Effect of Low Concentration Sn Doping on Optical Properties of CdS Films Grown by CBD Technique
Abstract
:1. Introduction
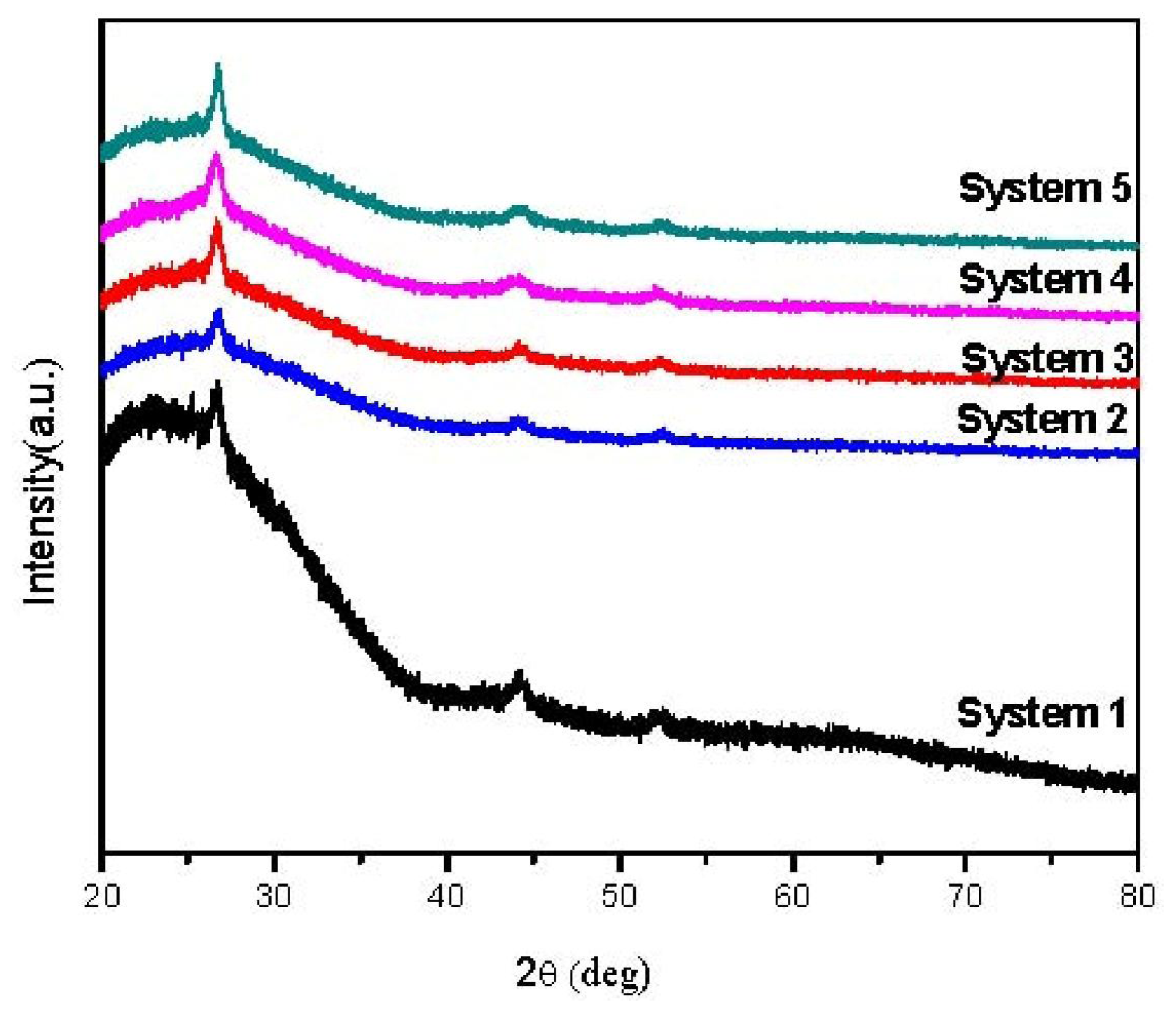
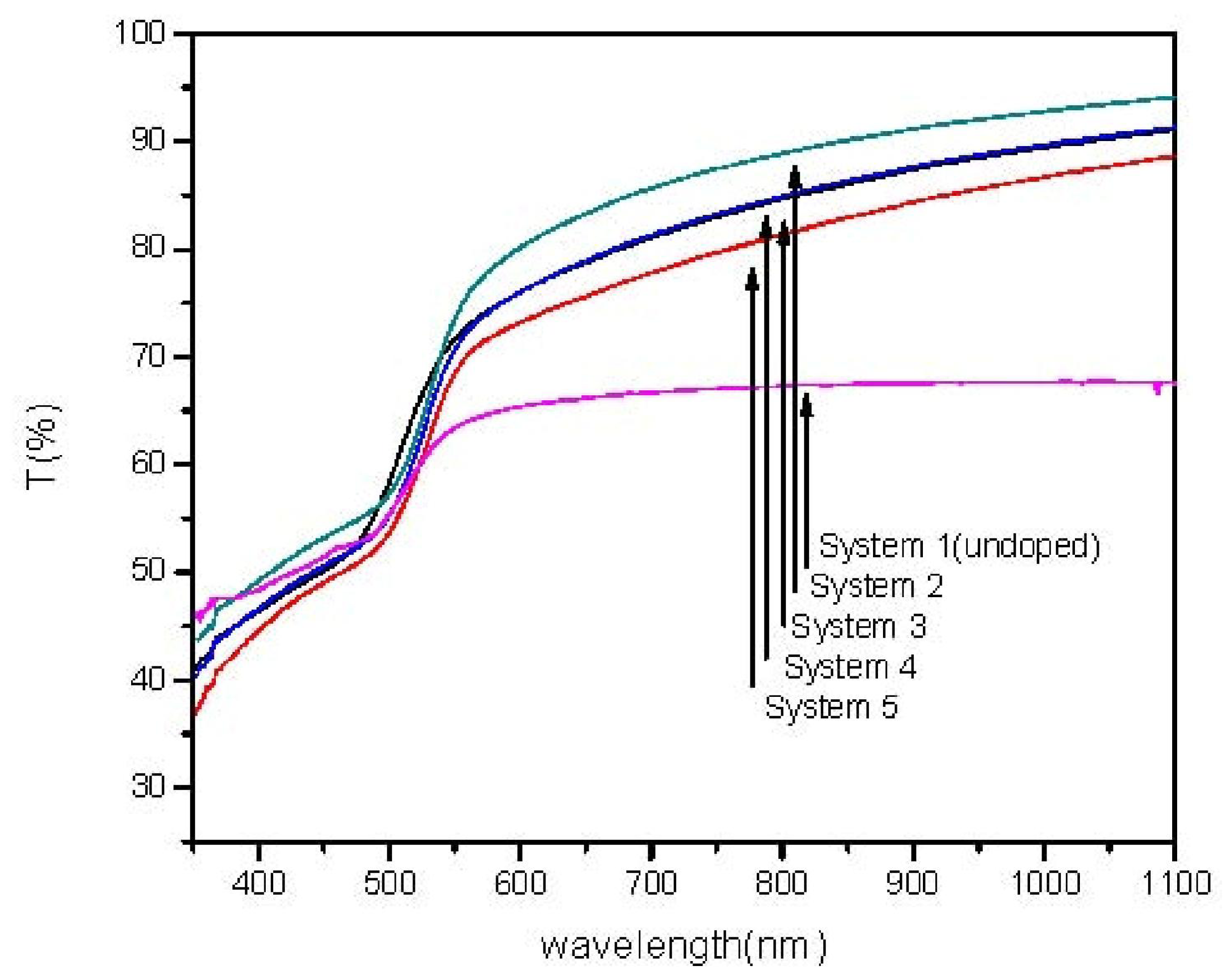
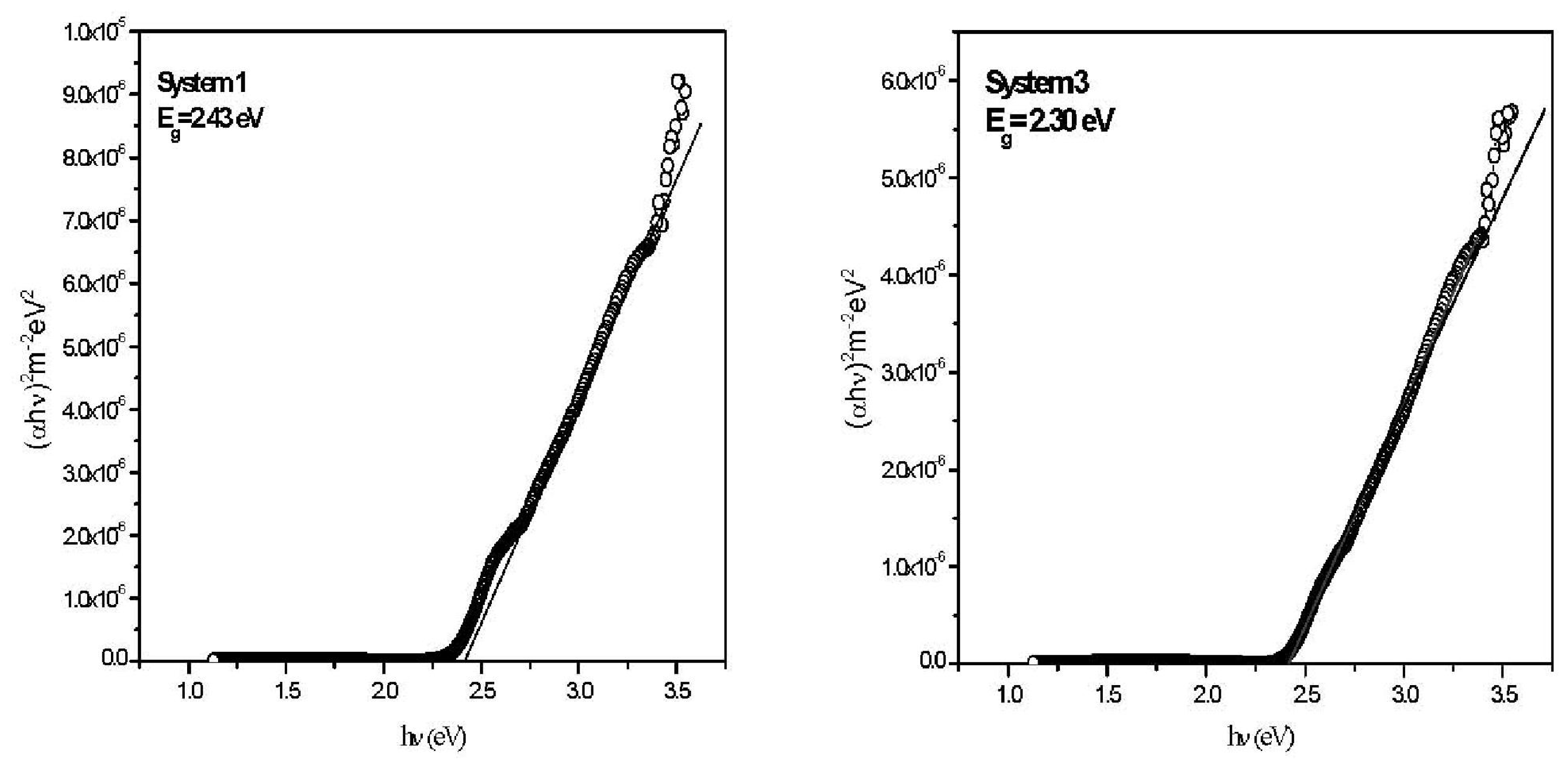
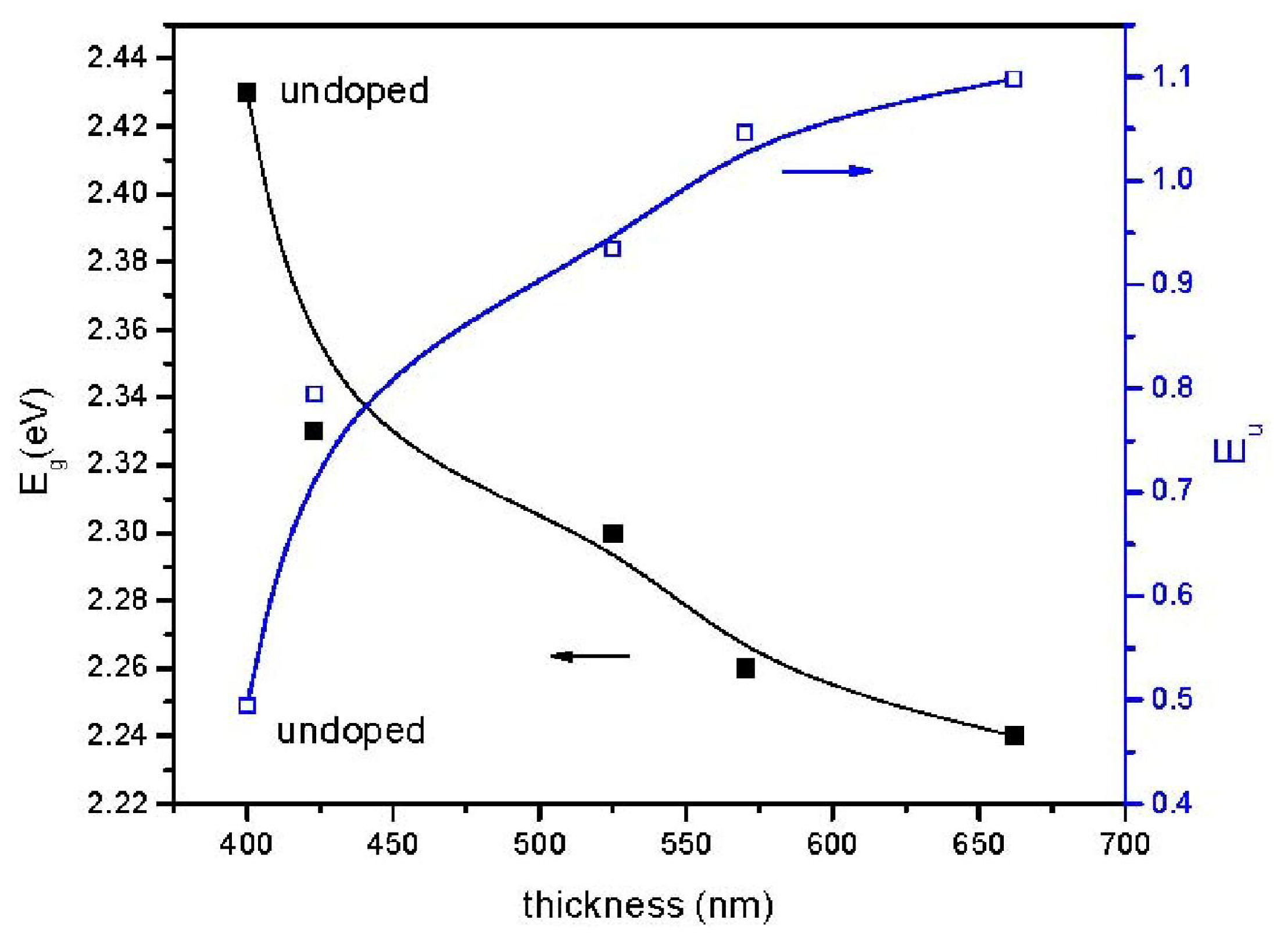
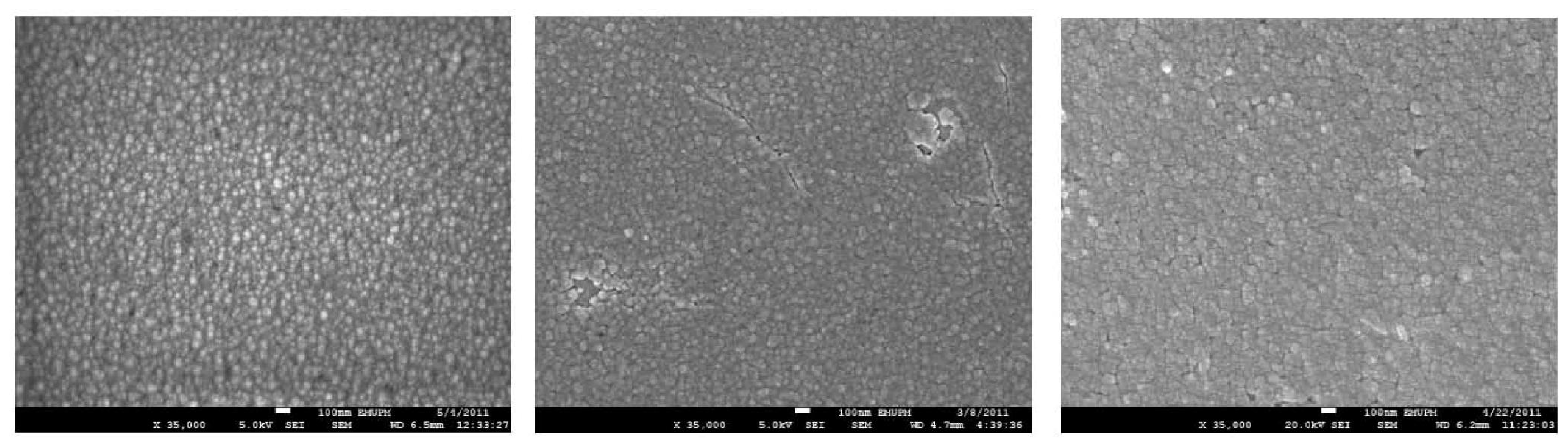
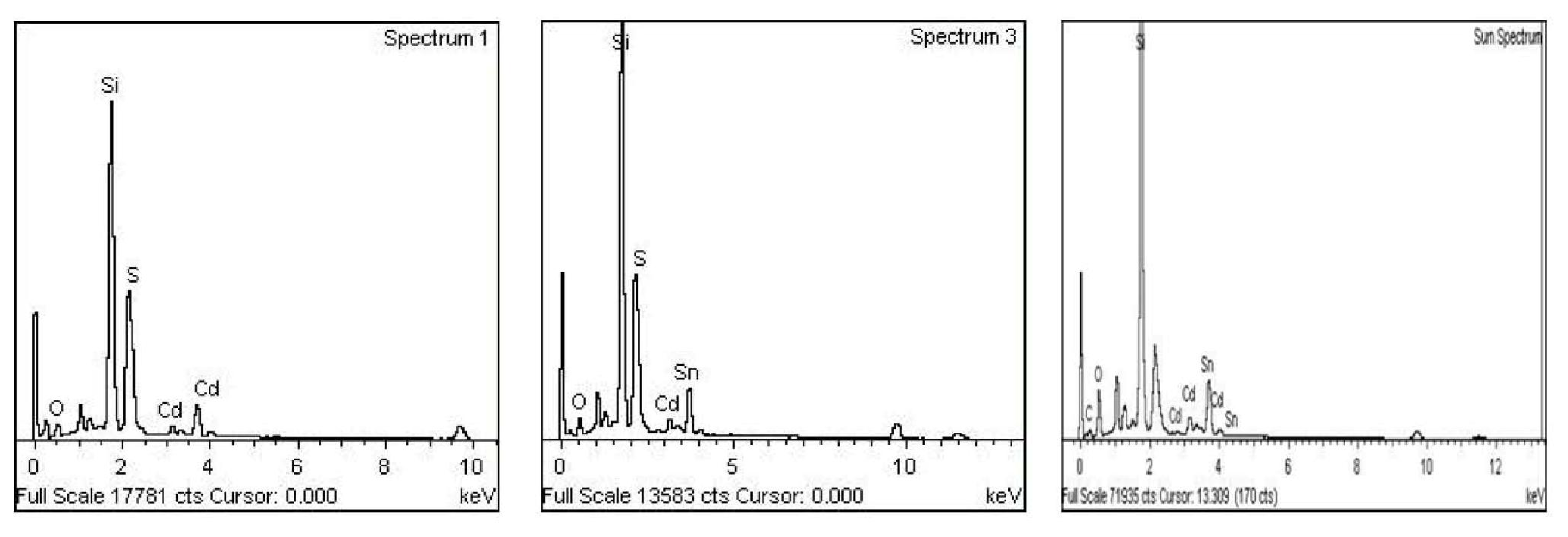
2. Results and Discussion
3. Experiment
4. Conclusions
Acknowledgments
References
- Schropp, REI; Zeman, M. Amorphous and Microcrystalline Silicon Solar Cells: Modelling. In Materials and Device Technology; Springer: Berlin, Germany, 1998. [Google Scholar]
- Zhang, H; Ma, X; Ji, Y; Xu, J; Yang, D. Single crystalline CdS nanorods fabricated by a novel hydrothermal method. Chem Phys Lett 2003, 377, 654–657. [Google Scholar]
