Enhanced Anti-Tumoral Activity of Methotrexate-Human Serum Albumin Conjugated Nanoparticles by Targeting with Luteinizing Hormone-Releasing Hormone (LHRH) Peptide
Abstract
:1. Introduction
2. Results
2.1. Characterization of LHRH Targeted MTX-HSA Nanoparticles
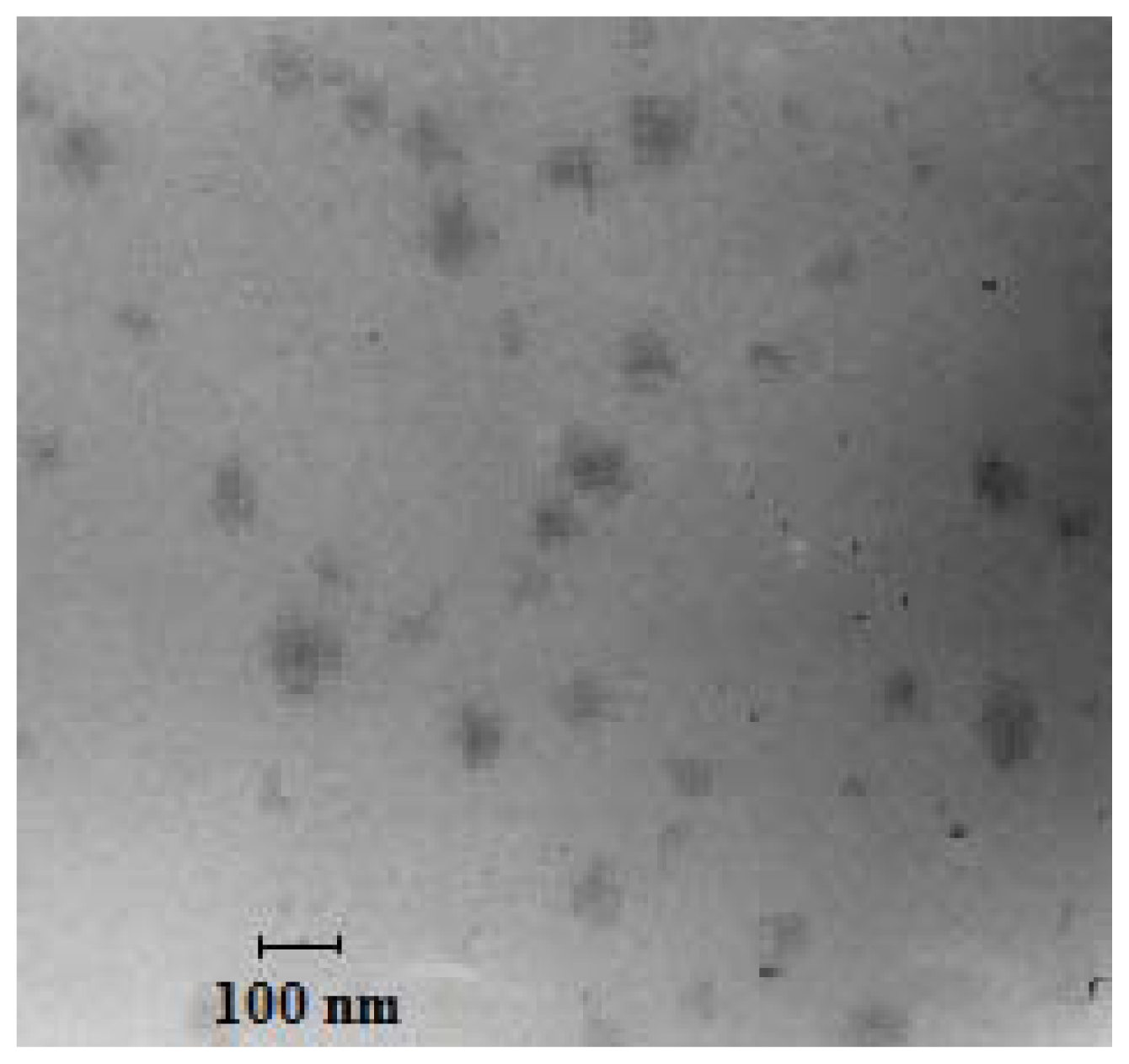
2.2. Transmission Electron Microscopy (TEM)
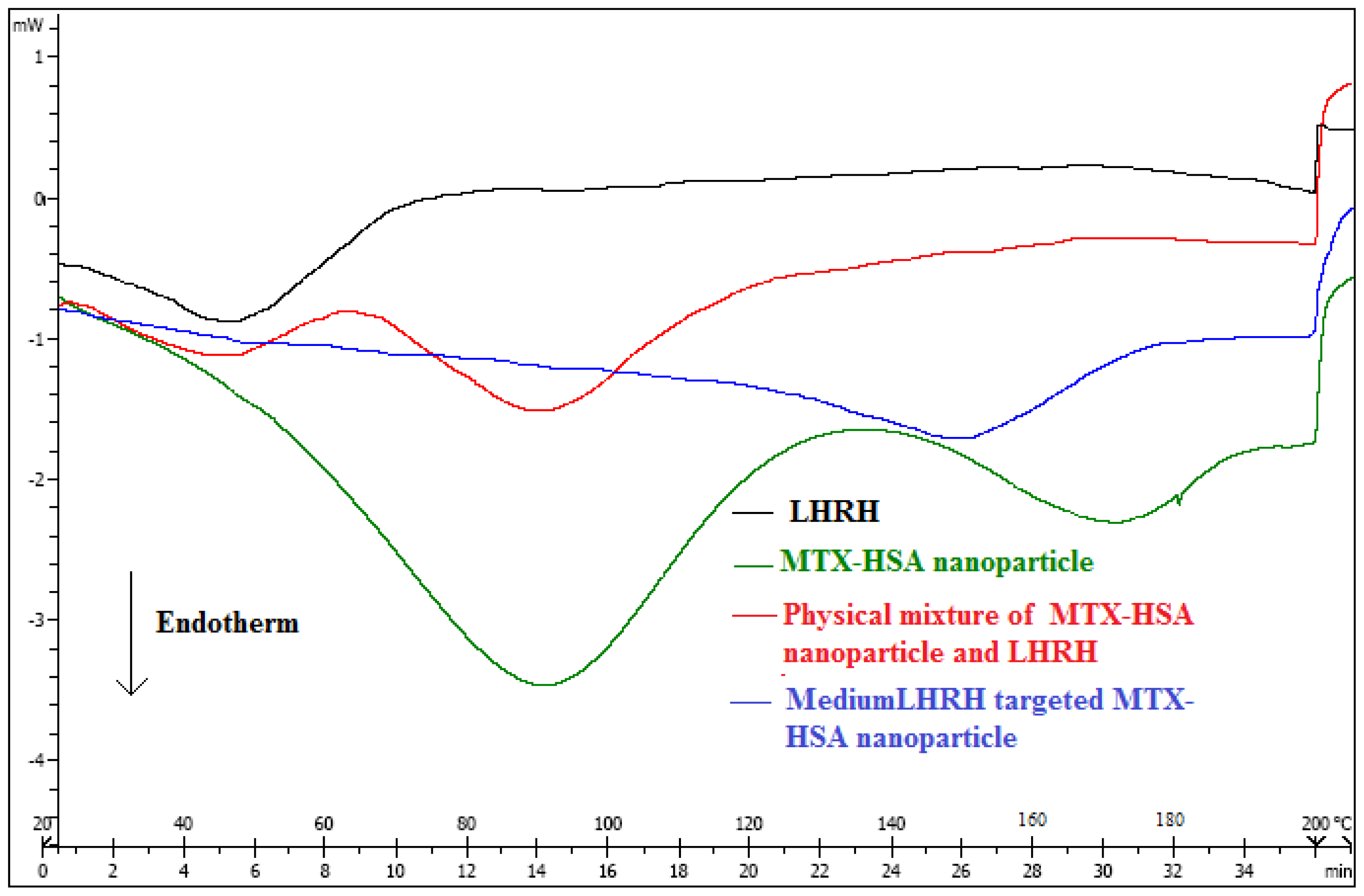
2.3. Stability of LHRH Targeted MTX-HSA Nanoparticles
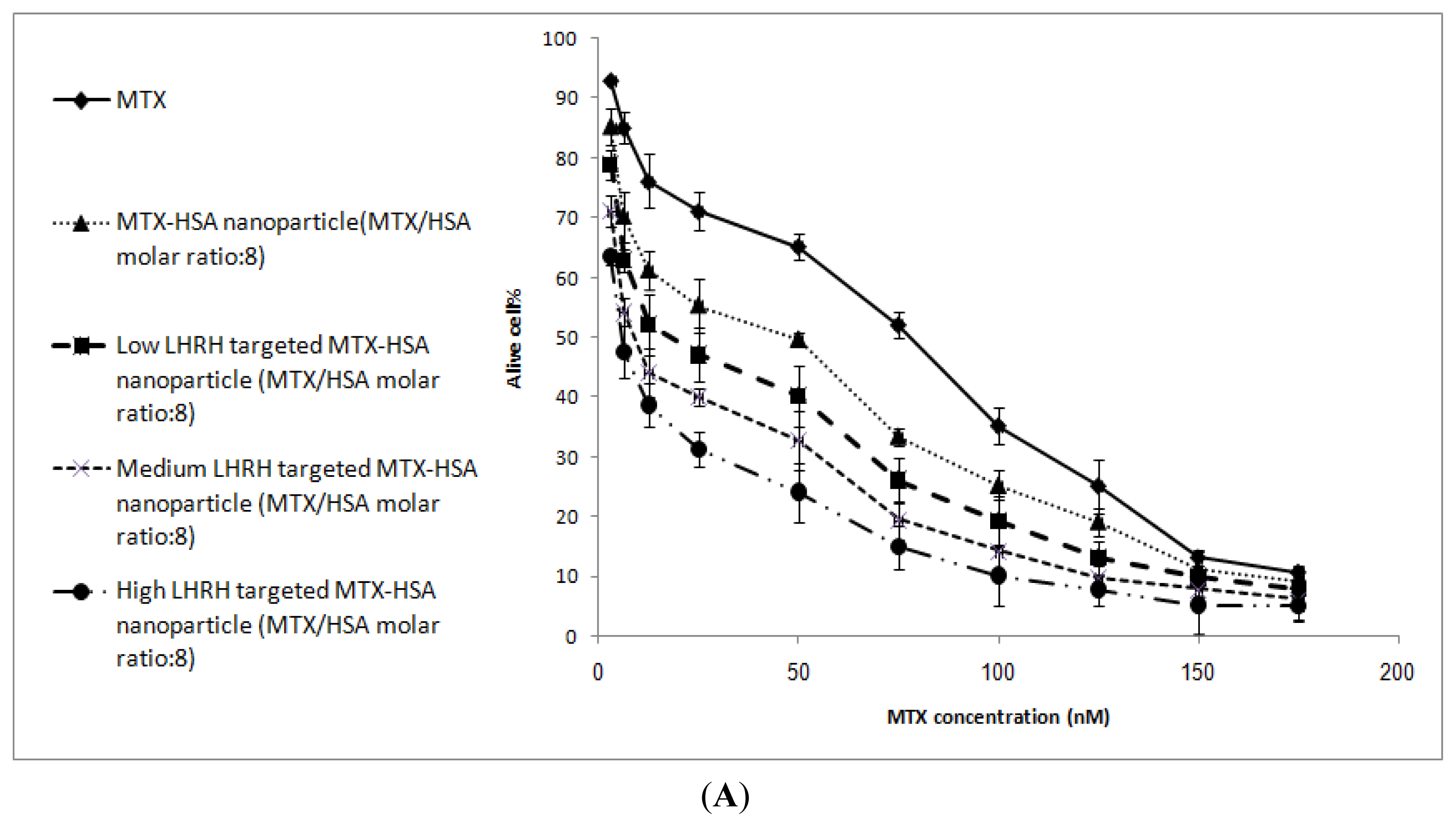
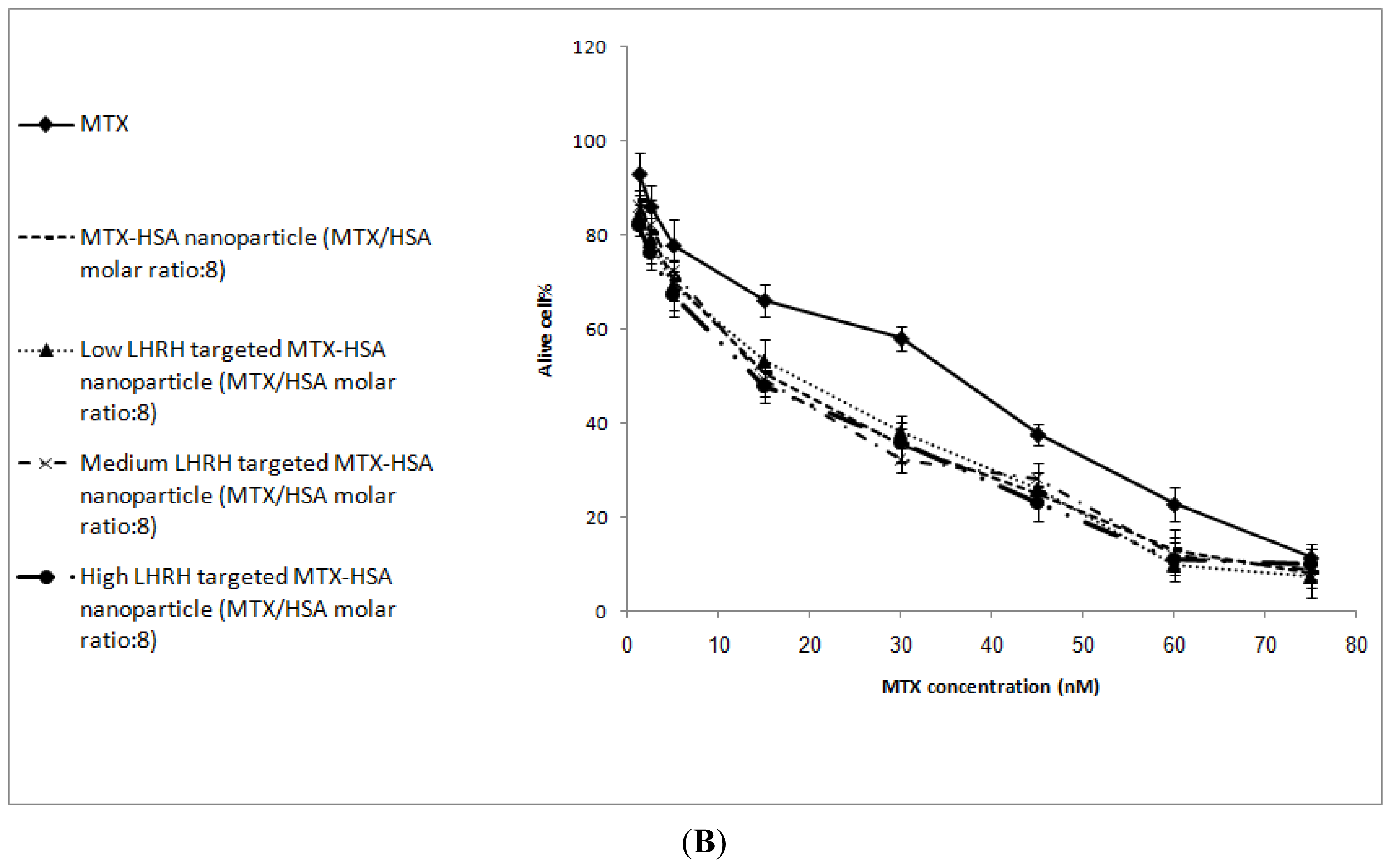
2.4. In Vitro Cytotoxicity
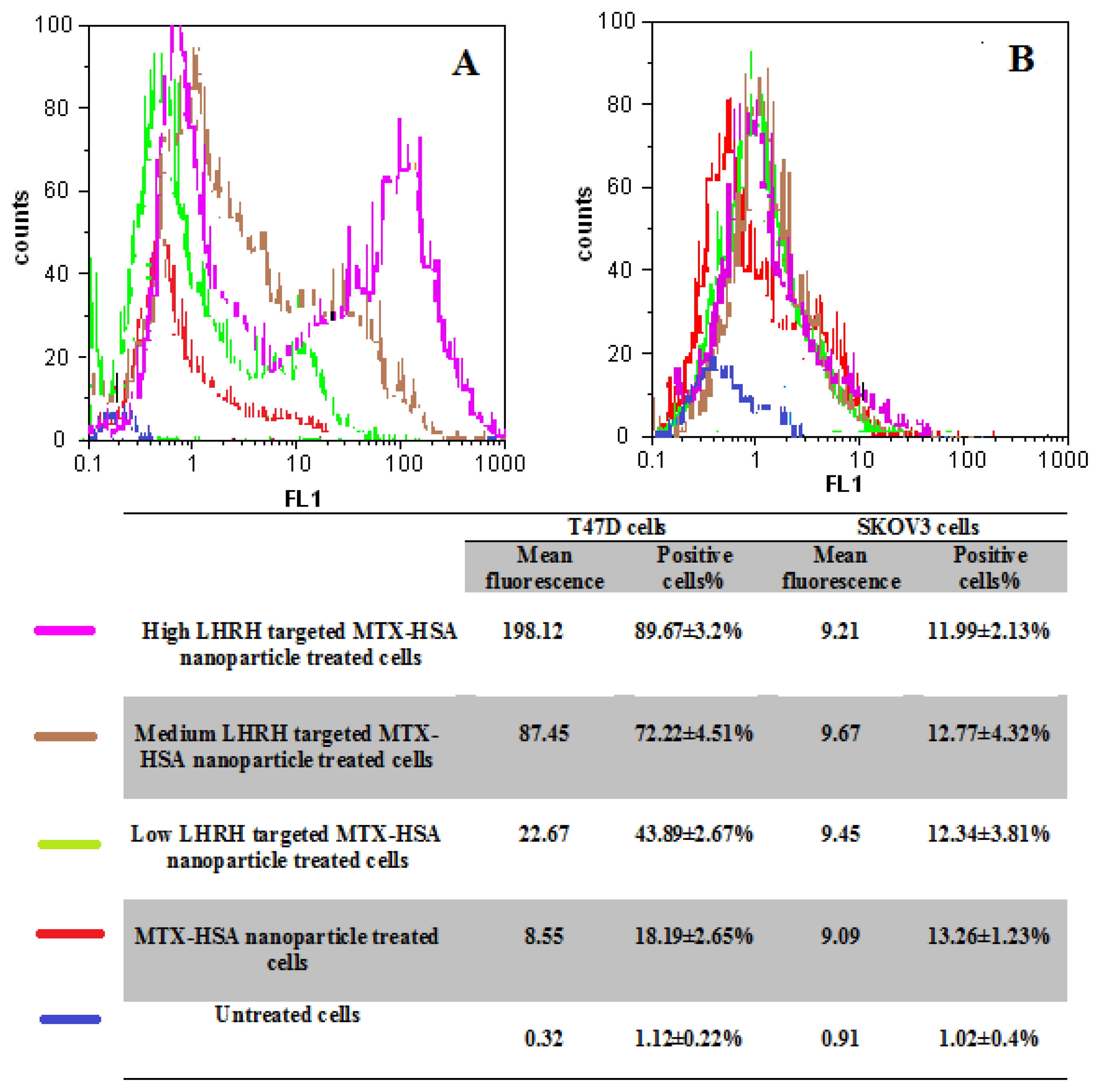
2.5. Cellular Uptake of LHRH Targeted MTX-HSA Nanoparticles
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Preparation of LHRH Targeted MTX-HSA Nanoparticles
4.3. Determination of LHRH on the Surface of LHRH Conjugated MTX-HSA Nanoparticles
4.4. Characterization of Nanoparticles
4.4.1. Particle Size and Zeta Potential Measurement
4.4.2. Transmission Electron Microscopy (TEM) Observation
4.4.3. Stability of LHRH Targeted MTX-HSA Nanoparticles
4.4.4. Differential Scanning Calorimetric (DSC) Analysis
4.5. Cell Culture
4.6. In Vitro Cytotoxicity Assay
4.7. Flow Cytometry Experiment
4.8. Fluorescence Microscopy
3. Conclusions
References
- Schally, A; Comaru-Schally, A; Nagy, A; Kovacs, M; Szepeshazi, K; Plonowski, A; Varga, J; Halmos, G. Hypothalamic hormones and cancer. Front. Neuroendocrinol 2001, 22, 248–291. [Google Scholar]
- Jemal, A; Murray, T; Ward, E; Samuels, A; Tiuari, RC; Ghafoor, A; Feuer, EJ; Thun, MJ. Cancer statistics. Ca-Cancer J. Clin 2005, 55, 10–30. [Google Scholar]
