The Hildebrand Solubility Parameters of Ionic Liquids—Part 2
Abstract
:1. Introduction
2. Results and Discussion
3. Calculation of Solubility Parameters
3.1. Experimental Procedure
3.2. Theoretical Basis
4. Conclusions
Acknowledgements
Appendix Electronic Supporting Information
| χ 12∞ | ||||||
|---|---|---|---|---|---|---|
| [N-C3OHPY][FAP] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | n-nonane | n-decane |
| 308.15 | 3.62 | 3.96 | 4.32 | 4.68 | 5.04 | 5.42 |
| 318.15 | 3.50 | 3.83 | 4.17 | 4.52 | 4.86 | 5.24 |
| 328.15 | 3.39 | 3.71 | 4.04 | 4.38 | 4.72 | 5.08 |
| 338.15 | 3.27 | 3.58 | 3.91 | 4.24 | 4.57 | 4.93 |
| 348.15 | 3.18 | 3.48 | 3.78 | 4.11 | 4.44 | 4.78 |
| 358.15 | 3.09 | 3.37 | 3.67 | 3.99 | 4.30 | 4.64 |
| T/K | cyclopentane | cyclohexane | cycloheptane | cyclooctane | 1-pentene | 1-hexene |
| 308.15 | 3.19 | 3.55 | 3.81 | 4.08 | 2.75 | 3.07 |
| 318.15 | 3.08 | 3.42 | 3.67 | 3.93 | 2.66 | 2.97 |
| 328.15 | 2.97 | 3.29 | 3.54 | 3.80 | 2.58 | 2.88 |
| 338.15 | 2.86 | 3.17 | 3.42 | 3.67 | 2.50 | 2.80 |
| 348.15 | 2.75 | 3.05 | 3.30 | 3.55 | 2.43 | 2.72 |
| 358.15 | 2.67 | 2.94 | 3.19 | 3.43 | 2.36 | 2.64 |
| T/K | 1-heptene | 1-octene | 1-hexyne | 1-heptyne | 1-octyne | benzene |
| 308.15 | 3.40 | 3.79 | 2.01 | 2.33 | 2.69 | 0.482 |
| 318.15 | 3.31 | 3.68 | 1.96 | 2.28 | 2.63 | 0.495 |
| 328.15 | 3.22 | 3.58 | 1.92 | 2.23 | 2.57 | 0.505 |
| 338.15 | 3.14 | 3.48 | 1.87 | 2.18 | 2.51 | 0.519 |
| 348.15 | 3.06 | 3.38 | 1.83 | 2.13 | 2.45 | 0.528 |
| 358.15 | 2.97 | 3.29 | 1.79 | 2.08 | 2.40 | 0.537 |
| T/K | toluene | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol |
| 308.15 | 0.732 | 1.12 | 0.944 | 1.01 | 1.06 | 1.01 |
| 318.15 | 0.743 | 1.12 | 0.950 | 1.02 | 1.07 | 0.979 |
| 328.15 | 0.751 | 1.12 | 0.955 | 1.03 | 1.07 | 0.944 |
| 338.15 | 0.761 | 1.12 | 0.961 | 1.04 | 1.08 | 0.913 |
| 348.15 | 0.769 | 1.12 | 0.966 | 1.04 | 1.08 | 0.882 |
| 358.15 | 0.777 | 1.11 | 0.971 | 1.05 | 1.09 | 0.855 |
| T/K | ethanol | 1-propanol | 1-butanol | water | thiophene | tetrahydrofuran |
| 308.15 | 0.873 | 1.01 | 1.20 | 2.95 | 0.557 | −0.967 |
| 318.15 | 0.832 | 0.965 | 1.14 | 2.85 | 0.564 | −0.859 |
| 328.15 | 0.796 | 0.916 | 1.08 | 2.77 | 0.572 | −0.776 |
| 338.15 | 0.761 | 0.878 | 1.03 | 2.69 | 0.578 | −0.683 |
| 348.15 | 0.730 | 0.836 | 0.974 | 2.61 | 0.585 | −0.601 |
| 358.15 | 0.699 | 0.803 | 0.926 | 2.54 | 0.591 | −0.529 |
| T/K | methyl tert-butyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether | 2-pentanone | 3-pentanone |
| 308.15 | −0.0104 | 0.168 | 1.29 | 2.14 | ||
| 318.15 | 0.083 | 0.245 | 1.32 | 2.15 | −1.09 | −0.988 |
| 328.15 | 0.170 | 0.324 | 1.36 | 2.15 | −1.01 | −0.907 |
| 338.15 | 0.262 | 0.390 | 1.39 | 2.16 | −0.933 | −0.831 |
| 348.15 | 0.335 | 0.450 | 1.43 | 2.16 | −0.860 | −0.759 |
| 358.15 | 0.414 | 0.504 | 1.45 | 2.16 | −0.792 | −0.694 |
| T/K | acetone | |||||
| 308.15 | −1.66 | |||||
| 318.15 | −1.56 | |||||
| 328.15 | −1.47 | |||||
| 338.15 | −1.38 | |||||
| 348.15 | −1.30 | |||||
| 358.15 | −1.23 | |||||
| [N-C3OHPY][NTf2] | ||||||
| T/K | n-pentane | n-hexane | 3-methylpentane | 2,2-dimethylbutane | n-heptane | n-octane |
| 318.15 | 3.67 | 4.04 | 3.90 | 3.81 | 4.45 | 4.86 |
| 328.15 | 3.59 | 3.94 | 3.80 | 3.71 | 4.34 | 4.73 |
| 338.15 | 3.50 | 3.84 | 3.70 | 3.61 | 4.22 | 4.61 |
| 348.15 | 3.43 | 3.76 | 3.62 | 3.53 | 4.13 | 4.50 |
| 358.15 | 3.35 | 3.67 | 3.54 | 3.44 | 4.03 | 4.40 |
| T/K | 2,2,4-trimethylpentane | n-nonane | n-decane | cyclopentane | cyclohexane | methylcyclohe xane |
| 318.15 | 4.40 | 5.27 | 5.70 | 3.08 | 3.45 | 3.78 |
| 328.15 | 4.30 | 5.13 | 5.54 | 2.99 | 3.35 | 3.68 |
| 338.15 | 4.20 | 5.00 | 5.41 | 2.91 | 3.26 | 3.58 |
| 348.15 | 4.11 | 4.89 | 5.29 | 2.84 | 3.18 | 3.49 |
| 358.15 | 4.03 | 4.77 | 5.16 | 2.78 | 3.10 | 3.41 |
| T/K | cycloheptane | cyclooctane | 1-pentene | 1-hexene | cyclohexene | 1-heptene |
| 318.15 | 3.73 | 4.03 | 2.89 | 3.28 | 2.75 | 3.67 |
| 328.15 | 3.63 | 3.92 | 2.83 | 3.20 | 2.68 | 3.59 |
| 338.15 | 3.53 | 3.81 | 2.76 | 3.12 | 2.62 | 3.51 |
| 348.15 | 3.44 | 3.71 | 2.71 | 3.06 | 2.57 | 3.44 |
| 358.15 | 3.36 | 3.63 | 2.65 | 3.00 | 2.52 | 3.38 |
| T/K | 1-octene | 1-decene | 1-hexyne | 1-heptyne | 1-octyne | benzene |
| 318.15 | 4.09 | 4.91 | 2.10 | 2.48 | 2.89 | 0.886 |
| 328.15 | 4.00 | 4.80 | 2.08 | 2.45 | 2.84 | 0.887 |
| 338.15 | 3.91 | 4.70 | 2.05 | 2.41 | 2.79 | 0.888 |
| 348.15 | 3.83 | 4.61 | 2.03 | 2.39 | 2.76 | 0.888 |
| 358.15 | 3.75 | 4.51 | 2.01 | 2.35 | 2.71 | 0.888 |
| T/K | toluene | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol |
| 318.15 | 1.18 | 1.62 | 1.38 | 1.51 | 1.51 | 0.896 |
| 328.15 | 1.18 | 1.61 | 1.38 | 1.51 | 1.51 | 0.850 |