- Palm, J; Probst, V; Stetter, W; Toelle, R; Visbeck, S; Calwer, H; Niesen, T; Vogt, H; Hernandez, O; Wendl, M; et al. CIGSSe thin films modules PV: From fundamental investigations to advance performance and stability. Thin Solid Films 2004, 451–452, 544–551. [Google Scholar]
- Punnoose, A; Marafi, M; Prabu, G; Elakkad, F. CdS thin films prepared by RF magnetron sputtering in Ar atmosphere. Phys States Solid 2000, 177, 453–458. [Google Scholar]
- Bertino, MF; Gadipalli, RR; Story, JG; Williams, CG; Zhang, G; Sotiriou-Leventis, C; Tokuhiro, AT; Guha, S; Leventis, N. Laser writing of semiconductor nanoparticles and quantum dots. Appl Phys Lett 2004, 85, 6007–6009. [Google Scholar]
- Liu, Q; Mao, G. Comparison of CdS and ZnS thin films prepared by Chemical Bath Deposition. Surf Rev Lett 2009, 16, 469–474. [Google Scholar]
- Garadkar, KM; Patil, AA; Korake, PV; Hankare, PP. Characterization of CdS thin films synthesized by SILAR method at room temperature. Arch Appl Sci Res 2010, 2, 429–437. [Google Scholar]
- Hodes, G. Chemical Solution Deposition of Semiconductor Films; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Altosaar, M; Ernits, K; Krustok, J; Varema, T; Raudoja, J; Mellikovc, E. Comparison of CdS films deposited from chemical baths containing different doping impurities. Thin Solid Films 2005, 480–481, 147–150. [Google Scholar]
- Çetinörgü, E; Gümüs, C; Esen, R. Effect of deposition time and temperature on the optical properties of air-annealed chemical bath deposited CdS films. Thin Solid Films 2006, 515, 1688–1693. [Google Scholar]
- Petre, D; Pintilie, I; Pentia, E; Pintilie, I; Botila, T. The influence of Cu doping on opto-electronic properties of chemically deposited CdS. Mater Sci Eng B 1999, 58, 238–243. [Google Scholar]
