Surface Photochemistry: 3,3'-Dialkylthia and Selenocarbocyanine Dyes Adsorbed onto Microcrystalline Cellulose
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ground State Diffuse Reflectance and Infrared (FTIR) Absorption Spectra
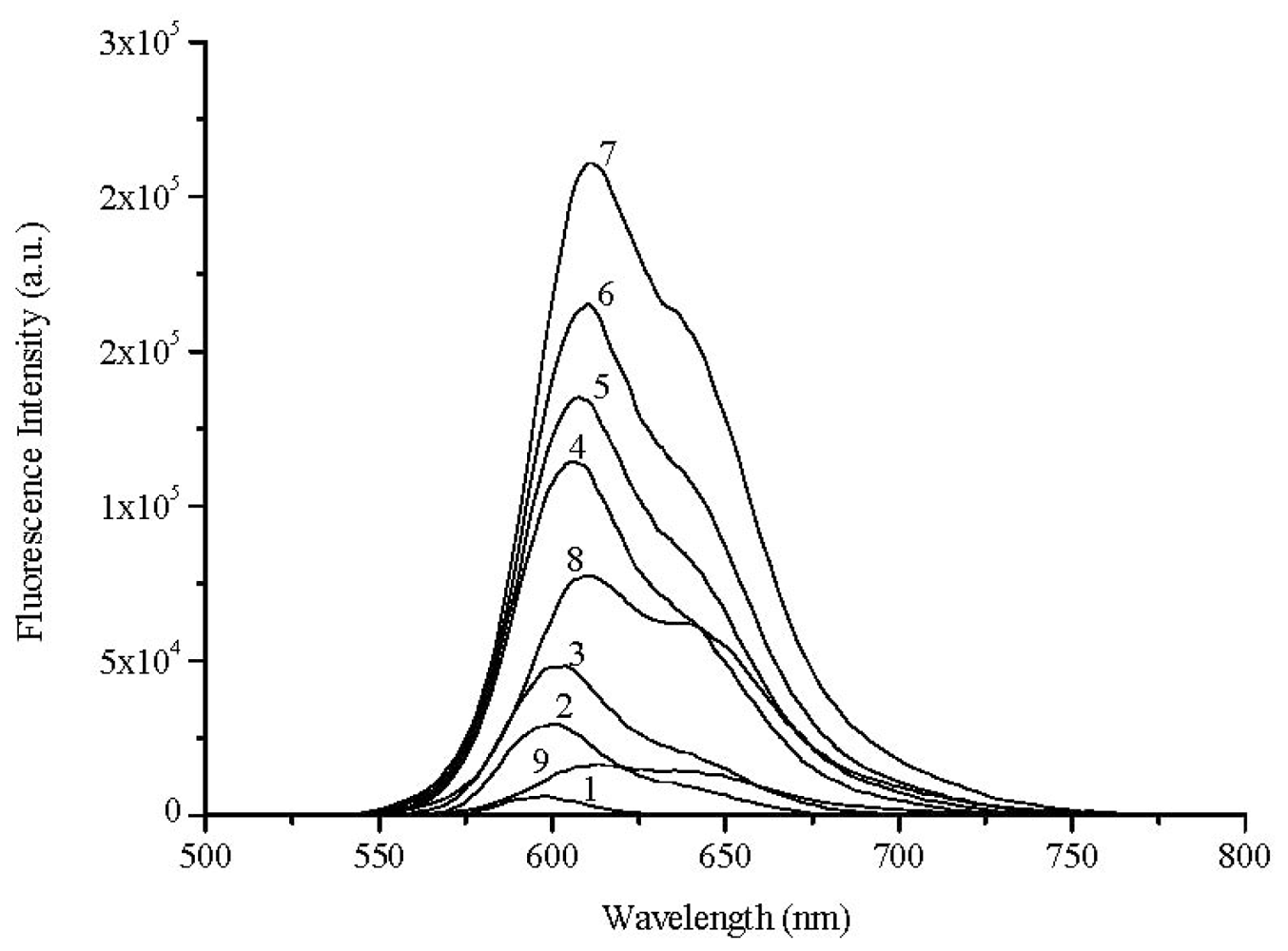
2.2. Laser Induced Fluorescence of Air Equilibrated Samples
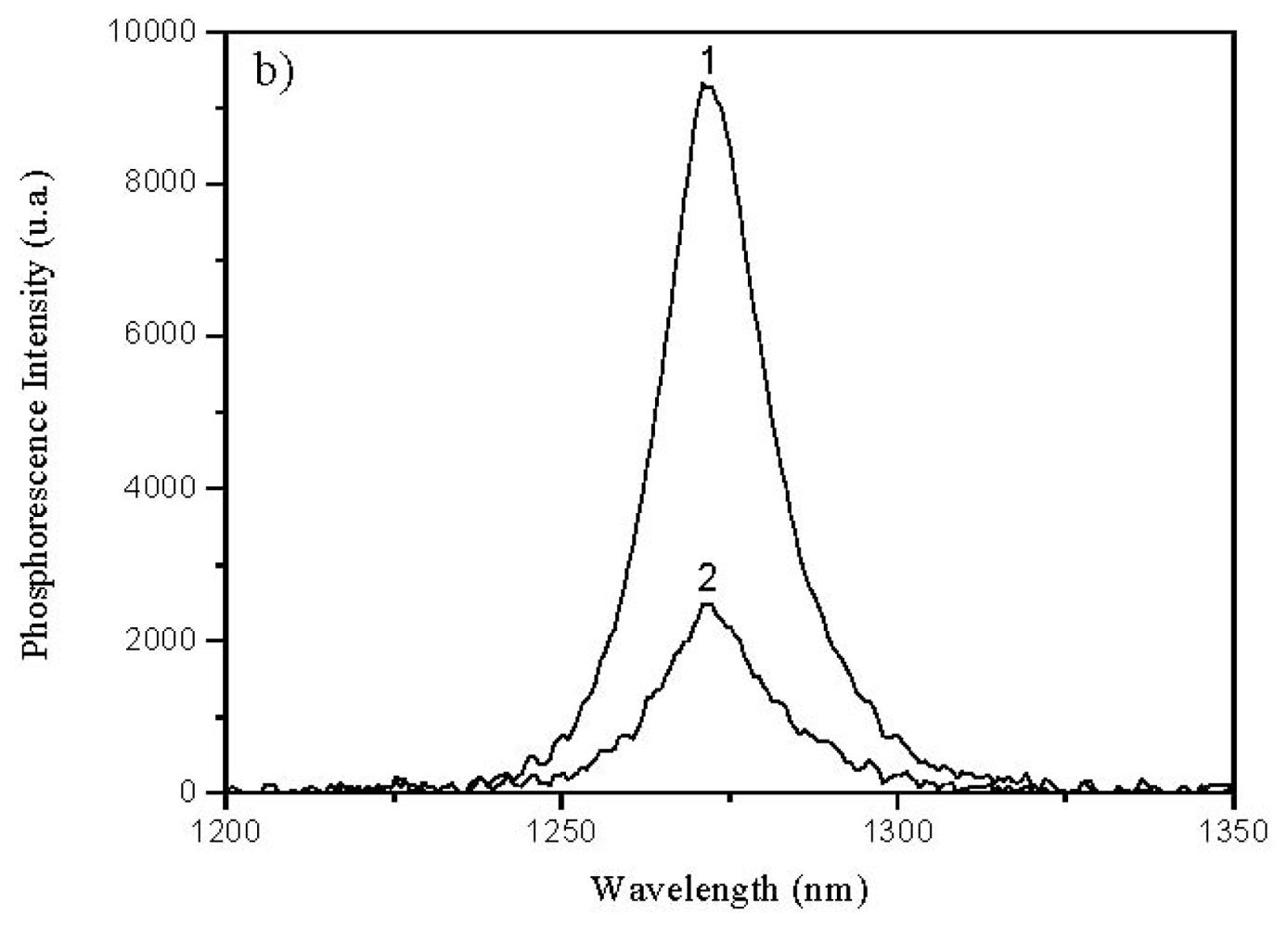
2.3. Quantum Yield of Singlet Oxygen
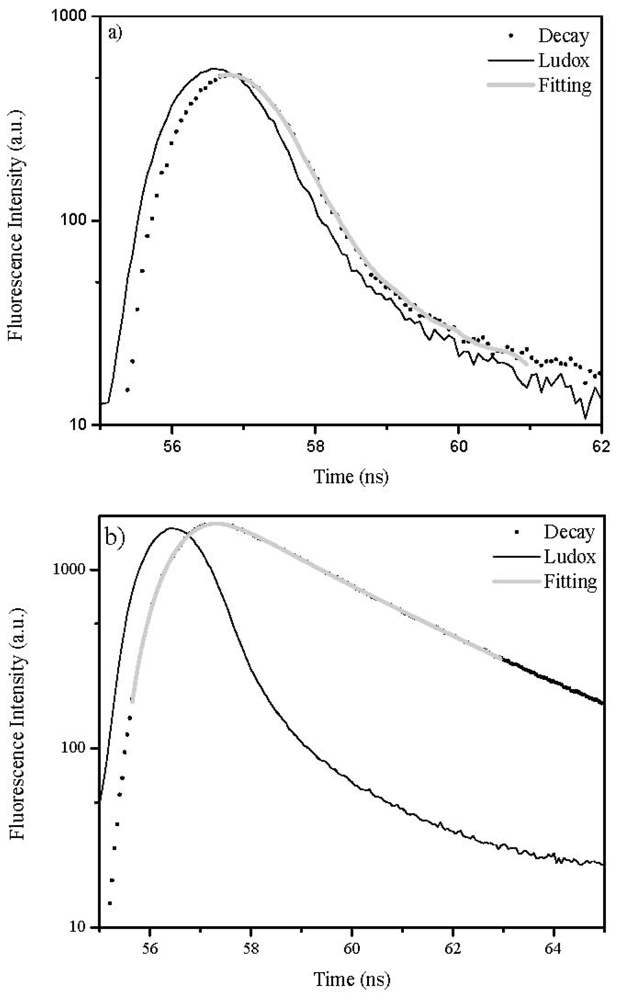
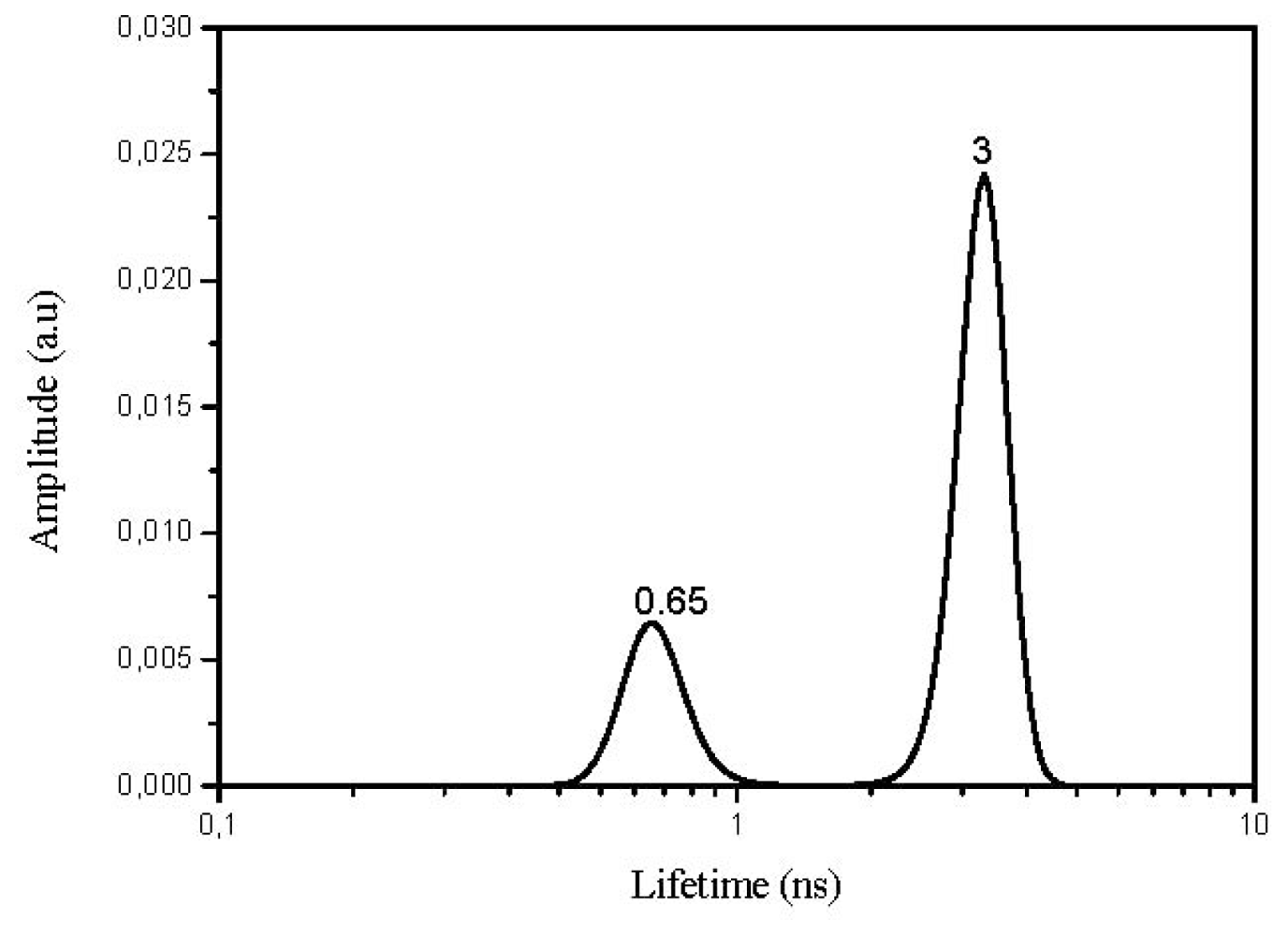
2.4. Lifetime Distribution Analysis
2.5. High Fluences of Laser Excitation
3. Experimental Section
3.1. Materials
3.2. Sample Preparation
3.3. Ground State Diffuse Reflectance Absorption Spectra (GSDR)
3.4. Absolute and Relative Fluorescence Emission Quantum Yield Determinations
3.5. Fluorescence Lifetime Determinations
3.6. Laser-Induced Luminescence (LIL)
3.7. Quantum Yield of Singlet Oxygen Determinations
4. Conclusions
Acknowledgments
References
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar]
- Salice, P.; Arnbjerg, J.; Pederson, B.W.; Toftegaard, R.; Beverina, L.; Pagani, G.A.; Ogilby, P.R. Photophysics of squaraine dyes: Role of charge transfer in singlet oxygen production and removal. J. Phys. Chem. A 2010, 114, 2518–2525. [Google Scholar]