| 338.15 | 1.18 | 1.60 | 1.38 | 1.51 | 1.51 | 0.805 |
| 348.15 | 1.18 | 1.58 | 1.38 | 1.51 | 1.51 | 0.761 |
| 358.15 | 1.18 | 1.58 | 1.38 | 1.51 | 1.51 | 0.719 |
| T/K | ethanol | 1-propanol | 1-butanol | water | acetic acid | thiophene |
| 318.15 | 0.885 | 1.02 | 1.23 | 2.21 | −0.614 | 0.754 |
| 328.15 | 0.836 | 0.968 | 1.17 | 2.13 | −0.534 | 0.756 |
| 338.15 | 0.788 | 0.918 | 1.11 | 2.06 | −0.464 | 0.756 |
| 348.15 | 0.745 | 0.868 | 1.05 | 1.99 | −0.397 | 0.757 |
| 358.15 | 0.698 | 0.822 | 0.995 | 1.94 | −0.334 | 0.756 |
| T/K | tetrahydrofuran | 1,4-dioxane | methyl tert-butyl ether | methyl tert-pentyl ether | diethyl ether | di-n-propyl ether |
| 318.15 | 0.125 | −0.205 | 1.26 | 1.69 | 1.34 | 2.43 |
| 328.15 | 0.166 | −0.157 | 1.29 | 1.70 | 1.35 | 2.40 |
| 338.15 | 0.201 | −0.112 | 1.31 | 1.71 | 1.36 | 2.38 |
| 348.15 | 0.230 | −0.070 | 1.33 | 1.73 | 1.37 | 2.36 |
| 358.15 | 0.260 | −0.032 | 1.35 | 1.74 | 1.38 | 2.35 |
| T/K | di-n-butyl ether | acetone | 2-pentanone | 3-pentanone | ||
| 318.15 | 3.30 | −0.351 | 0.193 | 0.253 | ||
| 328.15 | 3.25 | −0.314 | 0.217 | 0.277 | ||
| 338.15 | 3.20 | −0.284 | 0.242 | 0.301 | ||
| 348.15 | 3.16 | −0.255 | 0.261 | 0.322 | ||
| 358.15 | 3.12 | −0.229 | 0.281 | 0.341 | ||
| [emim][TCB] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | 2,2,4-trimethylpentane | n-nonane |
| 298.15 | 3.26 | 3.63 | 4.05 | 4.46 | 4.14 | 4.90 |
| 308.15 | 3.16 | 3.54 | 3.94 | 4.34 | 4.03 | 4.76 |
| 318.15 | 3.11 | 3.47 | 3.86 | 4.25 | 3.96 | 4.65 |
| 328.15 | 3.02 | 3.38 | 3.75 | 4.14 | 3.87 | 4.52 |
| 338.15 | 2.96 | 3.30 | 3.67 | 4.04 | 3.78 | 4.41 |
| 348.15 | 2.90 | 3.24 | 3.60 | 3.95 | 3.71 | 4.31 |
| 358.15 | 2.84 | 3.18 | 3.52 | 3.86 | 3.63 | 4.21 |
| T/K | n-decane | cyclopentane | cyclohexane | methylcyclohe xane | cycloheptane | cyclooctane |
| 298.15 | 5.32 | 2.64 | 3.01 | 3.35 | 3.23 | 3.49 |
| 308.15 | 5.18 | 2.57 | 2.92 | 3.24 | 3.13 | 3.38 |
| 318.15 | 5.06 | 2.52 | 2.86 | 3.17 | 3.07 | 3.31 |
| 328.15 | 4.92 | 2.44 | 2.77 | 3.08 | 2.98 | 3.21 |
| 338.15 | 4.81 | 2.39 | 2.70 | 3.00 | 2.90 | 3.14 |
| 348.15 | 4.69 | 2.34 | 2.64 | 2.94 | 2.83 | 3.06 |
| 358.15 | 4.58 | 2.28 | 2.57 | 2.88 | 2.77 | 2.98 |
| T/K | 1-pentene | 1-hexene | cyclohexene | 1-heptene | 1-octene | 1-hexyne |
| 298.15 | 2.42 | 2.80 | 2.21 | 3.18 | 3.61 | 1.50 |
| 308.15 | 2.37 | 2.73 | 2.16 | 3.10 | 3.52 | 1.49 |
| 318.15 | 2.34 | 2.69 | 2.14 | 3.06 | 3.46 | 1.49 |
| 328.15 | 2.29 | 2.62 | 2.09 | 2.99 | 3.37 | 1.48 |
| 338.15 | 2.25 | 2.57 | 2.04 | 2.92 | 3.30 | 1.48 |
| 348.15 | 2.22 | 2.53 | 2.01 | 2.89 | 3.24 | 1.47 |
| 358.15 | 2.15 | 2.48 | 1.98 | 2.83 | 3.18 | 1.47 |
| T/K | 1-heptyne | 1-octyne | benzene | toluene | ethylbenzene | o-xylene |
| 298.15 | 1.86 | 2.23 | 0.433 | 0.710 | 1.10 | 0.922 |
| 308.15 | 1.84 | 2.21 | 0.443 | 0.721 | 1.10 | 0.927 |
| 318.15 | 1.83 | 2.18 | 0.455 | 0.730 | 1.10 | 0.933 |
| 328.15 | 1.81 | 2.16 | 0.462 | 0.739 | 1.10 | 0.937 |
| 338.15 | 1.80 | 2.14 | 0.471 | 0.747 | 1.10 | 0.943 |
| 348.15 | 1.78 | 2.12 | 0.477 | 0.757 | 1.09 | 0.949 |
| 358.15 | 1.77 | 2.11 | 0.483 | 0.762 | 1.09 | 0.950 |
| T/K | m-xylene | p-xylene | methanol | ethanol | 1-propanol | 1-butanol |
| 298.15 | 1.08 | 1.02 | 0.968 | 1.04 | 1.11 | 1.29 |
| 308.15 | 1.08 | 1.03 | 0.886 | 0.944 | 1.01 | 1.17 |
| 318.15 | 1.09 | 1.03 | 0.812 | 0.856 | 0.909 | 1.06 |
| 328.15 | 1.09 | 1.04 | 0.739 | 0.770 | 0.816 | 0.953 |
| 338.15 | 1.10 | 1.05 | 0.674 | 0.693 | 0.734 | 0.861 |
| 348.15 | 1.10 | 1.06 | 0.612 | 0.620 | 0.660 | 0.780 |
| 358.15 | 1.10 | 1.06 | 0.553 | 0.551 | 0.591 | 0.701 |
| T/K | water | thiophene | tetrahydrofuran | methyl tert-butyl ether | methyl tert-pentyl ether | diethyl ether |
| 298.15 | 2.39 | 0.316 | −0.0164 | 1.19 | 1.53 | 1.21 |
| 308.15 | 2.27 | 0.325 | 0.0104 | 1.20 | 1.54 | 1.21 |
| 318.15 | 2.19 | 0.331 | 0.0335 | 1.21 | 1.54 | 1.21 |
| 328.15 | 2.10 | 0.337 | 0.0458 | 1.22 | 1.55 | 1.21 |
| 338.15 | 2.01 | 0.345 | 0.0626 | 1.23 | 1.55 | 1.21 |
| 348.15 | 1.92 | 0.348 | 0.0878 | 1.24 | 1.56 | 1.21 |
| 358.15 | 1.85 | 0.355 | 0.101 | 1.24 | 1.56 | 1.20 |
| T/K | di-n-propyl ether | di-n-butyl ether | acetone | 2-pentanone | 3-pentanone | 2-hexanone |
| 298.15 | 2.24 | 3.06 | −0.445 | −0.0425 | −0.0790 | 0.210 |
| 308.15 | 2.21 | 2.99 | −0.421 | −0.0239 | −0.0528 | 0.225 |
| 318.15 | 2.18 | 2.94 | −0.398 | −0.0018 | −0.0208 | 0.238 |
| 328.15 | 2.14 | 2.87 | −0.379 | 0.0155 | 0.0047 | 0.247 |
| 338.15 | 2.12 | 2.83 | −0.358 | 0.0298 | 0.0266 | 0.261 |
| 348.15 | 2.09 | 2.78 | −0.344 | 0.0427 | 0.0464 | 0.272 |
| 358.15 | 2.06 | 2.73 | −0.325 | 0.0601 | 0.0678 | 0.283 |
