Variable Frequencies of Apolipoprotein E Genotypes and Its Effect on Serum Lipids in the Guangxi Zhuang and Han Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Data Collection
2.2. Laboratory Analysis
2.3. APOE Genotyping
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
References
- Anoop, S; Misra, A; Meena, K; Luthra, K. Apolipoprotein E polymorphism in cerebrovascular & coronary heart diseases. Indian J Med Res 2010, 132, 363–378. [Google Scholar]
- Zhou, TB; Qin, YH; Lei, FY; Su, LN; Zhao, YJ; Huang, WF. All-trans retinoic acid regulates the expression of apolipoprotein E in rats with glomerulosclerosis induced by Adriamycin. Exp Mol Pathol 2011, 90, 287–294. [Google Scholar]
- Hu, P; Qin, YH; Jing, CX; Lei, FY; Chen, P; Li, MF. Association of polymorphisms at restriction enzyme recognition sites of apolipoprotein B and E gene with dyslipidemia in children undergoing primary nephrotic syndrome. Mol Biol Rep 2009, 36, 1015–1021. [Google Scholar]
- Mahley, RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar]
- Zhou, X; Li, D; Yan, W; Li, W. Pravastatin prevents aortic atherosclerosis via modulation of signal transduction and activation of transcription 3 (STAT3) to attenuate interleukin-6 (IL-6) action in ApoE knockout mice. Int J Mol Sci 2008, 9, 2253–2264. [Google Scholar]
- Davignon, J; Gregg, RE; Sing, CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 1988, 8, 1–21. [Google Scholar]
- Utermann, G; Steinmetz, A; Weber, W. Genetic control of human apolipoprotein E polymorphism: Comparison of one- and two-dimensional techniques of isoprotein analysis. Hum Genet 1982, 60, 344–351. [Google Scholar]
- Hu, P; Qin, YH; Lu, L; Hu, B; Jing, CX; Lei, FY; Li, MF. Genetic variation of apolipoprotein E does not contribute to the lipid abnormalities secondary to childhood minimal change nephrotic syndrome. Int Urol Nephrol 2010, 42, 453–460. [Google Scholar]
- Garcés, C; Cantos, M; Benavente, M; Granizo, JJ; Cano, B; Viturro, E; de Oya, M. Variations in APOE genotype distribution in children from areas with different adult cardiovascular disease mortality in Spain. Hum Biol 2004, 76, 615–621. [Google Scholar]