- Chabner, BA; Collins, JM. Cancer Chemotherapy: Principles and Practice; J.B. Lippincott Company: Philadelphia, PA, USA, 1990. [Google Scholar]
- Schally, A; Nagy, A. Chemotherapy targeted to cancers through tumoral hormone receptors. Trends Endocrinol. MeTab 2004, 15, 300–310. [Google Scholar]
- Minko, T; Dharap, SS; Pakunlu, RI; Wang, Y. Molecular targeting of drug delivery systems to cancer. Curr. Drug. Targets 2004, 5, 389–406. [Google Scholar]
- Singh, Y; Palombo, M; Sinko, PJ. Recent trends in targeted anticancer prodrug and conjugate design. Curr. Med. Chem 2008, 15, 1802–1826. [Google Scholar]
- Esmaeili, F; Ghahremani, MH; Ostad, SN; Atyabi, F; Seyedabadi, M; Malekshahi, MR; Amini, M; Dinarvand, R. Folate-receptor-targeted delivery of docetaxel nanoparticles prepared by PLGA-PEG-folate conjugate. J. Drug. Targets 2008, 16, 415–423. [Google Scholar]
- Allen, TM. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar]
- Reubi, JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev 2003, 24, 389–427. [Google Scholar]
- Schally, A; Nagy, A. New approaches to treatment of various cancers based on cytotoxic analogs of LHRH, somatostatin and bombesin. Life Sci 2003, 72, 2305–2320. [Google Scholar]
- Chatzistamou, L; Schally, A; Nagi, A; Halmos, G. Effective treatment of metastatic MDA-MB-435 human estrogen independent breast carcinomas with a targeted cytotoxic analogue of luteinizing hormone releasing hormone AN-207. Clin. Cancer Res 2000, 6, 4158–4168. [Google Scholar]
- Leuschner, C; Hansel, W. Targeting breast and prostate cancers through their hormone receptors. Biol. Reprod 2005, 73, 255–260. [Google Scholar]