| T/K | 3-hexanone | |||||
| 298.15 | 0.276 | |||||
| 308.15 | 0.294 | |||||
| 318.15 | 0.314 | |||||
| 328.15 | 0.330 | |||||
| 338.15 | 0.343 | |||||
| 348.15 | 0.355 | |||||
| 358.15 | 0.371 | |||||
| [dmim][TCB] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | 2,2,4-trimethylpentane | n-nonane |
| 328.15 | 1.98 | 2.11 | 2.27 | 2.44 | 2.35 | 2.62 |
| 338.15 | 1.94 | 2.07 | 2.23 | 2.39 | 2.30 | 2.57 |
| 348.15 | 1.90 | 2.03 | 2.18 | 2.34 | 2.25 | 2.52 |
| 358.15 | 1.85 | 1.99 | 2.13 | 2.30 | 2.21 | 2.47 |
| 368.15 | 1.81 | 1.94 | 2.09 | 2.25 | 2.17 | 2.42 |
| T/K | n-decane | cyclopentane | cyclohexane | methylcyclohe xane | cycloheptane | cyclooctane |
| 328.15 | 2.82 | 1.58 | 1.73 | 1.84 | 1.78 | 1.88 |
| 338.15 | 2.76 | 1.54 | 1.68 | 1.79 | 1.73 | 1.83 |
| 348.15 | 2.71 | 1.50 | 1.63 | 1.75 | 1.69 | 1.79 |
| 358.15 | 2.65 | 1.46 | 1.59 | 1.71 | 1.65 | 1.74 |
| 368.15 | 2.60 | 1.42 | 1.54 | 1.67 | 1.61 | 1.70 |
| T/K | 1-pentene | 1-hexene | cyclohexene | 1-heptene | 1-octene | 1-hexyne |
| 328.15 | 1.49 | 1.63 | 1.28 | 1.78 | 1.96 | 0.853 |
| 338.15 | 1.47 | 1.59 | 1.25 | 1.75 | 1.93 | 0.857 |
| 348.15 | 1.45 | 1.56 | 1.23 | 1.73 | 1.90 | 0.860 |
| 358.15 | 1.42 | 1.53 | 1.21 | 1.70 | 1.87 | 0.859 |
| 368.15 | 1.40 | 1.51 | 1.18 | 1.68 | 1.84 | 0.861 |
| T/K | 1-heptyne | 1-octyne | benzene | toluene | ethylbenzene | o-xylene |
| 328.15 | 0.983 | 1.14 | 0.0698 | 0.182 | 0.382 | 0.266 |
| 338.15 | 0.987 | 1.14 | 0.0826 | 0.201 | 0.396 | 0.283 |
| 348.15 | 0.990 | 1.14 | 0.0957 | 0.218 | 0.409 | 0.302 |
| 358.15 | 0.991 | 1.14 | 0.105 | 0.233 | 0.421 | 0.318 |
| 368.15 | 0.992 | 1.14 | 0.114 | 0.247 | 0.429 | 0.330 |
| T/K | m-xylene | p-xylene | methanol | ethanol | 1-propanol | 1-butanol |
| 328.15 | 0.361 | 0.343 | 0.997 | 0.835 | 0.682 | 0.635 |
| 338.15 | 0.381 | 0.366 | 0.929 | 0.759 | 0.613 | 0.565 |
| 348.15 | 0.402 | 0.386 | 0.870 | 0.693 | 0.555 | 0.507 |
| 358.15 | 0.416 | 0.401 | 0.803 | 0.625 | 0.497 | 0.452 |
| 368.15 | 0.437 | 0.422 | 0.752 | 0.566 | 0.441 | 0.392 |
| T/K | water | acetic acid | butyric acid | thiophene | tetrahydrofuran | methyl tert-butyl ether |
| 328.15 | 2.78 | −0.332 | 0.118 | 0.0626 | −0.338 | 0.547 |
| 338.15 | 2.68 | −0.284 | 0.116 | 0.0761 | −0.307 | 0.565 |
| 348.15 | 2.57 | −0.238 | 0.114 | 0.0845 | −0.279 | 0.585 |
| 358.15 | 2.49 | −0.198 | 0.111 | 0.0968 | −0.255 | 0.605 |
| 368.15 | 2.42 | −0.164 | 0.109 | 0.107 | −0.230 | 0.619 |
| T/K | methyl tert-pentyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether | acetone | 2-pentanone |
| 328.15 | 0.711 | 0.626 | 1.15 | 1.49 | −0.450 | −0.462 |
| 338.15 | 0.728 | 0.635 | 1.14 | 1.48 | −0.428 | −0.431 |
| 348.15 | 0.746 | 0.641 | 1.13 | 1.47 | −0.409 | −0.404 |
| 358.15 | 0.760 | 0.646 | 1.13 | 1.46 | −0.394 | −0.380 |
| 368.15 | 0.772 | 0.649 | 1.12 | 1.45 | −0.378 | −0.353 |
| T/K | 3-pentanone | |||||
| 328.15 | −0.497 | |||||
| 338.15 | −0.460 | |||||
| 348.15 | −0.426 | |||||
| 358.15 | −0.395 | |||||
| 368.15 | −0.364 | |||||
| [bmPIP][SCN] | ||||||
| T/K | n-hexane | n-heptane | n-octane | n-nonane | n-decane | cyclopentane |
| 318.15 | 4.90 | 5.19 | 5.49 | 5.82 | 6.19 | 3.55 |
| 328.15 | 4.73 | 5.00 | 5.36 | 5.69 | 6.07 | 3.42 |
| 338.15 | 4.57 | 4.89 | 5.24 | 5.60 | 5.97 | 3.32 |
| 348.15 | 4.44 | 4.75 | 5.11 | 5.46 | 5.84 | 3.21 |
| 358.15 | 4.30 | 4.67 | 5.03 | 5.37 | 5.74 | 3.15 |
| T/K | cyclohexane | cycloheptane | cyclooctane | 1-hexene | 1-heptene | 1-octene |
| 318.15 | 3.85 | 3.91 | 4.17 | 3.82 | 4.16 | 4.54 |
| 328.15 | 3.74 | 3.84 | 4.07 | 3.71 | 4.08 | 4.46 |
| 338.15 | 3.64 | 3.74 | 3.97 | 3.64 | 4.00 | 4.38 |
| 348.15 | 3.54 | 3.65 | 3.88 | 3.56 | 3.92 | 4.30 |
| 358.15 | 3.46 | 3.60 | 3.81 | 3.49 | 3.87 | 4.24 |
| T/K | 1-hexyne | 1-heptyne | 1-octyne | benzene | toluene | ethylbenzene |
| 318.15 | 1.94 | 2.30 | 2.66 | 0.907 | 1.32 | 1.76 |
| 328.15 | 1.94 | 2.30 | 2.66 | 0.916 | 1.33 | 1.76 |
| 338.15 | 1.95 | 2.30 | 2.66 | 0.924 | 1.33 | 1.75 |
| 348.15 | 1.95 | 2.30 | 2.66 | 0.930 | 1.33 | 1.75 |
| 358.15 | 1.95 | 2.30 | 2.66 | 0.938 | 1.34 | 1.74 |
| T/K | o-xylene | m-xylene | p-xylene | methanol | ethanol | water |
| 318.15 | 1.54 | 1.77 | 1.72 | −0.187 | 0.103 | 0.413 |
| 328.15 | 1.55 | 1.77 | 1.73 | −0.190 | 0.0822 | 0.429 |
| 338.15 | 1.55 | 1.77 | 1.73 | −0.191 | 0.0600 | 0.445 |
| 348.15 | 1.56 | 1.77 | 1.73 | −0.196 | 0.0405 | 0.460 |
| 358.15 | 1.56 | 1.77 | 1.74 | −0.198 | 0.0241 | 0.476 |
| T/K | thiophene | tetrahydrofuran | methyl tert-butyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether |
| 318.15 | 0.434 | 1.14 | 2.70 | 2.67 | 3.63 | 4.39 |