- Hu, P; Qin, YH; Jing, CX; Lu, L; Hu, B; Du, PF. Does the geographical gradient of ApoE4 allele exist in China? A systemic comparison among multiple Chinese populations. Mol Biol Rep 2011, 38, 489–494. [Google Scholar]
- Singh, PP; Singh, M; Mastana, SS. APOE distribution in world populations with new data from India and the UK. Ann. Hum Biol 2006, 33, 279–308. [Google Scholar]
- Luthra, K; Bharghav, B; Chabbra, S; Das, N; Misra, A; Agarwal, DP; Pandey, RM; Srivastava, LM. Apolipoprotein E polymorphism in Northern Indian patients with coronary heart disease: Phenotype distribution and relation to serum lipids and lipoproteins. Mol Cell Biochem 2002, 232, 97–102. [Google Scholar]
- Mendes-Lana, A; Pena, GG; Freitas, SN; Lima, AA; Nicolato, RL; Nascimento-Neto, RM; Machado-Coelho, GL; Freitas, RN. Apolipoprotein E polymorphism in Brazilian dyslipidemic individuals: Ouro Preto study. Braz J Med Biol Res 2007, 40, 49–56. [Google Scholar]
- Liang, S; Pan, M; Geng, HH; Chen, H; Gu, LQ; Qin, XT; Qian, JJ; Zhu, JH; Liu, CF. Apolipoprotein E polymorphism in normal Han Chinese population: Frequency and effect on lipid parameters. Mol Biol Rep 2009, 36, 1251–1256. [Google Scholar]
- Yang, J. An analysis of the longevous population in Bama. Chin J Popul Sci 1992, 4, 351–356. [Google Scholar]
- Schwab, US; Callaway, JC; Erkkilä, AT; Gynther, J; Uusitupa, MI; Järvinen, T. Effects of hempseed and flaxseed oils on the profile of serum lipids, serum total and lipoprotein lipid concentrations and haemostatic factors. Eur J Nutr 2006, 45, 470–477. [Google Scholar]
- Yin, R; Wang, Y; Chen, G; Lin, W; Yang, D; Pan, S. Lipoprotein lipase gene polymorphism at the PvuII locus and serum lipid levels in Guangxi Hei Yi Zhuang and Han populations. Clin Chem Lab Med 2006, 44, 1416–1421. [Google Scholar]