- Nagy, A; Schally, A. Targeted cytotoxic somatostatin analogs: A modern approach to the therapy of various cancers. Drugs Future 2001, 26, 261–270. [Google Scholar]
- Nagy, A; Schally, A. Cytotoxic analogs of luteinizing hormone-releasing hormone (LHRH): A new approach to targeted chemotherapy. Drugs Future 2002, 27, 359–270. [Google Scholar]
- Ben-Yehudah, A; Lorberboum-Galski, H. Targeted cancer therapy with gonadotropin-releasing hormone chimeric proteins. Anticancer Ther 2004, 4, 151–161. [Google Scholar]
- Leuschner, C; Enright, F; Gawronska-Kozak, B; Hansel, W. Human prostate cancer cells and xenografts are targeted and destroyed through luteinizing hormone-releasing hormone receptors. Prostate 2003, 56, 239–249. [Google Scholar]
- Qi, L; Nett, T; Allen, M; Sha, X; Harrison, G; Frederick, B; Crawford, D; Glode, M. Binding and cytotoxicity of conjugated and recombinant fusion proteins targeted to the gonadotropin-releasing hormone receptor. Cancer Res 2004, 64, 2090–2095. [Google Scholar]
- Szoke, B; Horvath, J; Halmos, G; Rekasi, Z; Groot, K; Nagy, A; Schally, AV. LHRH analogue carrying a cytotoxic radical is internalized by rat pituitary cells in vitro. Peptides 1994, 15, 359–366. [Google Scholar]
- Taratula, O; Garbuzenko, OB; Kirkpatrick, P; Pandya, I; Savla, R; Pozharov, VP; He, H; Minko, T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J. Control. Release 2009, 140, 284–293. [Google Scholar]
- Minko, T; Patil, ML; Zhang, M; Khandare, JJ; Saad, M; Chandna, P; Taratula, O. LHRH-Targeted nanoparticles for cancer therapeutics. Methods Mol. Biol 2010, 624, 281–294. [Google Scholar]