- Aramendia, P.F.; Negri, R.M.; San Roman, E. Temperature dependence of fluorescence and photoisomerization in symmetric carbocyanines. Influence of medium viscosity and molecular structure. J. Phys. Chem 1994, 98, 3165–3173. [Google Scholar]
- Krieg, M.; Redmond, R.W. Photophysical properties of 3,3′-Dialkylthiacarbocyanine dyes in homogeneous solution. Photochem. Photobiol 1993, 57, 472–479. [Google Scholar]
- Vieira Ferreira, L.F.; Oliveira, A.S.; Wilkinson, F.; Worrall, D.R. A new emission from aggregates of 2,2′-Cyanine dyes adsorbed onto microcrystalline Cellulose. J. Chem. Soc. Faraday Trans 1996, 92, 1217–1225. [Google Scholar]
- Oliveira, A.S.; Vieira Ferreira, L.F.; Worrall, D.R.; Wilkinson, F. Photophysics of oxacyanine dyes on surfaces: Re-examination of the origins of the ‘new emission’ observed with laser excitation and high concentrations of adsorbed dyes. J. Chem. Soc. Faraday Trans 1996, 23, 4809–4814. [Google Scholar]
- Pais, I.R.; Nunes, M.J.; Reis, L.V.; Santos, P.F.; Almeida, P. The synthesis of chloroheptamethinecyanine dyes in the absence of water. Dyes Pigments 2008, 77, 48–52. [Google Scholar]
- Santos, P.F.; Reis, L.V.; Duarte, I.; Serrano, J.P.; Almeida, P.; Oliveira, A.S.; Vieira Ferreira, L.F. Synthesis and photochemical evaluation of iodinated Squarylium Cyanine dyes. Helv. Chim. Acta 2005, 88, 1135–1143. [Google Scholar]