- Ruixing, Y; Fengping, H; Shangling, P; Dezhai, Y; Weixiong, L; Tangwei, L; Yuming, C; Jinzhen, W; Limei, Y; Jiandong, H. Prevalence of hyperlipidemia and its risk factors for the middle-aged and elderly in the Guangxi Hei Yi Zhuang and Han populations. J Investig Med 2006, 54, 191–200. [Google Scholar]
- Yin, R; Pan, S; Wu, J; Lin, W; Yang, D. Apolipoprotein E gene polymorphism and serum lipid levels in the Guangxi Hei Yi Zhuang and Han populations. Exp Biol Med (Maywood) 2008, 233, 409–418. [Google Scholar]
- Bairaktari, E; Hatzidimou, K; Tzallas, C; Vini, M; Katsaraki, A; Tselepis, A; Elisaf, M; Tsolas, O. Estimation of LDL cholesterol based on the Friedewald formula and on apo B levels. Clin Biochem 2000, 33, 549–555. [Google Scholar]
- Ciulla, TA; Sklar, RM; Hauser, SL. A simple method for DNA purification from peripheral blood. Anal Biochem 1988, 174, 485–488. [Google Scholar]
- Seet, WT; Mary Anne, TJ; Yen, TS. Apolipoprotein E genotyping in the Malay, Chinese and Indian ethnic groups in Malaysia-a study on the distribution of the different apoE alleles and genotypes. Clin Chim Acta 2004, 340, 201–205. [Google Scholar]
- Yasuda, N; Kimura, M. A gene-counting method of maximum likelihood for estimating gene frequencies in ABO and ABO-like systems. Ann Hum Genet 1968, 31, 409–420. [Google Scholar]
- McGill, HC, Jr; McMahan, CA; Herderick, EE; Malcom, GT; Tracy, RE; Strong, JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 2000, 72, 1307S–1315S. [Google Scholar]
- Hu, P; Qin, YH; Hu, B; Lu, L. Hypervariability in a minisatellite 3′ of the apolipoprotein B gene: Allelic distribution and influence on lipid profiles in Han Children from central China. Clin Chim Acta 2010, 411, 2092–2096. [Google Scholar]
- Xin, XY; Song, YY; Ma, JF; Fan, CN; Ding, JQ; Yang, GY; Chen, SD. Gene polymorphisms and risk of adult early-onset ischemic stroke: A meta-analysis. Thromb Res 2009, 124, 619–624. [Google Scholar]
- Mayila, W; Fang, MW; Cheng, ZH; Qiu, CC. Polymorphism of apolipoprotein E gene and natural longevity in the Xinjiang Uighur people: An association study. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2005, 22, 462–463. [Google Scholar]