- Janaky, T; Juhasz, A; Bajusz, S; Csernus, V; Srkalovic, G; Bokser, L; Milovanovic, S; Redding, TW; Rekasi, Z; Nagy, A; et al. Analogues of luteinizing hormone-releasing hormone containing cytotoxic groups. Proc. Natl. Acad. Sci. USA 1992, 89, 972–976. [Google Scholar]
- Hwa-Kim, S; Hoon-Jeong, J; Hyeon-Lee, S; Wan-Kim, S; Gwan-Park, T. LHRH receptor-mediated delivery of siRNA using polyelectrolyte complex micelles self-assembled from siRNA-PEG-LHRH conjugate and PEI. Bioconjugate Chem 2008, 19, 2156–2162. [Google Scholar]
- Krebs, L; Wang, X; Pudavar, H; Bergey, E; Schally, A; Nagy, A; Prasad, P; Liebow, C. Regulation of targeted chemotherapy with cytotoxic LH-RH analog by epidermal growth factor. Cancer Res 2000, 60, 4194–4199. [Google Scholar]
- Westphalen, S; Kotulla, G. Receptor mediated anti-proliferative effects of the cytotoxic LHRH agonist AN-152 in human ovarian and endometrial cancer cell lines. Int. J. Oncol 2000, 17, 1063–1069. [Google Scholar]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar]
- Zhao, J; Zheng, X; Xing, W; Huang, J; Li, G. Electrochemical studies of camptothecin and its interactionwith human serum albumin. Int. J. Mol. Sci 2007, 8, 42–50. [Google Scholar]
- Santhi, K; Dhanaraj, SA; Koshy, M. Study of biodistribution of methotrexate-loaded bovine serum albumin nanospheres in mice. Drug Dev. Ind. Pharm 2000, 26, 1293–1296. [Google Scholar]
- Taheri, A; Atyabi, F; Salman Nouri, F; Ahadi, F; Derakhshan, MA; Amini, A; Ghahremani, MH; Ostad, SN; Mansoori, P; Dinarvand, R. Nanoparticles of conjugated methotrexate-human serum albumin: Preparation and cytotoxicity evaluations. J Nanomater 2011, in press. [Google Scholar]
