Studies on Bioflocculant Production by Arthrobacter sp. Raats, a Freshwater Bacteria Isolated from Tyume River, South Africa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identity of the Bacteria
2.2. Effect of Culture Conditions on Bioflocculant Production
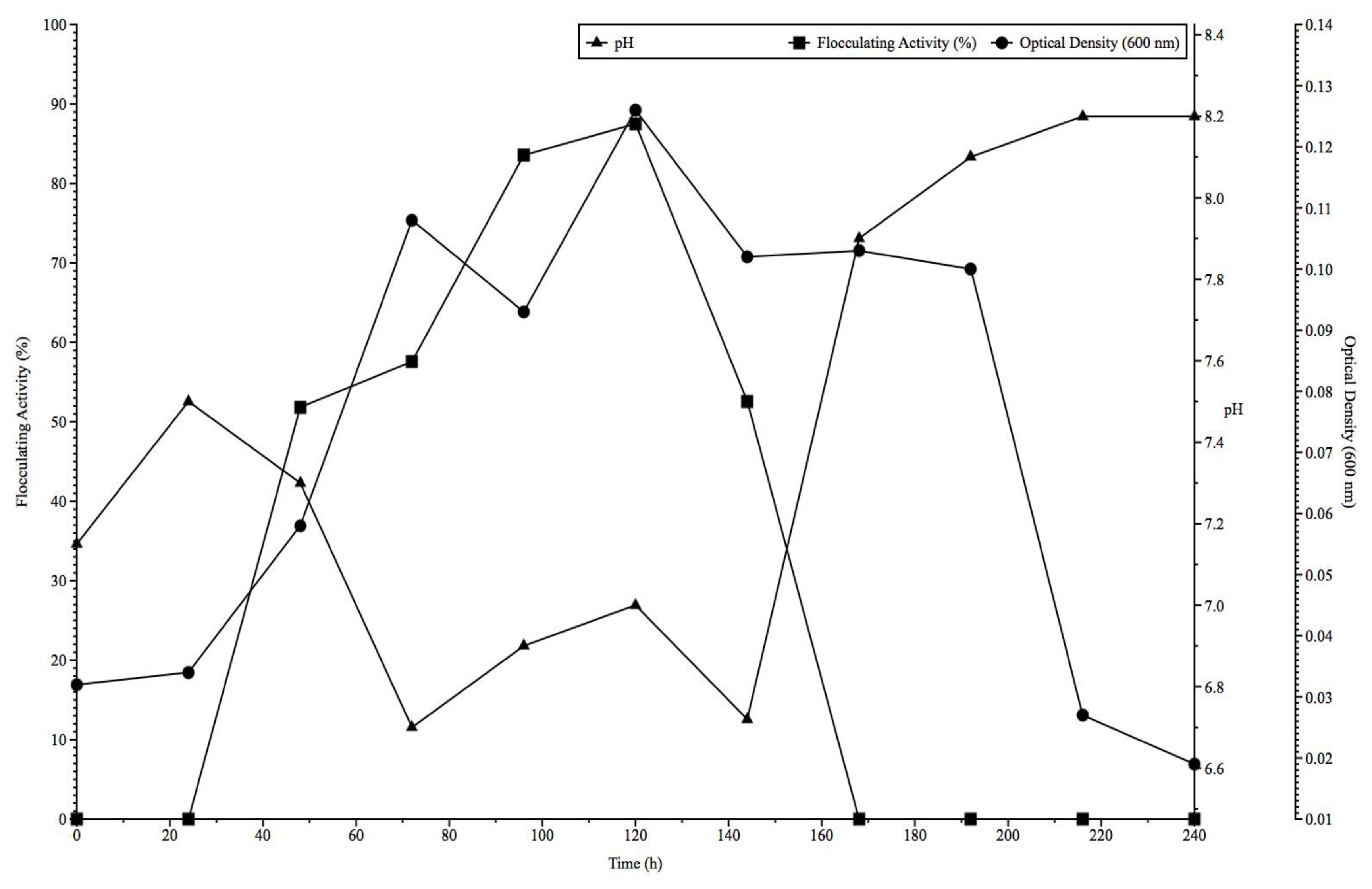
2.3. Time Course Assay of Bioflocculant Production
2.4. Chemical Analysis of Bioflocculant Composition
3. Experimental Section
3.1. Test Bacterium and Cultivation Conditions
3.2. Determination of Flocculating Activity
3.3. Effect of Carbon and Nitrogen Sources on Bioflocculant Production
3.4. Effect of Various Cations and pH on Bioflocculant Production
3.5. Time Course Assay of Bioflocculant Production
3.6. Extraction, Purification and Characterization of the Bioflocculant
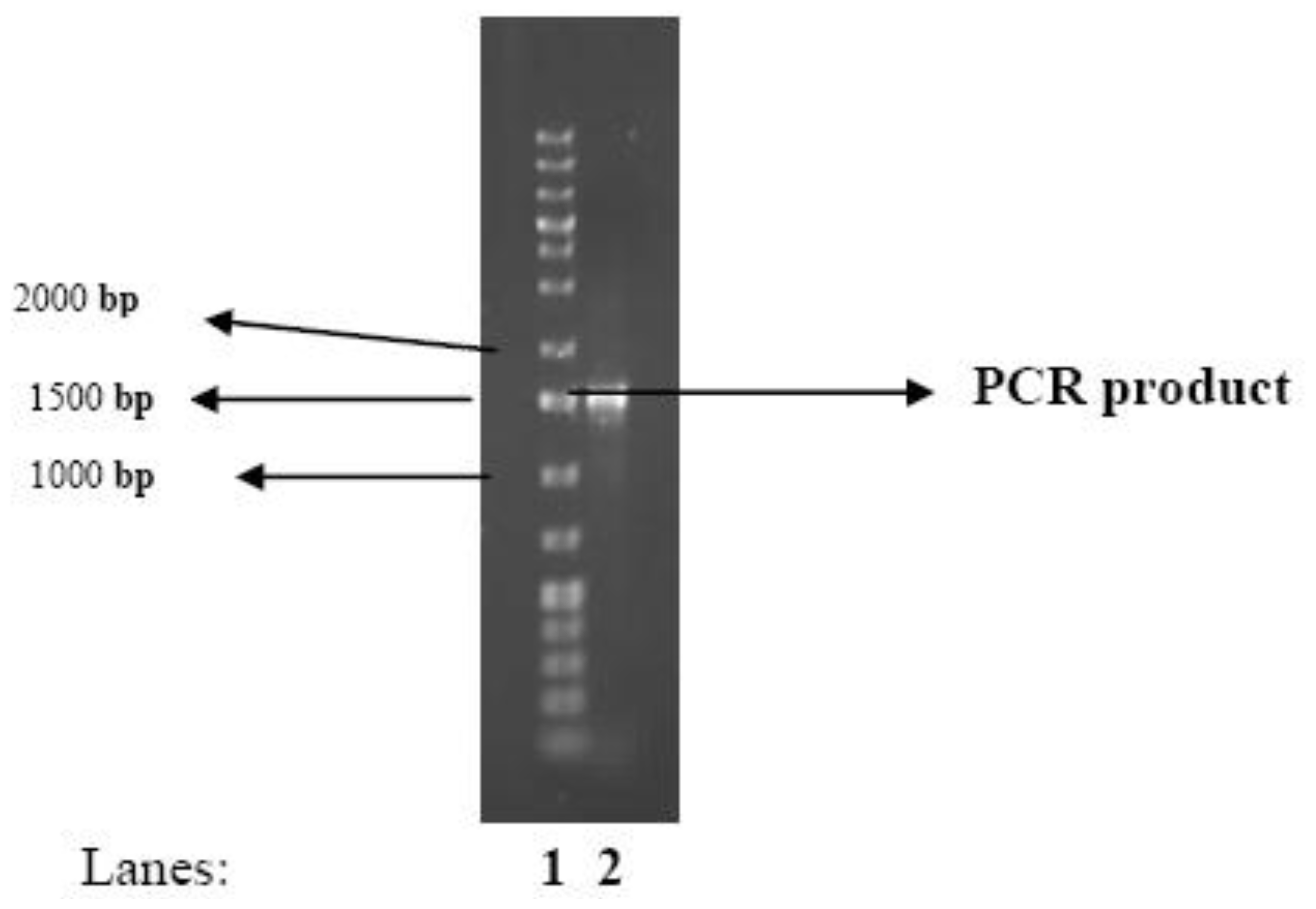
3.7. Identification of the Bioflocculant-Producing Microorganism
DNA extraction
PCR Amplification
4. Conclusions
Acknowledgments
References
- Salehizadeh, H.; Shojaosadati, S.A. Extracellular biopolymeric flocculants: Recent trends and biotechnology importance. Biotechnol. Adv 2001, 19, 371–385. [Google Scholar]
- Zheng, Y.; Ye, Z.-L.; Fang, X.-L.; Li, Y.-H.; Cai, W.-M. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour. Technol 2008, 99, 7686–7691. [Google Scholar]
- Li, Z.; Zhong, S.; Lei, H.-Y.; Chen, R.-W.; Yu, Q.; Li, H.-L. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresour. Technol 2009, 100, 3650–3656. [Google Scholar]
- Wang, L.; Ma, F.; Qu, Y.; Sun, D.; Li, A.; Guo, J.; Yu, B. Characterization of a compound bioflocculant produced by mixed culture of Rhizobium radiobacter F2 and Bacillus sphaeicus F6. World J. Microbiol. Biotechnol 2011, 27, 2559–2565. [Google Scholar]
- Feng, D.L.; Xu, S.H. Characterization of biofloculant MBF3-3 produced by an isolated Bacillus sp. World J. Microbiol. Biotechnol 2008, 24, 1627–1632. [Google Scholar]
- Wu, J.; Ye, H.-F. Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtillis DYU1 isolate. Process Biochem 2007, 42, 1114–1123. [Google Scholar]