- Pablos-Méndez, A; Mayeux, R; Ngai, C; Shea, S; Berglund, L. Association of apo E polymorphism with plasma lipid levels in a multiethnic elderly population. Arterioscler Thromb Vasc Biol 1997, 17, 3534–3541. [Google Scholar]
- Schwanke, CH; da Cruz, IB; Leal, NF; Scheibe, R; Moriguchi, Y; Moriguchi, EH. Analysis of the association between apolipoprotein E polymorphism and cardiovascular risk factors in an elderly population with longevity. Arq Bras Cardiol 2002, 78, 561–579. [Google Scholar]
- Ruiz, JR; Labayen, I; Ortega, FB; Moreno, LA; González-Lamuño, D; Martí, A; Nova, E; Fuentes, MG; Redondo-Figuero, C; Martínez, JA; et al. AVENA Study Group. Birth weight and blood lipid levels in Spanish adolescents: Influence of selected APOE, APOC3 and PPARgamma2 gene polymorphisms. The AVENA Study. BMC Med Genet 2008, 9, 98–108. [Google Scholar]
- Nascimento, H; Silva, L; Lourenço, P; Weinfurterová, R; Castro, E; Rego, C; Ferreira, H; Guerra, A; Quintanilha, A; Santos-Silva, A; et al. Lipid profile in Portuguese obese children and adolescents: Interaction of apolipoprotein E polymorphism with adiponectin levels. Arch Pediatr Adolesc Med 2009, 163, 1030–1036. [Google Scholar]
- Smart, MC; Dedoussis, G; Louizou, E; Yannakoulia, M; Drenos, F; Papoutsakis, C; Maniatis, N; Humphries, SE; Talmud, PJ. APOE, CETP and LPL genes show strong association with lipid levels in Greek children. Nutr Metab Cardiovasc Dis 2010, 20, 26–33. [Google Scholar]
| Group | Guangxi Zhuang children (n = 278) | Guangxi Han children (n = 200) | p-value |
|---|---|---|---|
| Male/female | 168/110 | 132/68 | NS |
| Age (years) | 8.5 ± 2.4 | 8.3 ± 3.8 | NS |
| BMI (kg/m2) | 15.3 ± 4.2 | 15.8 ± 3.6 | NS |
| SBP (mmHg) | 98.6 ± 9.5 | 100.7 ± 10.2 | NS |
| DBP (mmHg) | 65.8 ± 9.1 | 66.5 ± 8.4 | NS |
| PP (mmHg) | 34.7 ± 7.9 | 37.9 ± 8.7 | NS |
| Lp(a) (mg/L) | 246.0 ± 42.7 | 277.5 ± 37.7 | NS |
| TC (mmol/L) | 4.1 ± 0.8 | 4.5 ± 0.4 | NS |
| TG (mmol/L) | 1.1 ± 0.4 | 0.9 ± 0.3 | NS |
| HDL-C (mmol/L) | 1.5 ± 0.3 | 1.4 ± 0.1 | NS |