- Khallaf, H; Chai, G; Lupan, O; Chow, L; Park, S; Schulte, A. Characterization of gallium-doped CdS thin films grown by chemical bath deposition. Appl Surf Sci 2009, 255, 4129–4134. [Google Scholar]
- Davila-Pintle, JA; Lozada-Morales, RL; Palomino-Merino, MR; Rivera-Márquez, JA; Portillo-Moreno, OP; Zelaya-Angel, OZ. Electrical properties of Er-doped CdS thin films. J Appl Phys 2007, 101, 013712. [Google Scholar]
- Roy, P; Srivastava, SK. In situ deposition of Sn-doped CdS thin films by chemical bath deposition and their characterization. J Phys D 2009, 39, 4771–4776. [Google Scholar]
- Nair, PK; Daza, OG; Roadigos, AAC; Campos, J; Nair, MTS. Formation of conductive CdO layer on CdS thin films during air heating. Semicond Sci Technol 2001, 16, 651–656. [Google Scholar]
- Khallaf, H; Oladeji, I; Chai, G; Chow, L. Characterization of CdS thin films grown by chemical bath deposition using four different cadmium sources. Thin Solid Films 2008, 516, 7306–7312. [Google Scholar]
- Vanalakar, SA; Mali, SS; Suryavanshi, MP; Patil, PS. Quantum size effect in chemosynthesized nanostructured CdS thin films. Dig J Nanomater Biostruct 2010, 5, 805–810. [Google Scholar]
- Liu, F; Lai, Y; Liu, J; Wang, B; Kuang, S; Zhang, Z; Li, J; Liu, Y. Characterization of chemical bath deposited CdS thin films at different deposition temperature. J Alloys Compd 2010, 493, 305–308. [Google Scholar]
- Quiebrasa, JN; Puentea, GC; Moralesa, GR; Rodríguez, GS; Acevedo, AM. Properties of CdS thin films grown by CBD as a function of thiourea concentration. Sol Energy Mater Sol Cells 2006, 90, 727–732. [Google Scholar]