| 328.15 | 0.459 | 1.15 | 2.66 | 2.62 | 3.56 | 4.31 |
| 338.15 | 0.486 | 1.16 | 2.63 | 2.57 | 3.50 | 4.24 |
| 348.15 | 0.504 | 1.16 | 2.59 | 2.54 | 3.44 | 4.17 |
| 358.15 | 0.525 | 1.17 | 2.57 | 2.50 | 3.39 | 4.12 |
| T/K | acetone | 2-pentanone | 3-pentanone | |||
| 318.15 | 0.795 | 1.29 | 1.30 | |||
| 328.15 | 0.794 | 1.29 | 1.30 | |||
| 338.15 | 0.792 | 1.29 | 1.30 | |||
| 348.15 | 0.790 | 1.29 | 1.30 | |||
| 358.15 | 0.789 | 1.29 | 1.30 | |||
| [pmPIP][NTf2] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | n-nonane | n-decane |
| 308.15 | 3.31 | 3.40 | 3.58 | 3.81 | 4.08 | 4.38 |
| 318.15 | 3.02 | 3.21 | 3.44 | 3.70 | 3.98 | 4.30 |
| 328.15 | 2.98 | 3.14 | 3.35 | 3.60 | 3.88 | 4.18 |
| 338.15 | 2.85 | 3.06 | 3.29 | 3.53 | 3.80 | 4.09 |
| 348.15 | 2.75 | 2.95 | 3.19 | 3.43 | 3.70 | 3.99 |
| 358.15 | 2.72 | 2.93 | 3.14 | 3.37 | 3.63 | 3.91 |
| T/K | cyclopentane | cyclohexane | cycloheptane | cyclooctane | 1-pentene | 1-hexene |
| 308.15 | 2.72 | 2.93 | 3.05 | 3.23 | 2.48 | 2.64 |
| 318.15 | 2.52 | 2.77 | 2.94 | 3.15 | 2.30 | 2.52 |
| 328.15 | 2.47 | 2.71 | 2.86 | 3.04 | 2.27 | 2.48 |
| 338.15 | 2.38 | 2.62 | 2.78 | 2.98 | 2.32 | 2.41 |
| 348.15 | 2.29 | 2.51 | 2.70 | 2.89 | 2.13 | 2.34 |
| 358.15 | 2.26 | 2.48 | 2.64 | 2.82 | 2.13 | 2.31 |
| T/K | 1-heptene | 1-octene | 1-hexyne | 1-heptyne | 1-octyne | benzene |
| 308.15 | 2.84 | 3.11 | 1.47 | 1.71 | 1.98 | 0.418 |
| 318.15 | 2.76 | 3.04 | 1.46 | 1.70 | 1.98 | 0.427 |
| 328.15 | 2.71 | 2.96 | 1.45 | 1.68 | 1.95 | 0.430 |
| 338.15 | 2.65 | 2.92 | 1.46 | 1.69 | 1.94 | 0.433 |
| 348.15 | 2.59 | 2.85 | 1.44 | 1.67 | 1.95 | 0.444 |
| 358.15 | 2.55 | 2.81 | 1.44 | 1.67 | 1.91 | 0.456 |
| T/K | toluene | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol |
| 308.15 | 0.615 | 0.913 | 0.752 | 0.822 | 0.812 | 1.62 |
| 318.15 | 0.619 | 0.915 | 0.772 | 0.851 | 0.845 | 1.54 |
| 328.15 | 0.635 | 0.932 | 0.775 | 0.849 | 0.849 | 1.44 |
| 338.15 | 0.642 | 0.930 | 0.787 | 0.865 | 0.864 | 1.36 |
| 348.15 | 0.655 | 0.936 | 0.795 | 0.875 | 0.876 | 1.28 |
| 358.15 | 0.668 | 0.942 | 0.811 | 0.892 | 0.894 | 1.20 |
| T/K | ethanol | 1-propanol | 1-butanol | water | thiophene | tetrahydrofuran |
| 308.15 | 1.54 | 1.55 | 1.64 | 3.36 | 0.377 | 0.355 |
| 318.15 | 1.45 | 1.46 | 1.56 | 3.27 | 0.386 | 0.363 |
| 328.15 | 1.35 | 1.35 | 1.42 | 3.14 | 0.389 | 0.370 |
| 338.15 | 1.26 | 1.26 | 1.32 | 3.04 | 0.401 | 0.385 |
| 348.15 | 1.18 | 1.17 | 1.23 | 2.90 | 0.404 | 0.384 |
| 358.15 | 1.10 | 1.10 | 1.15 | 2.78 | 0.416 | 0.398 |
| T/K | methyl tert-butyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether | acetone | 2-pentanone |
| 308.15 | 1.38 | 1.47 | 2.16 | 2.78 | −0.0329 | 0.181 |
| 318.15 | 1.37 | 1.41 | 2.12 | 2.72 | −0.0217 | 0.200 |
| 328.15 | 1.36 | 1.41 | 2.10 | 2.68 | −0.0151 | 0.209 |
| 338.15 | 1.35 | 1.39 | 2.07 | 2.63 | 0.0007 | 0.225 |
| 348.15 | 1.34 | 1.36 | 2.02 | 2.57 | −0.0029 | 0.229 |
| 358.15 | 1.33 | 1.36 | 2.01 | 2.53 | 0.0071 | 0.244 |
| T/K | 3-pentanone | |||||
| 308.15 | 0.161 | |||||
| 318.15 | 0.178 | |||||
| 328.15 | 0.208 | |||||
| 338.15 | 0.225 | |||||
| 348.15 | 0.235 | |||||
| 358.15 | 0.256 | |||||
| [bmPIP][NTf2] | ||||||
| T/K | n-pentane | n-hexane | 3-methylpentane | 2,2-dimethylbutane | n-heptane | n-octane |
| 308.15 | 2.62 | 2.85 | 2.74 | 2.66 | 3.13 | 3.42 |
| 318.15 | 2.51 | 2.72 | 2.62 | 2.54 | 3.00 | 3.27 |
| 328.15 | 2.39 | 2.62 | 2.50 | 2.42 | 2.89 | 3.16 |
| 338.15 | 2.34 | 2.56 | 2.45 | 2.37 | 2.82 | 3.09 |
| 348.15 | 2.29 | 2.51 | 2.40 | 2.33 | 2.76 | 3.02 |
| 358.15 | 2.23 | 2.47 | 2.35 | 2.28 | 2.69 | 2.95 |
| T/K | 2,2,4-trimethylpentane | n-nonane | n-decane | cyclopentane | cyclohexane | methylcyclohe xane |
| 308.15 | 3.03 | 3.71 | 4.01 | 2.22 | 2.48 | 2.67 |
| 318.15 | 2.92 | 3.55 | 3.84 | 2.11 | 2.36 | 2.54 |
| 328.15 | 2.81 | 3.42 | 3.71 | 2.02 | 2.26 | 2.44 |
| 338.15 | 2.74 | 3.35 | 3.63 | 1.96 | 2.20 | 2.38 |
| 348.15 | 2.69 | 3.28 | 3.54 | 1.90 | 2.12 | 2.31 |
| 358.15 | 2.63 | 3.20 | 3.47 | 1.87 | 2.09 | 2.27 |
| T/K | cycloheptane | cyclooctane | 1-pentene | 1-hexene | cyclohexene | 1-heptene |
| 308.15 | 2.70 | 2.91 | 2.00 | 2.26 | 1.95 | 2.51 |
| 318.15 | 2.57 | 2.78 | 1.91 | 2.16 | 1.87 | 2.41 |
| 328.15 | 2.47 | 2.67 | 1.83 | 2.08 | 1.79 | 2.33 |
| 338.15 | 2.40 | 2.60 | 1.81 | 2.03 | 1.75 | 2.29 |
| 348.15 | 2.32 | 2.51 | 1.76 | 1.97 | 1.70 | 2.23 |
| 358.15 | 2.28 | 2.47 | 1.73 | 1.94 | 1.64 | 2.21 |
| T/K | 1-octene | 1-hexyne | 1-heptyne | 1-octyne | benzene | toluene |
| 308.15 | 2.80 | 1.28 | 1.52 | 1.79 | 0.304 | 0.486 |
| 318.15 | 2.70 | 1.24 | 1.47 | 1.72 | 0.315 | 0.498 |
| 328.15 | 2.60 | 1.20 | 1.43 | 1.67 | 0.317 | 0.508 |