- Dempster, D.N.; Morrow, T.; Rankin, R.; Thompson, G.F. Photochemical characteristics of cyanine dyes. Part 1—3,3′-Diethyloxadicarbocyanine iodide and 3,3′-Diethylothiadicarbocyanine iodide. J. Chem. Soc. Faraday Trans. 1972, 1479–1496. [Google Scholar]
- Vieira Ferreira, L.F.; Netto-Ferreira, J.C.; Kmelinskii, I.V.; Garcia, A.R.; Costa, S.M.B. Photochemistry on Surfaces: Matrix isolation mechanisms for the study of interactions of Benzophenone adsorbed on Microcrystalline Cellulose investigated by diffuse reflectance and luminescence techniques. Langmuir 1995, 11, 231–236. [Google Scholar]
- Botelho do Rego, A.M.; Vieira Ferreira, L.F. Nalwa, H., Ed.; Photonic and Electronic Spectroscopies for the Characterization of Organic Surfaces and Organic Molecules Adsorbed on Surfaces. In Handbook of Surfaces and Interfaces of Materials; Academic Press: Maryland Heights, MO, USA, 2001; Volume 2, Chapter 7; pp. 275–313. [Google Scholar]
- Vieira Ferreira, L.F.; Ferreira Machado, I.L. Surface Photochemistry: Organic molecules within nanocavities of Calixarenes. Curr. Drug. Discov. Technol 2007, 4, 229–245. [Google Scholar]