- Li, Y.; He, N.; Guan, H.; Du, G.; Chen, J. A novel polygalacturonic acid bioflocculant REA-11 produced by Corynebacterium glutamicum: A proposed biosynthetic pathway and experimental confirmation. Appl. Microbiol. Biotechnol 2003, 100, 3650–3656. [Google Scholar]
- Wu, H.; Li, Q.; Lu, R.; Wang, Y.; Zhuang, X.; He, N. Fed-batch production of a bioflocculant from Corynebacterium glutamicum. J. Ind. Microbiol. Biotechnol 2010, 37, 1203–1209. [Google Scholar]
- Eschbach, M.; Möbitz, H.; Rompf, A.; Jahn, D. Members of the genus Arthrobacter grow anaerobically using nitrate ammonification and fermentative processes: Anaerobic adaptation of aerobic bacteria abundant in soil. FEMS Microbiol. Lett 2003, 223, 227–230. [Google Scholar]
- Piñar, G.; Ramos, J.L. A strain of Arthrobacter that tolerates high concentrations of nitrate. Biodegradation 1998, 8, 393–399. [Google Scholar]
- Shen, M.; Zeng, Y.-G.; Shen, Y.-C. Isolation and characterization of a novel Arthrobacter nitroguajacolicus ZJUTB06-99, capable of converting acrylonitrile to acrylic acid. Process Biochem 2009, 44, 781–785. [Google Scholar]
- Su, X.; Shen, X.; Ding, L.; Yokota, A. Study on the flocculability of the Arthrobacter sp., an actinomycete resuscitated from the VBNC state. World J. Microbiol. Biotechnol 2011. [Google Scholar] [CrossRef]
- He, N.; Li, Y.; Chen, J. Production of a polygalacturonic acid bioflocculant REA-11 by Corynebacterium glutamicum. Bioresour. Technol 2004, 100, 99–105. [Google Scholar]
- Patil, S.V.; Salunkhe, R.B.; Patil, C.D.; Patil, D.M.; Salunke, B.K. Bioflocculant exopolysaccharide production by Azotobacter indicus using flower extract of Madhuca latifolia L. Appl. Biochem. Biotechnol 2010, 162, 1095–1108. [Google Scholar]
- He, J.; Zhen, Q.; Qiu, N.; Liu, Z.; Wang, B.; Shao, Z.; Yu, Z. Medium optimization for the production of a novel bioflocculant from Halomonas sp. V3a’ using response surface methodology. Bioresour. Technol 2009, 100, 5922–5927. [Google Scholar]
- Piyo, N.; Cosa, S.; Mabinya, V.L.; Okoh, I.A. Assessment of bioflocculant production by Bacillus sp. Gilbert, a marine bacterium isolated from the bottom sediment of Algoa Bay. Mar. Drugs 2011, 9, 1232–1242. [Google Scholar]