| 338.15 | 2.55 | 1.20 | 1.42 | 1.66 | 0.324 | 0.518 |
| 348.15 | 2.49 | 1.18 | 1.40 | 1.65 | 0.338 | 0.534 |
| 358.15 | 2.46 | 1.20 | 1.40 | 1.63 | 0.354 | 0.550 |
| T/K | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol | ethanol |
| 308.15 | 0.793 | 0.632 | 0.702 | 0.698 | 1.60 | 1.49 |
| 318.15 | 0.800 | 0.646 | 0.723 | 0.716 | 1.52 | 1.40 |
| 328.15 | 0.795 | 0.644 | 0.718 | 0.719 | 1.42 | 1.31 |
| 338.15 | 0.797 | 0.650 | 0.729 | 0.733 | 1.34 | 1.21 |
| 348.15 | 0.807 | 0.667 | 0.748 | 0.747 | 1.25 | 1.12 |
| 358.15 | 0.816 | 0.671 | 0.759 | 0.758 | 1.16 | 1.04 |
| T/K | 1-propanol | 1-butanol | water | thiophene | tetrahydrofuran | methyl tert-butyl ether |
| 308.15 | 1.47 | 1.54 | 3.49 | 0.299 | 0.206 | 1.06 |
| 318.15 | 1.38 | 1.42 | 3.34 | 0.302 | 0.215 | 1.06 |
| 328.15 | 1.28 | 1.33 | 3.21 | 0.301 | 0.205 | 1.05 |
| 338.15 | 1.18 | 1.22 | 3.07 | 0.306 | 0.207 | 1.04 |
| 348.15 | 1.09 | 1.12 | 2.94 | 0.308 | 0.207 | 1.04 |
| 358.15 | 1.01 | 1.04 | 2.81 | 0.323 | 0.219 | 1.04 |
| T/K | diethyl ether | di-n-propyl ether | di-n-butyl ether | acetone | 2-pentanone | 3-pentanone |
| 308.15 | 1.19 | 1.86 | 2.46 | −0.0841 | 0.0558 | 0.0284 |
| 318.15 | 1.18 | 1.85 | 2.43 | −0.0782 | 0.0667 | 0.0492 |
| 328.15 | 1.11 | 1.75 | 2.33 | −0.0764 | 0.0885 | 0.0770 |
| 338.15 | 1.12 | 1.75 | 2.29 | −0.0739 | 0.0943 | 0.0842 |
| 348.15 | 1.11 | 1.73 | 2.25 | −0.0732 | 0.106 | 0.114 |
| 358.15 | 1.10 | 1.70 | 2.21 | −0.0706 | 0.115 | 0.124 |
| [OQuin][NTf2] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | n-nonane | n-decane |
| 328.15 | 1.90 | 2.00 | 2.17 | 2.30 | 2.46 | 2.65 |
| 338.15 | 1.86 | 1.97 | 2.12 | 2.26 | 2.42 | 2.60 |
| 348.15 | 1.82 | 1.93 | 2.07 | 2.21 | 2.37 | 2.54 |
| 358.15 | 1.79 | 1.90 | 2.03 | 2.18 | 2.33 | 2.50 |
| 368.15 | 1.75 | 1.87 | 1.99 | 2.14 | 2.29 | 2.45 |
| T/K | cyclopentane | cyclohexane | cycloheptane | cyclooctane | 1-pentene | 1-hexene |
| 328.15 | 1.57 | 1.70 | 1.76 | 1.85 | 1.50 | 1.60 |
| 338.15 | 1.54 | 1.66 | 1.72 | 1.81 | 1.48 | 1.58 |
| 348.15 | 1.50 | 1.61 | 1.68 | 1.76 | 1.46 | 1.55 |
| 358.15 | 1.47 | 1.58 | 1.64 | 1.72 | 1.43 | 1.53 |
| 368.15 | 1.43 | 1.54 | 1.61 | 1.68 | 1.41 | 1.51 |
| T/K | 1-heptene | 1-octene | 1-hexyne | 1-heptyne | 1-octyne | benzene |
| 328.15 | 1.74 | 1.89 | 0.977 | 1.09 | 1.22 | 0.188 |
| 338.15 | 1.72 | 1.87 | 0.982 | 1.09 | 1.22 | 0.194 |
| 348.15 | 1.70 | 1.84 | 0.981 | 1.09 | 1.21 | 0.206 |
| 358.15 | 1.68 | 1.82 | 0.983 | 1.09 | 1.22 | 0.216 |
| 368.15 | 1.66 | 1.80 | 0.987 | 1.08 | 1.22 | 0.224 |
| T/K | toluene | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol |
| 328.15 | 0.247 | 0.471 | 0.255 | 0.386 | 0.399 | 1.62 |
| 338.15 | 0.267 | 0.485 | 0.282 | 0.402 | 0.416 | 1.54 |
| 348.15 | 0.285 | 0.495 | 0.303 | 0.419 | 0.424 | 1.43 |
| 358.15 | 0.302 | 0.505 | 0.322 | 0.430 | 0.437 | 1.35 |
| 368.15 | 0.320 | 0.517 | 0.340 | 0.445 | 0.447 | 1.27 |
| T/K | ethanol | 1-propanol | 1-butanol | 1-pentanol | water | thiophene |
| 328.15 | 1.43 | 1.29 | 1.24 | 1.15 | 3.53 | 0.215 |
| 338.15 | 1.32 | 1.20 | 1.14 | 1.07 | 3.40 | 0.223 |
| 348.15 | 1.22 | 1.10 | 1.02 | 0.979 | 3.24 | 0.227 |
| 358.15 | 1.14 | 1.02 | 0.943 | 0.896 | 3.12 | 0.231 |
| 368.15 | 1.06 | 0.942 | 0.865 | 0.827 | 2.98 | 0.236 |
| T/K | tetrahydrofuran | methyl tert-butyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether | acetone |
| 328.15 | 0.0326 | 0.778 | 0.879 | 1.33 | 1.65 | −0.0485 |
| 338.15 | 0.0506 | 0.787 | 0.880 | 1.31 | 1.63 | −0.0465 |
| 348.15 | 0.0653 | 0.793 | 0.881 | 1.30 | 1.60 | −0.0456 |
| 358.15 | 0.0742 | 0.796 | 0.879 | 1.28 | 1.57 | −0.0458 |
| 368.15 | 0.0853 | 0.803 | 0.877 | 1.26 | 1.54 | −0.0465 |
| T/K | 2-pentanone | 3-pentanone | ||||
| 328.15 | −0.0778 | −0.0596 | ||||
| 338.15 | −0.0694 | −0.0483 | ||||
| 348.15 | −0.0621 | −0.0372 | ||||
| 358.15 | −0.0521 | −0.0285 | ||||
| 368.15 | −0.0461 | −0.0216 | ||||
Example of Calculation of the Solubility Parameter
- T = 298.15 K
- pi = 137423 Pa
- po = 97423 Pa
- Tf = 297.15 K
- U = 41.2 mL·min−1
- tR − tG = 270.66 s
- m2 = 2.1053 g
- Uo = 6.679·10−7 m3·s−1 (from Equation 6)
- J23 = 1.217 (from Equation 5)
- VN = 1.485·10−4 m3 (from Equation 4)
- P1* = 1871.0 Pa (from [30])
- M1 = 114.2285 g·mol−1 (from [30])
- B11 = −4.496·10−3 m3·mol−1 (from [30])
- V1* = 1.6256·10−4 m3·mol−1 (from [30])
- V2* = 2.1818·10−4 m3·mol−1 (calculated from density from [19])
- ρ1 = 0.70268 g·cm−3 (from [30])
- ρ2 = 1.03627 g·cm−3 (from [19])
- χ12∞ = 4.463 (from Equation 2)
- δ1 = 15486 (J·m3)0.5 (from [30])
 ) n-octane, (
) n-octane, (
 ) rest of solutes.
) rest of solutes.
 ) n-octane, (
) n-octane, (
 ) rest of solutes.