- Esmaeili, F; Dinarvand, R; Ghahremani, MH; Amini, M; Rouhani, H; Sepehri, N; Ostad, SN; Atyabi, F. Docetaxel-albumin conjugates: Preparation, in vitro evaluation and biodistribution studies. J. Pharm. Sci 2009, 98, 2718–2730. [Google Scholar]
- Kreuter, J; Hekmatara, T; Dreis, S; Vogel, T; Gelperina, S; Langer, K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J. Control. Release 2007, 118, 54–58. [Google Scholar]
- Steinhauser, I; Spankuchb, B; Strebhardt, K; Langer, K. Trastuzumab-modified nanoparticles: Optimisation of preparation and uptake in cancer cells. Biomaterials 2006, 27, 4975–4983. [Google Scholar]
- Zhanga, L; Houb, S; Maob, S; Wei, D; Songb, X; Lub, Y. Uptake of folate-conjugated albumin nanoparticles to the SKOV3 cells. Int. J. Pharm 2004, 287, 155–162. [Google Scholar]
- Tuan, V; Chuang, G; Kragh-Hansen, U; Otagiri, M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm. Res 2002, 19, 569–577. [Google Scholar]
- Günthert, A; Gründker, C; Bongertz, T; Schlott, T; Nagy, A; Schally, A; Emons, G. Internalisation of cytotoxic luteinizing hormone-releasing hormone analog AN-152 induces multi drug resistance 1(MDR-1)-independent apoptosis in human endometrial and ovarian cancer cell lines. Am. J. Obstet. Gynecol 2004, 191, 1164–1172. [Google Scholar]
- Yousefi, G; Foroutan, SM; Zarghi, A; Shafaati, A. Synthesis and characterization of methotrexate polyethylene glycol esters as a drug delivery system. Chem. Pharm. Bull 2010, 58, 147–153. [Google Scholar]