- Cosa, S.; Mabinya, L.V.; Olaniran, A.O.; Okoh, O.O.; Bernard, K.; Deyzel, S.; Okoh, A.I. Bioflocculant production by Virgibacillus sp. Rob isolated from the bottom sediment of Algoa Bay in the Eastern Cape, South Africa. Molecules 2011, 16, 2431–2442. [Google Scholar]
- Yokoi, H.; Hirose, J.; Hayashi, S.; Takasaki, Y. Simultaneous production of hydrogen and bioflocculant by Enterobacter sp. BY-29. World J. Microbiol. Biotechnol 2001, 17, 609–613. [Google Scholar]
- Sam, S.; Kucukasik, F.; Yenigun, O.; Nicolaus, B.; Oner, E.B.; Yukselen, M.A. Flocculating performances of exopolysaccharides produced by a halophilic bacterial strain cultivated on agro-industrial waste. Bioresour. Technol 2011, 102, 1788–1794. [Google Scholar]
- Gong, W.-X.; Wang, S.-G.; Sun, X.-F.; Liu, X.-W.; Yue, Q.-Y.; Gao, B.-Y. Bioflocculant production by culture of Serratia ficaria and its application in wastewater treatment. Bioresour. Technol 2008, 99, 4668–4674. [Google Scholar]
- Xia, S.; Zhang, Z.; Wang, X.; Yang, A.; Chen, L.; Zhao, J.; Leonard, D.; Jaffrezic-Renault, N. Production and characterization of bioflocculant by Proteus mirabilis TJ-1. Bioresour. Technol 2008, 99, 6520–6527. [Google Scholar]
- Gao, J.; Bao, H.-Y.; Xin, M.-X.; Liu, Y.-X.; Li, Q.; Zhang, Y.-F. Characterization of a bioflocculant from a newly isolated Vagococcus sp. W31. J. Zhejiang Univ. Sci. B 2006, 7, 186–192. [Google Scholar]
- He, J.; Zou, J.; Shao, Z.; Zhang, J.; Liu, Z.; Yu, Z. Characteristics and flocculating mechanism of a novel bioflocculant HBF-3 produced by deep-sea bacterium mutant Halomonas sp. V3a’. World J. Microbiol. Biotechnol 2010, 26, 1135–1141. [Google Scholar]
- Li, X.-M.; Yang, Q.; Huang, K.; Zeng, G.-M.; Liao, D.-X.; Liu, J.-J.; Long, W.-F. Screening and characterization of a bioflocculant produced by Aeromonas sp. Biomed. Environ. Sci 2007, 20, 274–278. [Google Scholar]
- Li, W.W.; Zhou, W.Z.; Zhang, W.Z.; Wang, J.; Zhu, X.B. Flocculation behaviour and mechanism of an exopolysaccharide from the deep-sea psychrophilic bacterium Pseudoalteromonas sp. SM9913. Bioresour. Technol 2008, 99, 6893–6899. [Google Scholar]
- Yokoi, H.; Arima, T.; Hirose, J.; Hayashi, S.; Takasaki, Y. Flocculation properties of poly (γ-glutamic acid) produced by Bacillus subtilis. J. Ferment. Bioeng 1996, 82, 84–87. [Google Scholar]