- Vieira Ferreira, L.F.; Ferreira Machado, I.; Da Silva, J.P.; Oliveira, A.S. A diffuse reflectance comparative study of Benzil inclusion within Microcrystalline Cellulose and β-Cyclodextrin. Photochem. Photobiol. Sci 2004, 3, 174–181. [Google Scholar]
- Vieira Ferreira, L.F.; Cabral, P.V.; Almeida, P.; Oliveira, A.S.; Reis, M.J.; Botelho do Rego, A.M. UV/VIS absorption and luminescence and X-Ray Photoelectron Spectroscopic studies of Rhodamine B covalently bound to Microcrystalline Cellulose. Macromolecules 1998, 31, 3936–3944. [Google Scholar]
- Oliveira, A.S.; Almeida, P.; Vieira Ferreira, L.F. Photophysics of cyanine dyes on surfaces: Laser-induced photoisomer emission of 3,3-dialkylthiacarbocyanines adsorbed on microcrystalline cellulose. Collect. Czech. Chem. Commun 1999, 64, 459–473. [Google Scholar]
- Kuzmin, V.A.; Darmanyan, A.P. Study of sterically hindered short-lived isomers of polymerthine dyes by laser photolysis. Chem. Phys. Lett 1978, 54, 159–163. [Google Scholar]
- Rentsh, S.; Danielus, R.; Gadonas, R. Bestimmung von lebensdauern und transientabsorptionsspektren von polymethynfarbstoffen aus pikosekunden spektroskopischen messungen. J. Signalaufz.-Mater 1984, 12, 319–328. [Google Scholar]