| LDL-C (mmol/L) | 2.2 ± 0.6 | 2.1 ± 0.4 | NS |
| apoA1 (g/L) | 1.5 ± 0.3 | 1.2 ± 0.1 | NS |
| apoB (g/L) | 0.8 ± 0.3 | 0.9 ± 0.3 | NS |
| apoA1/B | 1.7 ± 0.5 | 1.4 ± 0.4 | NS |
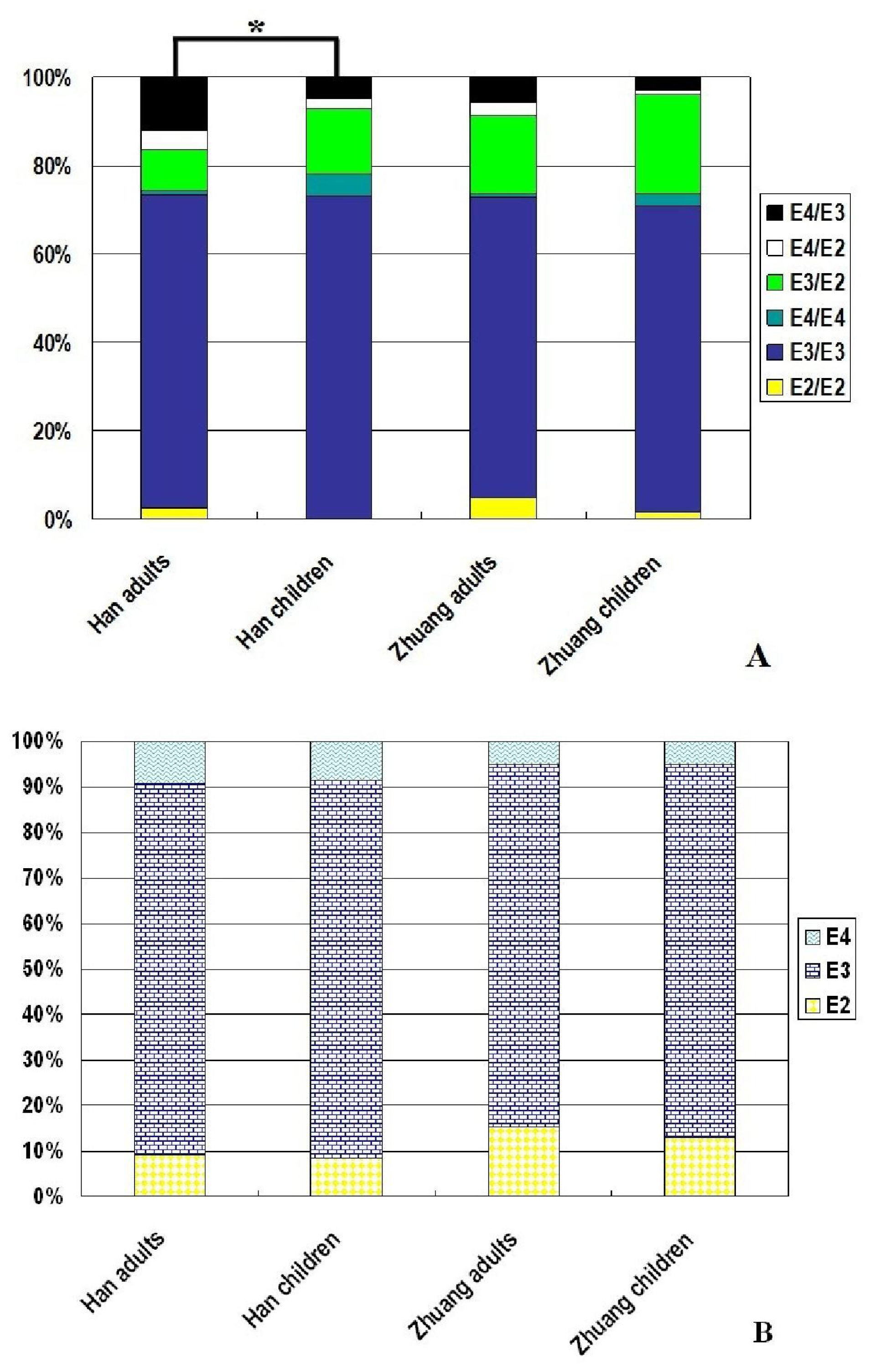
| Genotype or allele | Guangxi Zhuang children n = 278 (%) | Guangxi Han children n = 200 (%) | X2 | p-value |
|---|---|---|---|---|
| E2/E2 | 4 (1.4) | 0 (0) | ||
| E3/E3 | 193 (69.4) | 146 (73.0) | ||
| E4/E4 | 8 (2.9) | 10 (5.0) | ||
| E3/E2 | 62 (22.3) | 30 (15.0) | ||
| E4/E2 | 3 (1.1) | 4 (2.0) | ||
| E4/E3 | 8 (2.9) | 10 (5.0) | 11.25 | 0.04 |
| E2 allele | 36.5 (13.1) | 17 (8.5) | ||
| E3 allele | 228 (82.0) | 166 (83.0) | ||
| E4 allele | 13.5 (4.9) | 17 (8.5) | 4.59 | 0.10 |
| E2/E2 | E3/E3 | E4/E4 | E3/E2 | E4/E2 | E4/E3 | E2 | E3 | E4 | |
|---|---|---|---|---|---|---|---|---|---|
| Guangxi Zhuang children | |||||||||
| N = 278 | 4 | 193 | 8 | 62 | 3 | 8 | 36.5 | 228 | 13.5 |
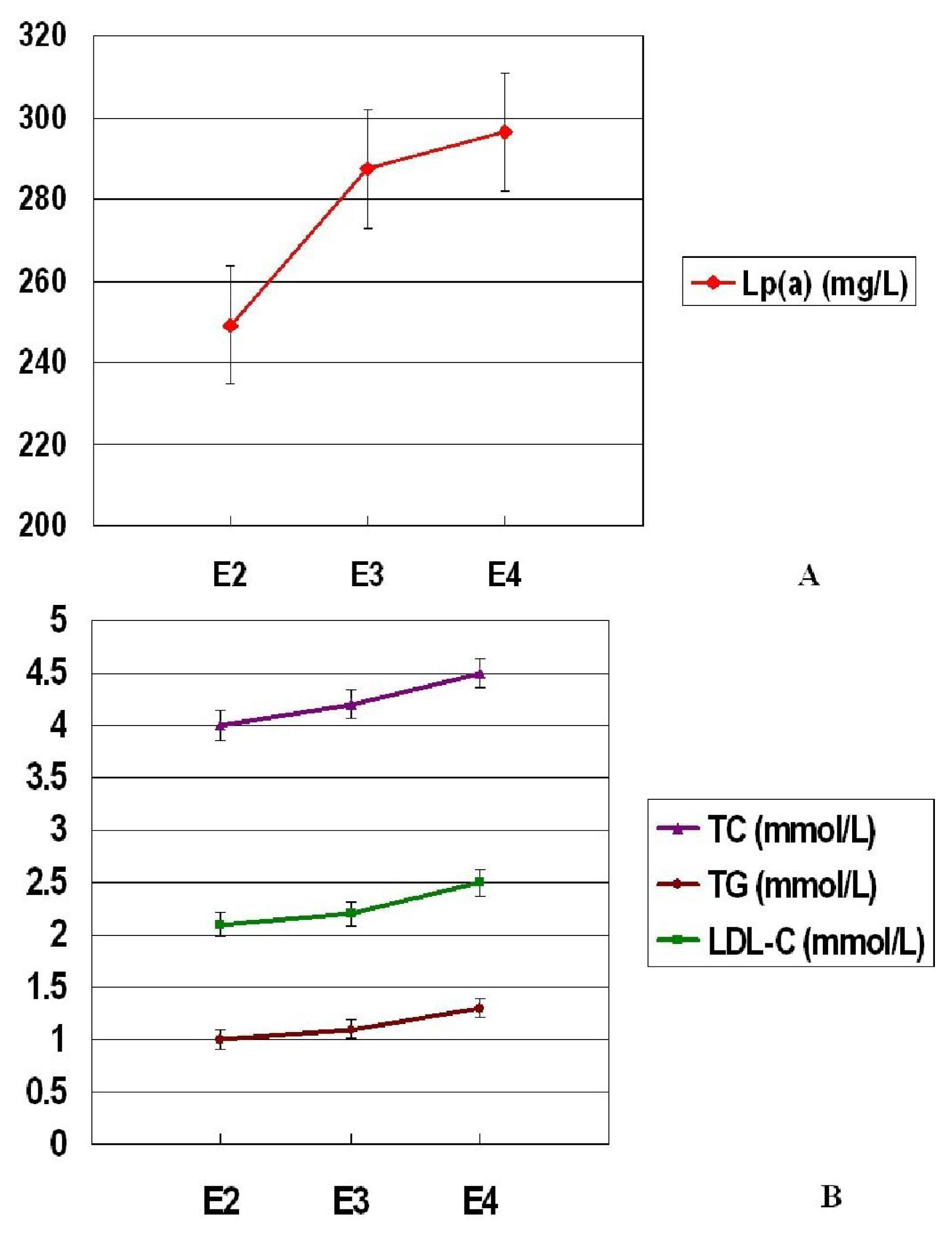
| Lp(a) (mg/L) | 233.7 ± 39.1 | 287.5 ± 40.6 | 298.3 ± 56.5 | 264.3 ± 35.7 | 300.6 ± 48.9294.2 ± 36.8 | 249.1 ± 37.5 | 287.5 ± 40.6 | 296.3 ± 45.9 | |
| TC (mmol/L) | 3.9 ± 0.9 | 4.2 ± 0.5 | 4.5 ± 0.7 | 4.0 ± 0.5 | 4.5 ± 0.8 | 4.3 ± 0.6 | 4.0 ± 0.7 | 4.2 ± 0.5 | 4.5 ± 0.7 |
| TG (mmol/L) | 0.9 ± 0.5 | 1.1 ± 0.3 | 1.3 ± 0.4 | 1.1 ± 0.4 | 1.2 ± 0.5 | 1.2 ± 0.4 | 1.0 ± 0.4 | 1.1 ± 0.3 | 1.3 ± 0.3 |
| HDL-C (mmol/L) | 1.3 ± 0.6 | 1.6 ± 0.3 | 1.6 ± 0.5 | 1.5 ± 0.4 | 1.5 ± 0.6 | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.6 ± 0.3 | 1.5 ± 0.5 |
| LDL-C (mmol/L) | 2.0 ± 0.7 | 2.2 ± 0.4 | 2.5 ± 0.9 | 2.1 ± 0.6 | 2.3 ± 0.8 | 2.5 ± 0.7 | 2.1 ± 0.6 | 2.2 ± 0.4 | 2.5 ± 0.7 |
| apoA1 (g/L) | 1.4 ± 0.4 | 1.4 ± 0.3 | 1.6 ± 0.5 | 1.5 ± 0.3 | 1.4 ± 0.5 | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.3 | 1.5 ± 0.4 |
| apoB (g/L) | 0.7 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.4 | 0.9 ± 0.4 | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.3 |
| apoA1/B | 1.6 ± 0.5 | 1.7 ± 0.5 | 1.7 ± 0.6 | 1.7 ± 0.5 | 1.6 ± 0.6 | 1.7 ± 0.51.7 ± 0.5 | 1.7 ± 0.5 | 1.7 ± 0.6 | |