- Bouchotroch, S.; Quesada, E.; Del Moral, A.; Llamas, I.; Béjar, V. Halomonas maura sp. nov., a novel moderately halophilic, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbiol 2001, 51, 1625–1632. [Google Scholar]
- Choi, C.W.; Yoo, S.-A.; Oh, I.-H.; Park, S.H. Characterization of an extracellular flocculating substance produced by a planktonic cyanobacterium, Anabaena sp. Biotechnol. Lett 1998, 20, 643–646. [Google Scholar]
- Yokoi, H.; Natsuda, O.; Hirose, J.; Hayashi, S.; Takasaki, Y. Characteristics of a biopolymer flocculant produced by Bacillus sp. PY-90. J. Ferment. Bioeng 1995, 79, 378–380. [Google Scholar]
- Jang, J.-H.; Ike, M.; Kim, S.M.; Fujita, M. Production of a novel bioflocculant by fed-batch culture of Citrobacter sp. Biotechnol. Lett 2001, 23, 593–597. [Google Scholar]
- Kallos, M.S.; Behie, L.A. Inoculation and growth conditions for high-cell-density expansion of mammalian neural stem cells in suspension bioreactors. Biotechnol. Bioeng 1999, 63, 473–483. [Google Scholar]
- Shih, I.L.; Van, Y.T.; Yeh, L.C.; Lin, H.G.; Chang, Y.N. Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour. Technol 2001, 78, 267–272. [Google Scholar]
- Lors, C.; Chehade, M.H.; Damidot, D. pH variations during growth of Acidithiobacillus thiooxidans in buffered media designed for an assay to evaluate concrete biodeterioration. Int. Biodeterior. Biodegrad 2009, 63, 880–883. [Google Scholar]
- Kovárová-Kovar, K.; Egli, T. Growth kinetics of suspended microbial cells: From single-substrate-controlled growth, to mixed-substrate kinetics. Microbiol. Mol. Biol. Rev 1998, 62, 646–666. [Google Scholar]
- Inoue, K.; Korenaga, H.; Kadoya, S. A sulfated polysaccharide produced by an Arthrobacter species. J. Biochem 1982, 92, 1775–1784. [Google Scholar]
- Zhang, Z.-Q.; Lin, B.; Xia, S.-Q.; Wang, X.-J.; Yang, A.-M. Production and application of a novel bioflocculant by multiple microorganism consortia using brewery wastewater as carbon source. J. Environ. Sci 2007, 19, 667–673. [Google Scholar]
- Kurane, R.; Takeda, K.; Suzuki, T. Screening and characteristics of microbial flocculants. Agric. Biol. Chem 1986, 50, 2301–2307. [Google Scholar]