- Lagorio, M.G.; Dicelio, L.E.; Litter, M.I.; San Roman, E. Modeling of fluorescence quantum yields of supported dyes—Aluminium carboxyphthalocyanine on cellulose. J. Chem. Soc. Faraday Trans 1998, 94, 419–425. [Google Scholar]
- Branco, T.J.F.; Botelho do Rego, A.M.; Ferreira Machado, I.; Vieira Ferreira, L.F. A luminescence lifetime distributions analysis in heterogeneous systems by the use of Excel’s Solver. J. Phys. Chem. B 2005, 109, 15958–15967. [Google Scholar]
- Vieira Ferreira, L.F.; Duarte, P.; Ferreira, D.; Ferreira Machado, I.; Oliveira, A.S.; Prukała, D.; Sikorski, M. Surface Photochemistry: p-Hydroxystilbazol within nanochannels of Na+ and H+ ZSM-5 Zeolites. Micropor. Mesopor. Mater 2012, 151, 317–324. [Google Scholar]
- Hamer, F.M. A general method for the preparation of carbocyanine dyes. J. Chem. Soc 1927, 2796–2804. [Google Scholar]
- Barbieri, A.; Accorsi, G. Absolute Photoluminescence Quantum Yield Determination of Solid- State Samples. In Technical Report from EPA Newsletter; Francis Perrin Laboratory: Gif-sur-Yvette, France, December 2006. [Google Scholar]
- Mello, J.C.; Wittman, H.F.; Friend, R.H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater 1997, 9, 230–232. [Google Scholar]