) rest of solutes.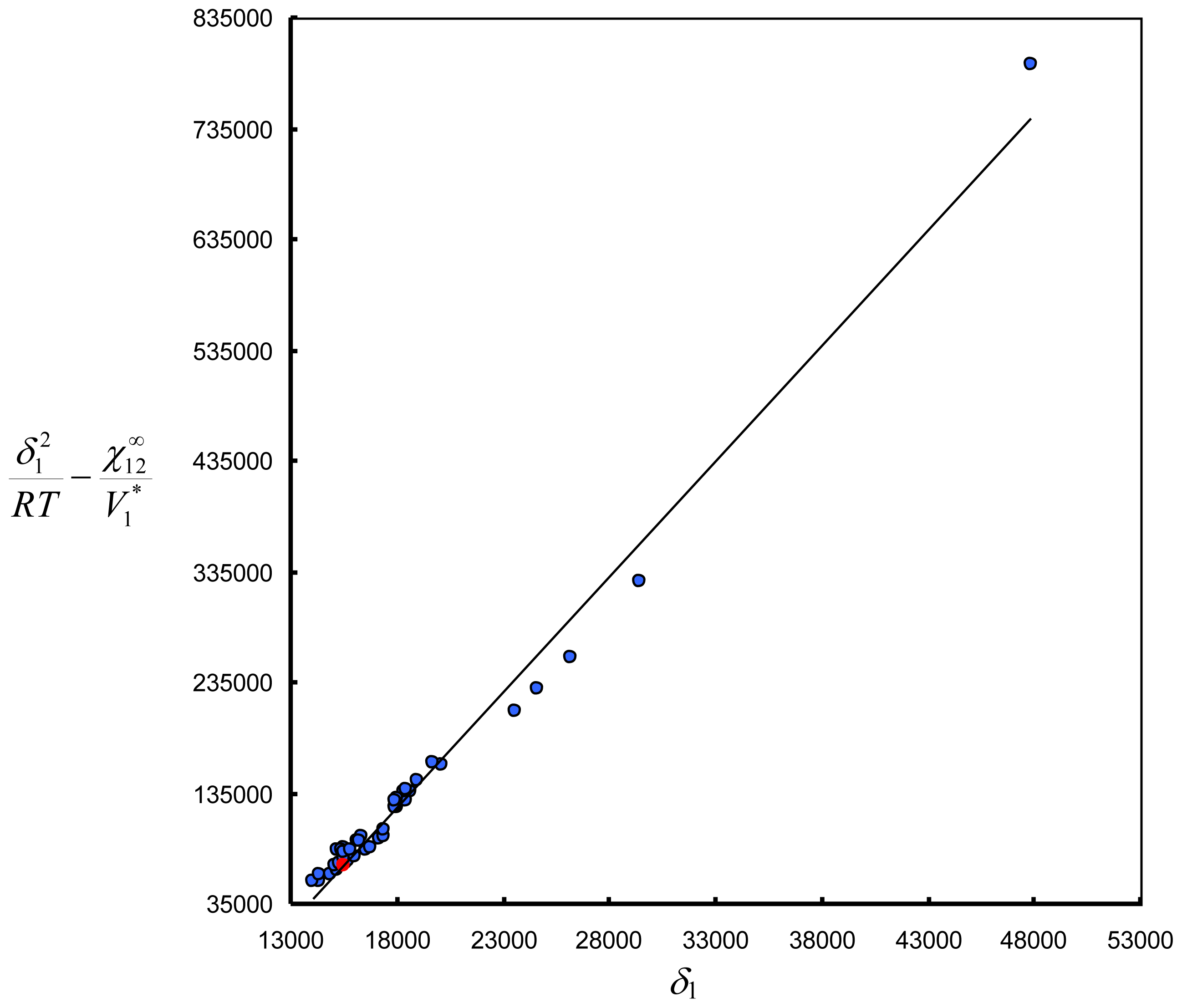
Calculation of the Kv Constant from Equation 2
| Ionic Liquid | ρ/g·cm−3 | M/g·mol−1 | μ/mPa·s | υ/cm3·mol−1 | δ2/MPa0.5 |
|---|---|---|---|---|---|
| [emim][NTf2] | 1.5192 | 391.32 | 34.29 | 257.6 | 21.3 |
| 1.5192 | 391.32 | 34.29 | 257.6 | 22.6 | |
| 1.5192 | 391.32 | 34.29 | 257.6 | 22.7 | |
| [bmim][NTf2] | 1.4366 | 419.37 | 50.70 | 291.9 | 21.2 |
| 1.4366 | 419.37 | 50.70 | 291.9 | 19.8 | |
| 1.4366 | 419.37 | 50.70 | 291.9 | 22.9 | |
| [hmim][NTf2] | 1.3706 | 447.42 | 70.96 | 326.5 | 20.5 |
| 1.3706 | 447.42 | 70.96 | 326.5 | 19.0 | |
| 1.3706 | 447.42 | 70.96 | 326.5 | 22.9 | |
| [omim][NTf2] | 1.3206 | 475.48 | 92.51 | 360.1 | 20.2 |
| 1.3206 | 475.48 | 92.51 | 360.1 | 20.2 | |
| 1.3206 | 475.48 | 92.51 | 360.1 | 23.0 | |
| 1.3206 | 475.48 | 92.51 | 360.1 | 18.9 | |
| [dmim][NTf2] | 1.2780 | 499.50 | 108.20 | 390.8 | 17.8 |
| [bmPYR][NTf2] | 1.3940 | 422.41 | 76.92 | 303.0 | 22.2 |
| Ionic Liquid | T/K | ρ/g·cm−3a | μ/m Pa·s b |
|---|---|---|---|
| [N-C3OHPY][NTf2] | 308.15 | 1.5451 | 67.03 |
| 318.15 | 1.5357 | 43.85 | |
| 328.15 | 1.5266 | 30.56 | |
| 338.15 | 1.5175 | 22.21 | |
| 348.15 | 1.5085 | 16.90 | |
| [pmPIP][NTf2] | 308.15 | 1.4010 | 86.70 |
| 318.15 | 1.3923 | 55.24 | |
| 328.15 | 1.3837 | 37.75 | |
| 338.15 | 1.3751 | 27.17 | |
| 348.15 | 1.3666 | 20.32 | |
| [bmPIP][NTf2] | 308.15 | 1.3706 | 97.76 |
| 318.15 | 1.3621 | 61.05 | |
| 328.15 | 1.3536 | 40.92 | |
| 338.15 | 1.3452 | 29.01 | |
| 348.15 | 1.3369 | 21.28 | |
References
- Voelkel, A; Strzemiecka, B; Adamska, K; Milczewska, K. Inverse gas chromatography as a source of physiochemical data. J. Chromatogr. A 2009, 1216, 1551–1566. [Google Scholar]
- Marciniak, A. The solubility parameters of ionic liquids. Int. J. Mol. Sci 2010, 11, 1973–1990. [Google Scholar]
- Revelli, A-L; Mutelet, F; Jaubert, J-N. Partition coefficients of organic compounds in new imidazolium based ionic liquids using inverse gas chromatography. J. Chromatogr. A 2009, 1216, 4775–4786. [Google Scholar]
- Mutelet, F; Jaubert, J-N. Measurement of activity coefficients at infinite dilution in 1-hexadecyl- 3-methylimidazolium tetrafluoroborate ionic liquid. J. Chem. Thermodyn 2007, 39, 1144–1150. [Google Scholar]
- Mutelet, F; Butet, V; Jaubert, J-N. Application of inverse gas chromatography and regular solution theory for characterization of ionic liquids. Ind. Eng. Chem. Res 2005, 44, 4120–4127. [Google Scholar]
- Foco, GM; Bottini, SB; Quezada, N; de la Fuente, JC; Peters, CJ. Activity coefficients at infinite dilution in 1-alkyl-3-methylimidazolium tetrafluoroborate ionic liquids. J. Chem. Eng. Data 2006, 51, 1088–1091. [Google Scholar]
- Barton, AFM. Solubility parameters. Chem. Rev 1975, 75, 731–753. [Google Scholar]
- Hansen, CM. Hansen Solubility Parameters: A User’s Handbook, 2nd ed; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Camper, D; Scovazzo, P; Koval, C; Noble, R. Gas Solubilities in room-temperature ionic liquids. Ind. Eng. Chem. Res 2004, 43, 3049–3054. [Google Scholar]
