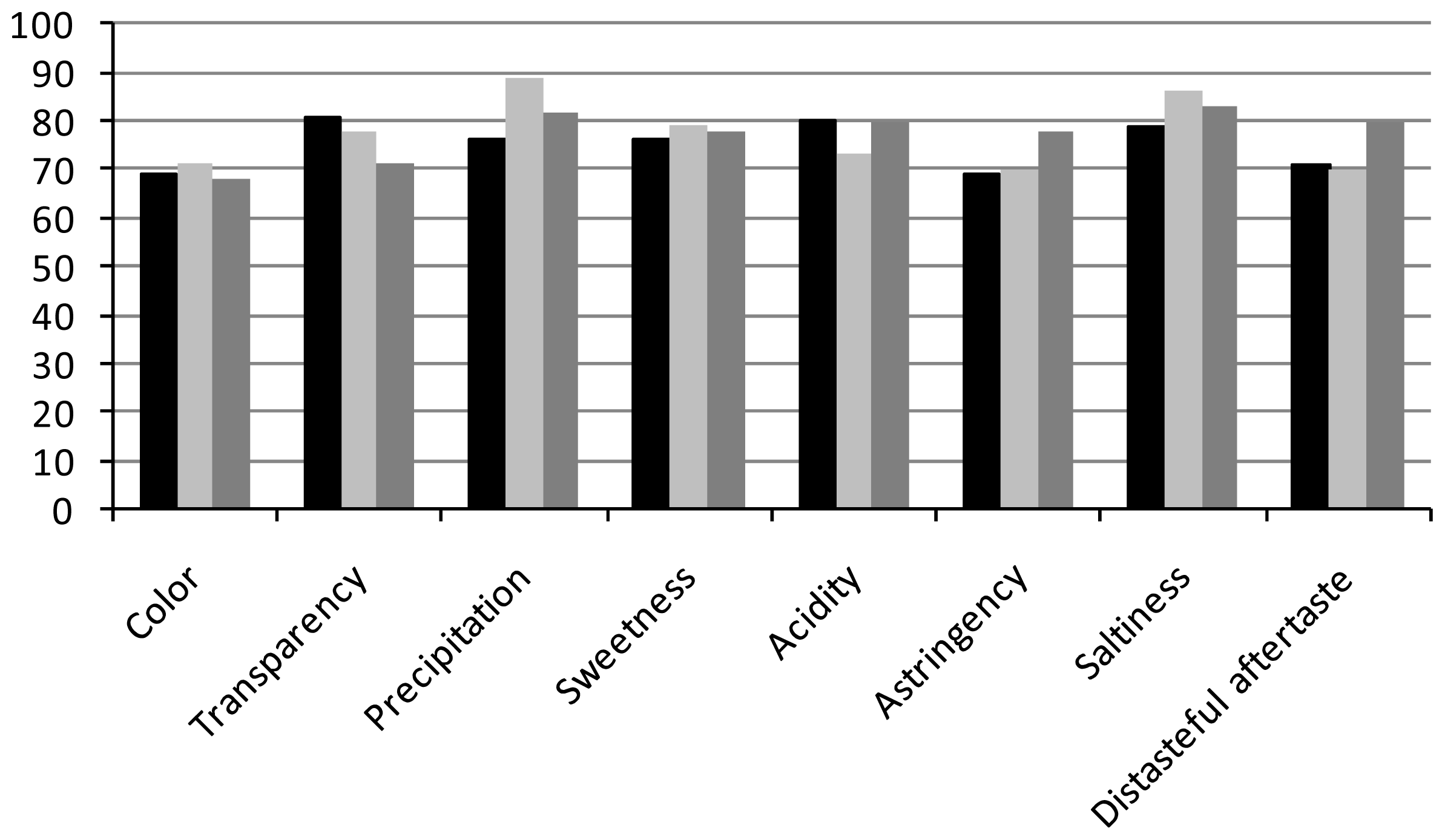
| Attributes (AF) | Commercial Mate Tea A | Commercial Mate Tea B | Probiotic Mate Beverage |
|---|---|---|---|
| Color | 6.2 ± 1.4 a (69) | 6.4 ± 1.3 a (71) | 5.4 ± 1.3 b (68) |
| Transparency | 7.3 ± 1.3 a (81) | 7.0 ± 1.2 a (78) | 5.0 ± 1.2 b (71) |
| Precipitation | 7.8 ± 1.1 a (76) | 8.0 ± 1.1 a (89) | 4.9 ± 1.3 b (82) |
| Sweetness | 6.1 ± 1.4 a (76) | 6.3 ± 1.3 a (79) | 5.5 ± 1.4 a (78) |
| Acidity | 6.4 ± 1.1 a (80) | 6.6 ± 1.3 a (73) | 5.6 ± 1.0 a (80) |
| Astringency | 6.2 ± 1.7 a (69) | 6.3 ± 1.7 a (70) | 5.5 ± 1.3 a (78) |
| Saltiness | 7.1 ± 1.1 a (79) | 6.9 ± 1.0 ab (86) | 5.8 ± 1.0 b (83) |
| Distasteful aftertaste | 6.4 ± 1.3 a (71) | 6.3 ± 1.5 b (70) | 6.4 ± 1.2 a (80) |
| Source of variation | Degree of freedom | Sum of squares | Mean of squares | F ratio |
|---|---|---|---|---|
| Color | 2 | 5.6000 | 2.8000 | 1.7571 * |
| Transparency | 2 | 31.2666 | 15.6333 | 9.8106 ** |
| Precipitation | 2 | 60.2000 | 30.1000 | 18.8890 ** |
| Sweetness | 2 | 3.4666 | 1.7333 | 1.0877 ns |
| Acidity | 2 | 5.6000 | 2.8000 | 1.7571 ns |
| Astringency | 2 | 3.8000 | 1.9000 | 1.1923 ns |
| Saltiness | 2 | 9.8000 | 4.9000 | 3.0750 * |
| Distasteful aftertaste | 2 | 0.0666 | 0.0333 | 0.0209 * |
| Residue | 216 | 344.2000 | 1.5935 | |
| Total | 232 | 464.0000 | ||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferrari Pereira Lima, I.; De Dea Lindner, J.; Soccol, V.T.; Parada, J.L.; Soccol, C.R. Development of an Innovative Nutraceutical Fermented Beverage from Herbal Mate (Ilex paraguariensis A.St.-Hil.) Extract. Int. J. Mol. Sci. 2012, 13, 788-800. https://doi.org/10.3390/ijms13010788
Ferrari Pereira Lima I, De Dea Lindner J, Soccol VT, Parada JL, Soccol CR. Development of an Innovative Nutraceutical Fermented Beverage from Herbal Mate (Ilex paraguariensis A.St.-Hil.) Extract. International Journal of Molecular Sciences. 2012; 13(1):788-800. https://doi.org/10.3390/ijms13010788
Chicago/Turabian StyleFerrari Pereira Lima, Isabela, Juliano De Dea Lindner, Vanete Thomaz Soccol, José Luiz Parada, and Carlos Ricardo Soccol. 2012. "Development of an Innovative Nutraceutical Fermented Beverage from Herbal Mate (Ilex paraguariensis A.St.-Hil.) Extract" International Journal of Molecular Sciences 13, no. 1: 788-800. https://doi.org/10.3390/ijms13010788