- Rurack, K.; Spieles, M. Fluorescence quantum yields of a series of NIR dyes emitting at 600–1000 nm. Anal. Chem 2011, 83, 1232–1242. [Google Scholar]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshiara, T.; Hishida, H.; Shiina, Y.; Oishi, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating shere and a back-tinned CCD detector. Phys. Chem. Chem. Phys 2009, 11, 9850–9860. [Google Scholar]
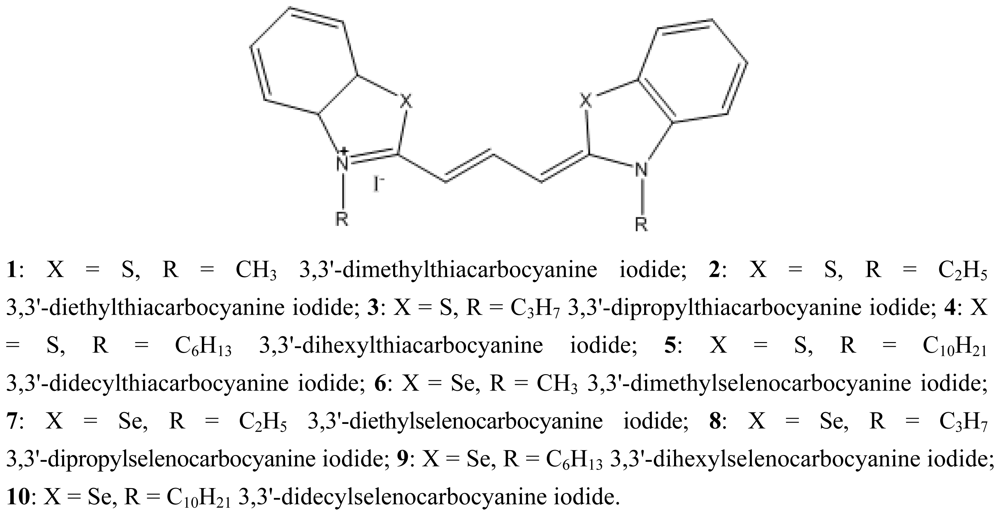




| Dye | ΦF in CHCl3 | Dye | ΦF in CHCl3 |
|---|---|---|---|
| 1 (std.) | 0.042 | 1 (std.) | 0.042 |
| 1 | 0.029 | 6 | 0.023 |
| 2 | 0.042 | 7 | 0.038 |
| 3 | 0.046 | 8 | 0.044 |
| 4 | 0.046 | 9 | 0.033 |
| 5 | 0.040 | 10 | 0.034 |
| Dye | ΦF abs | Dye | ΦF abs |
|---|---|---|---|
| 1 | 0.99 | 6 | 0.55 |
| 2 | 0.73 | 7 | 0.54 |
| 3 | 0.80 | 8 | 0.35 |
| 4 | 0.67 | 9 | 0.38 |
| 5 | 0.53 | 10 | 0.21 |
| Dye | ΦΔ | Dye | ΦΔ |
|---|---|---|---|
| Phenazine | 0.84 | Phenazine | 0.84 |
| 1 | 0.11 | 6 | 0.23 |
| 2 | 0.14 | 7 | 0.21 |
| 3 | 0.15 | 8 | 0.25 |
| 4 | 0.10 | 9 | 0.21 |
| 5 | 0.12 | 10 | 0.21 |
| Dye | τsolution (ns) | χ2 | τ1 (solid) (ns) | τ2 (solid) (ns) | χ2 |
|---|---|---|---|---|---|
| 1 | 0.20 | 1.80 | 3.78 | 0.53 | 1.56 |
| 2 | 0.32 | 1.11 | 3.7 | 0.56 | 1.49 |
| 3 | 0.39 | 1.17 | 3.53 | 0.84 | 1.04 |
| 4 | 0.35 | 1.59 | 3.37 | 0.82 | 1.15 |
| 5 | 0.31 | 0.83 | 3.22 | 0.43 | 1.14 |
| 6 | 0.20 | 1.27 | 3.45 | 0.74 | 1.14 |