- Lee, SH; Lee, SB. The Hildebrand solubility parameters, cohesive energy densities and internal energies of 1-alkyl-3-methylimidazolium-based room temperature ionic liquids. Chem Commun 2005, 3469–3471. [Google Scholar]
- Kilaru, PK; Scovazzo, P. Correlations of low-pressure carbon dioxide and hydrocarbon solubilities in imidazolium-, phosphonium-, and ammonium-based room-temperature ionic liquids. Part 2. Using activation energy of viscosity. Ind. Eng. Chem. Res 2008, 47, 910–919. [Google Scholar]
- Moganty, SS; Baltus, RE. Regular solution theory for low pressure carbon dioxide solubility in room temperature ionic liquids: Ionic liquid solubility parameter from activation energy of viscosity. Ind. Eng. Chem. Res 2010, 49, 5846–5853. [Google Scholar]
- Jin, H; O’Hare, B; Dong, J; Arzhantsev, S; Baker, GA; Wishart, JF; Benesi, AJ; Maroncelli, M. Physical properties of ionic liquids consisting of the 1-butyl-3-methylimidazolium cation with various anions and the bis(trifluoromethylsulfonyl)imide anion with various cations. J. Phys. Chem. B 2008, 112, 81–92. [Google Scholar]
- Swiderski, K; McLean, A; Gordon, CM; Vaughan, DH. Estimates of internal energies of vaporisation of some room temperature ionic liquids. Chem Commun 2004, 2178–2179. [Google Scholar]
- Paduszyński, K; Chiyen, J; Ramjugernath, D; Letcher, TM; Domańska, U. Liquid-liquid phase equilibrium of (piperidinium-based ionic liquid + an alcohol) binary systems and modelling with NRHB and PCP-SAFT. Fluid Phase Equilib 2011, 305, 43–52. [Google Scholar]
- Camper, D; Becker, C; Koval, C; Noble, R. Low pressure hydrocarbon solubility in room temperature ionic liquids containing imidazolium rings interpreted using regular solution theory. Ind. Eng. Chem. Res 2005, 44, 1928–1933. [Google Scholar]
- Marciniak, A; Wlazło, M. Activity Coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-(3-hydroxypropyl)pyridinium trifluorotris(perfluoroethyl) phosphate. J. Phys. Chem. B 2010, 114, 6990–6994. [Google Scholar]
- Marciniak, A. Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 1-(3-hydroxypropyl)pyridinium bis(trifluoromethylsulfonyl)- amide. J Chem Thermodyn 2011. [Google Scholar] [CrossRef]
- Domańska, U; Królikowska, M; William, EA; Baker, GA. Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-ethyl-3- methylimidazolium tetracyanoborate. J. Chem. Thermodyn 2011, 43, 1050–1057. [Google Scholar]
- Domańska, U; Marciniak, A. Physicochemical properties and activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-decyl-3-methylimidazolium tetracyanoborate. J. Phys. Chem. B 2010, 114, 16542–16547. [Google Scholar]
- Domańska, U; Królikowska, M. Measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-butyl-1-methylpiperidinium thiocyanate. J. Chem. Eng. Data 2011, 56, 124–129. [Google Scholar]
- Domańska, U; Paduszyński, K. Measurements of activity coefficients at infinite dilution of organic solutes and water in 1-propyl-1-methylpiperidinium bis{(trifluoromethyl)sulfonyl}imide ionic liquid using g.l.c. J. Chem. Thermodyn 2010, 42, 1361–1366. [Google Scholar]
- Paduszyński, K; Domańska, U. Limiting activity coefficients and gas-liquid partition coefficients of various solutes in piperidinium ionic liquids–measurements and LSER calculations. J Phys Chem B 2011. in submit. [Google Scholar]