- Lachhwani, P. Studies on Polymeric Bioflocculant Producing Microorganisms. Master Thesis, Thapar Institute of Engineering and Technology, Patiala, India, 2005. [Google Scholar]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodiumimpudicum KG03. Bioresour. Technol 2006, 98, 361–367. [Google Scholar]
- Cook, A.E.; Meyers, P.R. Rapid identification of filamentous actinomycetes to the genus level using genus-specific 16 S rRNA gene restriction fragment patterns. Int. J. Syst. Evol. Microbiol 2003, 53, 1907–1915. [Google Scholar]

| Carbon Source | Lactose | Sucrose | Fructose | Starch |
| Flocculating Activity % | 75.4 | 73.4 | 2.8 | 2.1 |
| Nitrogen Source | Ammonium Chloride | Ammonium Sulphate | Urea | |
| Flocculating Activity % | 60.9 | 78.5 | 83.4 | |
| Cations | Ferric Chloride | Magnesium Chloride | Ferrous Sulphate | Potassium Chloride |
| Flocculating Activity % | - | 76.9 | - | 2.8 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mabinya, L.V.; Cosa, S.; Nwodo, U.; Okoh, A.I. Studies on Bioflocculant Production by Arthrobacter sp. Raats, a Freshwater Bacteria Isolated from Tyume River, South Africa. Int. J. Mol. Sci. 2012, 13, 1054-1065. https://doi.org/10.3390/ijms13011054
Mabinya LV, Cosa S, Nwodo U, Okoh AI. Studies on Bioflocculant Production by Arthrobacter sp. Raats, a Freshwater Bacteria Isolated from Tyume River, South Africa. International Journal of Molecular Sciences. 2012; 13(1):1054-1065. https://doi.org/10.3390/ijms13011054
Chicago/Turabian StyleMabinya, Leonard V., Sekelwa Cosa, Uchechukwu Nwodo, and Anthony I. Okoh. 2012. "Studies on Bioflocculant Production by Arthrobacter sp. Raats, a Freshwater Bacteria Isolated from Tyume River, South Africa" International Journal of Molecular Sciences 13, no. 1: 1054-1065. https://doi.org/10.3390/ijms13011054
APA StyleMabinya, L. V., Cosa, S., Nwodo, U., & Okoh, A. I. (2012). Studies on Bioflocculant Production by Arthrobacter sp. Raats, a Freshwater Bacteria Isolated from Tyume River, South Africa. International Journal of Molecular Sciences, 13(1), 1054-1065. https://doi.org/10.3390/ijms13011054