- Anande, NM; Jain, SK; Jain, NK. Con-A conjugated mucoadhesive microspheres for the colonic delivery of diloxanide furoate. Int. J. Pharm 2008, 359, 182–189. [Google Scholar]
- Sahoo, SK; Panyam, J; Prabha, S; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar]
- Marcucci, F; Lefoulon, F. Active targeting with particulate drug carriers in tumor therapy: Fundamentals and recent progress. Drug Discovery Today 2004, 9, 219–228. [Google Scholar]
- Kakar, S; Jennes, L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non reproductive human tissues. Cancer Lett 1995, 98, 57–62. [Google Scholar]
- Dharap, SS; Qiu, B; Williams, GC; Sinko, P; Stein, S; Minko, T. Molecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptides. J. Control. Release 2003, 91, 61–73. [Google Scholar]
- Xu, C; Yuan, Z; Kohler, N; Ki, J; Chung, M; Sun, S. FePt Nanoparticles as Fe reservoir for controlled Fe release and tumor inhibition. J. Am. Chem. Soc 2009, 131, 15346–15351. [Google Scholar]
- Bajo, AM; Schally, AV; Halmos, G; Nagy, A. Targeted doxorubicin-containing luteinizing hormone-releasing hormone analogue AN-152 inhibits the growth of doxorubicin-resistant MX-1 human breast cancers. Clin. Cancer Res 2003, 9, 3742–3748. [Google Scholar]
- Wagner, S; Rothweiler, F; Anhorn, MG; Sauer, D; Riemann, I; Weiss, EC; Katsen-Globa, A; Michaelis, M; Cinatl, J; Schwartz, D; et al. Enhanced drug targeting by attachment of an anti av integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials 2010, 31, 2388–2398. [Google Scholar]