| 7 | 0.30 | 1.89 | 4.55 | 0.10 | 1.39 |
| 8 | 0.33 | 1.58 | 3.37 | 0.71 | 1.38 |
| 9 | 0.27 | 1.52 | 3.07 | 0.57 | 1.43 |
| 10 | 0.27 | 1.60 | 3 | 0.65 | 1.06 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vieira Ferreira, L.F.; Ferreira, D.P.; Duarte, P.; Oliveira, A.S.; Torres, E.; Ferreira Machado, I.; Almeida, P.; Reis, L.V.; Santos, P.F. Surface Photochemistry: 3,3'-Dialkylthia and Selenocarbocyanine Dyes Adsorbed onto Microcrystalline Cellulose. Int. J. Mol. Sci. 2012, 13, 596-611. https://doi.org/10.3390/ijms13010596
Vieira Ferreira LF, Ferreira DP, Duarte P, Oliveira AS, Torres E, Ferreira Machado I, Almeida P, Reis LV, Santos PF. Surface Photochemistry: 3,3'-Dialkylthia and Selenocarbocyanine Dyes Adsorbed onto Microcrystalline Cellulose. International Journal of Molecular Sciences. 2012; 13(1):596-611. https://doi.org/10.3390/ijms13010596
Chicago/Turabian StyleVieira Ferreira, Luís F., Diana P. Ferreira, Paulo Duarte, A. S. Oliveira, E. Torres, I. Ferreira Machado, P. Almeida, Lucinda V. Reis, and Paulo F. Santos. 2012. "Surface Photochemistry: 3,3'-Dialkylthia and Selenocarbocyanine Dyes Adsorbed onto Microcrystalline Cellulose" International Journal of Molecular Sciences 13, no. 1: 596-611. https://doi.org/10.3390/ijms13010596