| Guangxi Han children | |||||||||
| n = 200 | 0 | 146 | 10 | 30 | 4 | 10 | 17 | 166 | 17 |
| Lp(a) (mg/L) | 236.5 ± 34.1 | 306 ± 43.2 | 296.2 ± 33.4 | 311.3 ± 52.1 | 305 ± 32.4 | 296.2 ± 33.4 | 236.5 ± 34.1 | 305.3 ± 41.5 | |
| TC (mmol/L) | 4.1 ± 0.8 | 4.9 ± 1.3 | 4.3 ± 0.6 | 4.9 ± 2.1 | 4.5 ± 1.6 | 4.3 ± 0.6 | 4.1 ± 0.8 | 4.7 ± 1.1 | |
| TG (mmol/L) | 0.8 ± 0.2 | 1.0 ± 0.4 | 0.9 ± 0.2 | 0.9 ± 0.5 | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.3 | |
| HDL-C (mmol/L) | 1.3 ± 0.7 | 1.7 ± 0.8 | 1.5 ± 0.6 | 1.3 ± 0.7 | 1.5 ± 0.8 | 1.3 ± 0.7 | 1.3 ± 0.7 | 1.6 ± 0.8 | |
| LDL-C (mmol/L) | 1.9 ± 0.3 | 2.4 ± 0.7 | 2.2 ± 0.5 | 2.2 ± 1.0 | 2.3 ± 0.6 | 2.2 ± 1.0 | 1.9 ± 0.3 | 2.3 ± 0.6 | |
| apoA1 (g/L) | 1.1 ± 0.1 | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.4 ± 0.5 | 1.3 ± 0.4 | 1.2 ± 0.3 | 1.1 ± 0.1 | 1.3 ± 0.3 | |
| apoB (g/L) | 0.8 ± 0.2 | 1.2 ± 0.4 | 0.8 ± 0.2 | 1.0 ± 0.4 | 1.1 ± 0.4 | 0.8 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.4 | |
| apoA1/B | 1.4 ± 0.2 | 1.1 ± 0.4 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.2 ± 0.4 | 1.4 ± 0.31.4 ± 0.2 | 1.2 ± 0.4 | ||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, P.; Qin, Y.H.; Lei, F.Y.; Pei, J.; Hu, B.; Lu, L. Variable Frequencies of Apolipoprotein E Genotypes and Its Effect on Serum Lipids in the Guangxi Zhuang and Han Children. Int. J. Mol. Sci. 2011, 12, 5604-5615. https://doi.org/10.3390/ijms12095604
Hu P, Qin YH, Lei FY, Pei J, Hu B, Lu L. Variable Frequencies of Apolipoprotein E Genotypes and Its Effect on Serum Lipids in the Guangxi Zhuang and Han Children. International Journal of Molecular Sciences. 2011; 12(9):5604-5615. https://doi.org/10.3390/ijms12095604
Chicago/Turabian StyleHu, Peng, Yuan Han Qin, Feng Ying Lei, Juan Pei, Bo Hu, and Ling Lu. 2011. "Variable Frequencies of Apolipoprotein E Genotypes and Its Effect on Serum Lipids in the Guangxi Zhuang and Han Children" International Journal of Molecular Sciences 12, no. 9: 5604-5615. https://doi.org/10.3390/ijms12095604