- Anhorn, MG; Wagner, S; Kreuter, J; Langer, K; Briesen, H. Specific targeting of HER2 overexpressing breast cancer cells with doxorubicin-loaded trastuzumab-modified Human serum albumin nanoparticles. Bioconjugate Chem 2008, 19, 2321–2333. [Google Scholar]
- Desai, N; Trieu, V; Yao, Z. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI 007, compared with cremophor-based paclitaxel. Clin. Cancer Res 2006, 12, 1317–1324. [Google Scholar]
- Miele, E; Spinelli, GP; Miele, E; Tomao, F; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed 2009, 4, 99–105. [Google Scholar]
- Michaelis, K; Hoffmann, MM; Dreis, S; Herbert, E; Alyautdin, RN; Michaelis, M; Kreuter, J; Lange, K. Covalent linkage of apolipoprotein E to albumin nanoparticles strongly enhances drug transport into the brain. J. Pharmacol. Exp. Ther 2006, 317, 1246–1253. [Google Scholar]
- Nagy, A; Schally, A; Armatis, P; Szepeshazi, K; Halmos, G; Kovacs, M. Cytotoxic analogs of luteinizing hormone-releasing hormone containing doxorubicin or 2-pyrrolino-doxorubicin, a derivative 500–1000 times more potent. Proc. Natl. Acad. Sci. USA 1996, 93, 7269–7273. [Google Scholar]


| Non Targeted MTX-HSA Nanoparticle | Low LHRH Targeted MTX-HSA Nanoparticles | Medium LHRH Targeted MTX-HSA Nanoparticles | High LHRH Targeted MTX-HSA Nanoparticles | |
|---|---|---|---|---|
| Particle Size (nm) | 111.7 ± 4.6 | 120.5 ± 2.7 | 128.45 ± 4.4 | 138.56 ± 3.2 |
| Poly Dispersity | 0.10 ± 0.01 | 0.12 ± 0.09 | 0.2 ± 0.04 | 0.14 ± 0.05 |
| Zeta Potential (mV) | −12.1 ± 0.5 | −10.45 ± 1.23 | −10.1 ± 1.1 | −10.04 ± 0.65 |
| LHRH/HSA Molar Ratio | ------ | 5.8 ± 0.12 | 17.3 ± 0.23 | 29.1 ± 0.2 |