- Morris, GC; Vanderveen, R. Cadmium sulphide films prepared by pulsed electrodeposition. Sol Energy Mater Sol Cells 1992, 27, 305–319. [Google Scholar]
- Rakhshani, AE. Study of Urbach tail, bandgap energy and grain-boundary characteristics in CdS by modulated photocurrent. J Phys Condens Matter 2000, 12, 4391–4400. [Google Scholar]

| System | Volume (mL)
| Molar ratio
| ||||
|---|---|---|---|---|---|---|
| CdSO4 | Thiourea | SnCl2 | Cd | S | Sn | |
| 1 (undoped) | 50 | 50 | 0 | 5 | 1 | 0 |
| 2 | 50 | 50 | 1 | 5 | 1 | 0.003 |
| 3 | 50 | 50 | 3 | 5 | 1 | 0.018 |
| 4 | 50 | 50 | 5 | 5 | 1 | 0.030 |
| 5 | 50 | 50 | 7 | 5 | 1 | 0.042 |
| System | d spacing of plane (111) (JCPDS) (Å) | d spacing of plane (111) (Å) | Crystallite size (nm) |
|---|---|---|---|
| 1 (undoped) | 3.35 | 3.35 | 18.06 |
| 2 | 3.35 | 3.33 | 21.68 |
| 3 | 3.36 | 3.32 | 27.10 |
| 4 | 3.35 | 3.31 | 36.14 |
| 5 | 3.35 | 3.30 | 37.09 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jafari, A.; Zakaria, A.; Rizwan, Z.; Ghazali, M.S.M. Effect of Low Concentration Sn Doping on Optical Properties of CdS Films Grown by CBD Technique. Int. J. Mol. Sci. 2011, 12, 6320-6328. https://doi.org/10.3390/ijms12096320
Jafari A, Zakaria A, Rizwan Z, Ghazali MSM. Effect of Low Concentration Sn Doping on Optical Properties of CdS Films Grown by CBD Technique. International Journal of Molecular Sciences. 2011; 12(9):6320-6328. https://doi.org/10.3390/ijms12096320
Chicago/Turabian StyleJafari, Atefeh, Azmi Zakaria, Zahid Rizwan, and Mohd Sabri Mohd Ghazali. 2011. "Effect of Low Concentration Sn Doping on Optical Properties of CdS Films Grown by CBD Technique" International Journal of Molecular Sciences 12, no. 9: 6320-6328. https://doi.org/10.3390/ijms12096320