| Free MTX | Non Targeted MTX-HSA Nanoparticles | Low LHRH Targeted MTX-HSA Nanoparticles | Medium LHRH Targeted MTX-HSA Nanoparticles | High LHRH Targeted MTX-HSA Nanoparticle | |
|---|---|---|---|---|---|
| T47D cells | 78.23 ± 3.12 | 49.2 ± 2.12 | 19.3 ± 1.98 | 9.12 ± 1.36 | 5.82 ± 1.08 |
| SKOV3 cells | 35.89 ± 1.46 | 15.12 ± 2.19 | 17.64 ± 2.23 | 14.89 ± 2.78 | 14.53 ± 1.46 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Taheri, A.; Dinarvand, R.; Atyabi, F.; Ahadi, F.; Nouri, F.S.; Ghahremani, M.H.; Ostad, S.N.; Borougeni, A.T.; Mansoori, P. Enhanced Anti-Tumoral Activity of Methotrexate-Human Serum Albumin Conjugated Nanoparticles by Targeting with Luteinizing Hormone-Releasing Hormone (LHRH) Peptide. Int. J. Mol. Sci. 2011, 12, 4591-4608. https://doi.org/10.3390/ijms12074591
Taheri A, Dinarvand R, Atyabi F, Ahadi F, Nouri FS, Ghahremani MH, Ostad SN, Borougeni AT, Mansoori P. Enhanced Anti-Tumoral Activity of Methotrexate-Human Serum Albumin Conjugated Nanoparticles by Targeting with Luteinizing Hormone-Releasing Hormone (LHRH) Peptide. International Journal of Molecular Sciences. 2011; 12(7):4591-4608. https://doi.org/10.3390/ijms12074591
Chicago/Turabian StyleTaheri, Azade, Rassoul Dinarvand, Fatemeh Atyabi, Fatemeh Ahadi, Farank Salman Nouri, Mohammad Hossein Ghahremani, Seyed Nasser Ostad, Atefeh Taheri Borougeni, and Pooria Mansoori. 2011. "Enhanced Anti-Tumoral Activity of Methotrexate-Human Serum Albumin Conjugated Nanoparticles by Targeting with Luteinizing Hormone-Releasing Hormone (LHRH) Peptide" International Journal of Molecular Sciences 12, no. 7: 4591-4608. https://doi.org/10.3390/ijms12074591