- Domańska, U; Zawadzki, M; Królikowska, M; Tshibangu, MM; Ramjugernath, D; Letcher, TM. Measurements of activity coefficients at infinite dilution of organic compounds and water in isoquinolinium-based ionic liquid [C8iQuin][NTf2] using GLC. J. Chem. Thermodyn 2011, 43, 499–504. [Google Scholar]
- Luo, H; Baker, GA; Dai, S. Isothermogravimetric determination of the enthalpies of vaporization of 1-alkyl-3-methylimidazolium ionic liquids. J. Phys. Chem. B 2008, 112, 10077–10081. [Google Scholar]
- Armstrong, JP; Hurst, C; Jones, RG; Licence, P; Lovelock, KRJ; Satterley, CJ; Villar-Garcia, IJ. Vapourisation of ionic liquids. Phys. Chem. Chem. Phys 2007, 9, 982–990. [Google Scholar]
- Santos, LMNBF; Lopes, JNC; Coutinho, JAP; Esperança, JMSS; Gomes, LR; Marrucho, IM; Rebelo, LPN. Ionic liquids: First direct determination of their cohesive energy. J. Am. Chem. Soc 2007, 129, 284–285. [Google Scholar]
- Zaitsau, DH; Kabo, GJ; Strechan, AA; Paulechka, YU; Tschersich, A; Verevkin, SP; Heintz, A. Experimental vapor pressures of 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imides and a correlation scheme for estimation of vaporization enthalpies of ionic liquids. J. Phys. Chem. A 2006, 110, 7303–7306. [Google Scholar]
- Deyko, A; Lovelock, KRJ; Corfield, J-A; Taylor, AW; Gooden, PN; Villar-Garcia, IJ; Licence, P; Jones, RG; Krasovskiy, VG; Chernikova, EA; et al. Measuring and predicting ΔvapH298 values of ionic liquids. Phys. Chem. Chem. Phys 2009, 11, 8544–8555. [Google Scholar]
- DIPPR Project 801-Full Version; Design Institute for Physical Property Data/AIChE: New York, NY, USA, 2010.
| Abbreviation, Name, Source, Purity | Structure | Reference |
|---|---|---|
| abbreviation: [N-C3OHPY][FAP] name: 1-(3-hydroxypropyl)pyridinium trifluorotris(perfluoroethyl)phosphate source: MERCK purity > 0.999 mass fraction water content < 100 ppm halide content < 100 ppm | 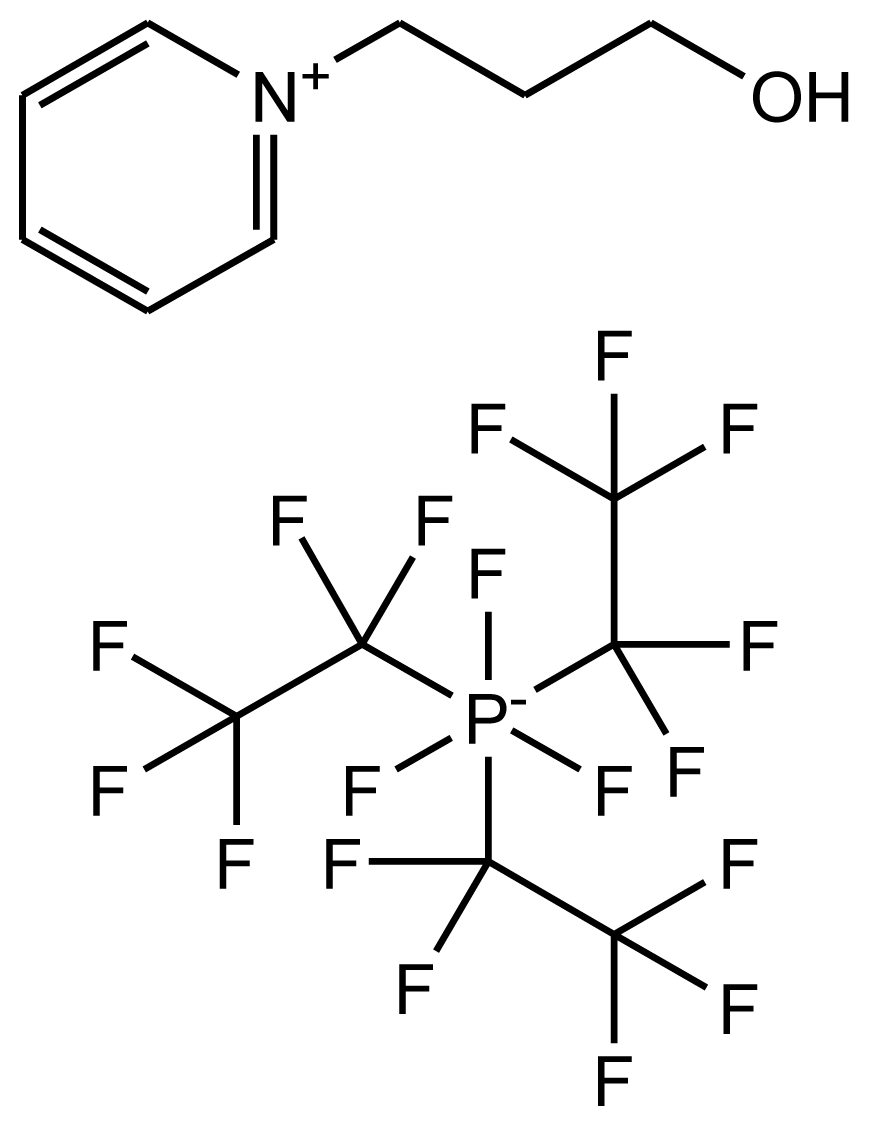 | [17] |
| abbreviation: [N-C3OHPY][NTf2] name: 1-(3-hydroxypropyl)pyridinium bis(trifluoromethylsulfonyl)-amide source: MERCK purity > 0.999 mass fraction water content < 100 ppm halide content < 100 ppm | 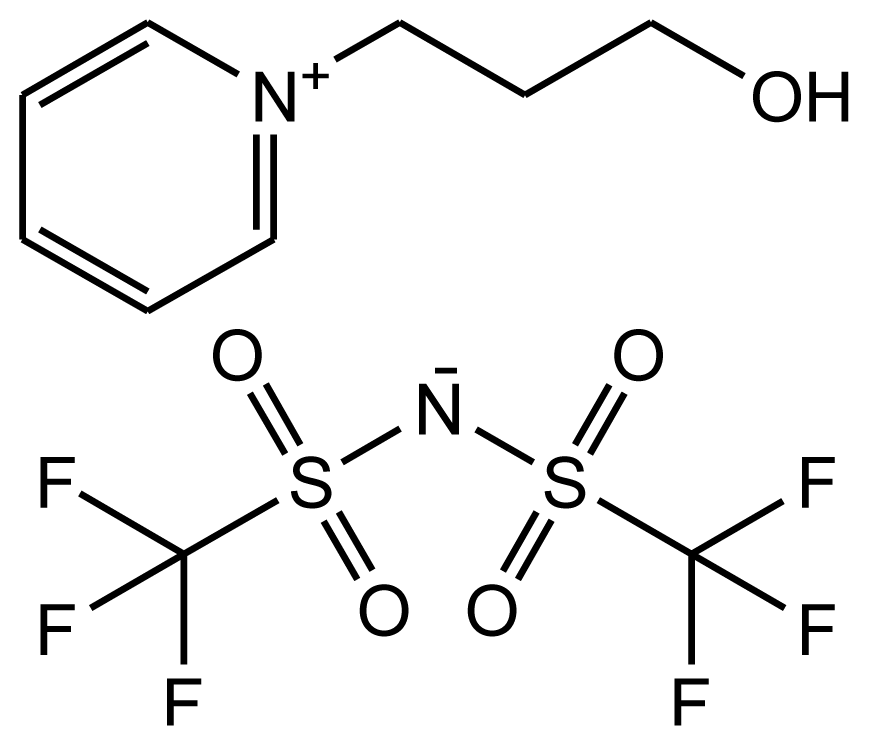 | [18] |
| abbreviation: [emim][TCB] name: 1-ethyl-3-methylimidazolium tetracyanoborate source: MERCK purity > 0.99 mass fraction water content < 200 ppm halide content < 100 ppm | 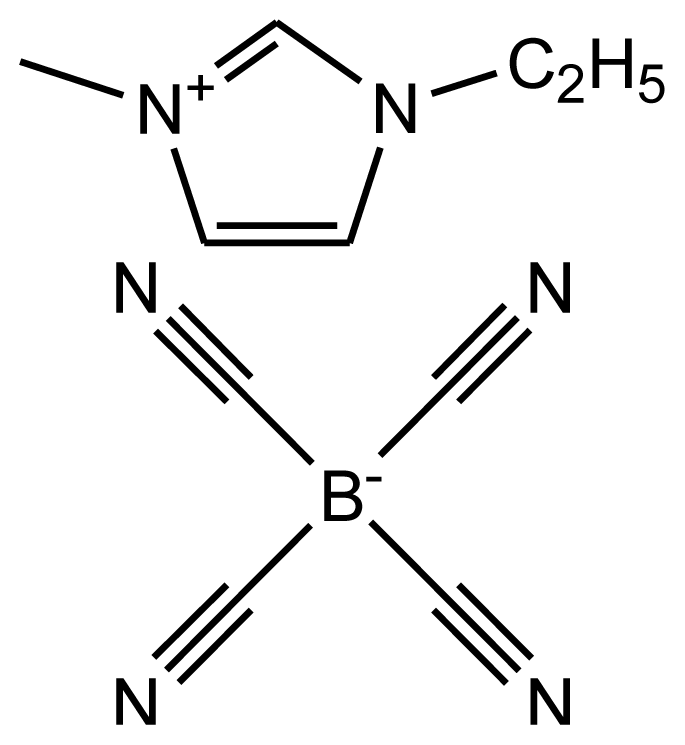 | [19] |
| abbreviation: [dmim][TCB] name: 1-decyl-3-methylimidazolium tetracyanoborate source: MERCK purity > 0.9996 mass fraction water content: < 100 ppm halide content < 100 ppm | 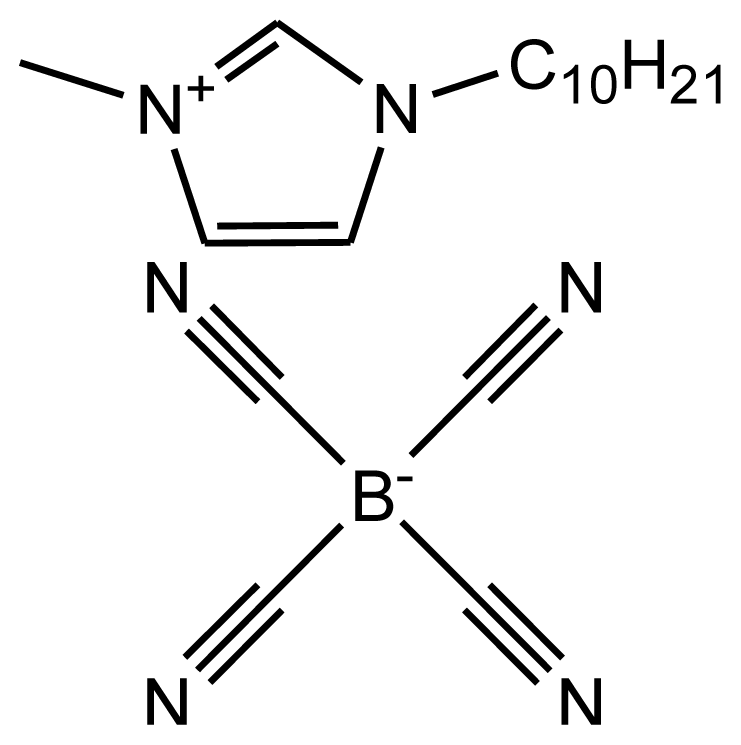 | [20] |
| abbreviation: [bmPIP][SCN] name: 1-butyl-1-methylpiperidinium thiocyanate source: IoLiTec purity > 0.98 mass fraction water content: < 100 ppm halide content < 100 ppm | 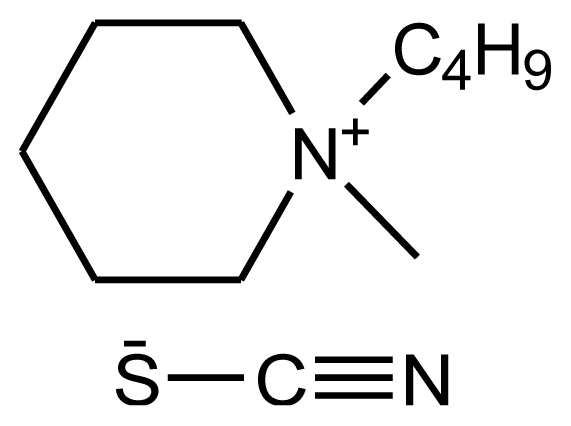 | [21] |
| abbreviation: [pmPIP][NTf2] name: 1-propyl-1-methylpiperidinium bis(trifluoromethylsulfonyl)-amide source: IoLiTec purity > 0.99 mass fraction water content: < 100 ppm halide content < 100 ppm | 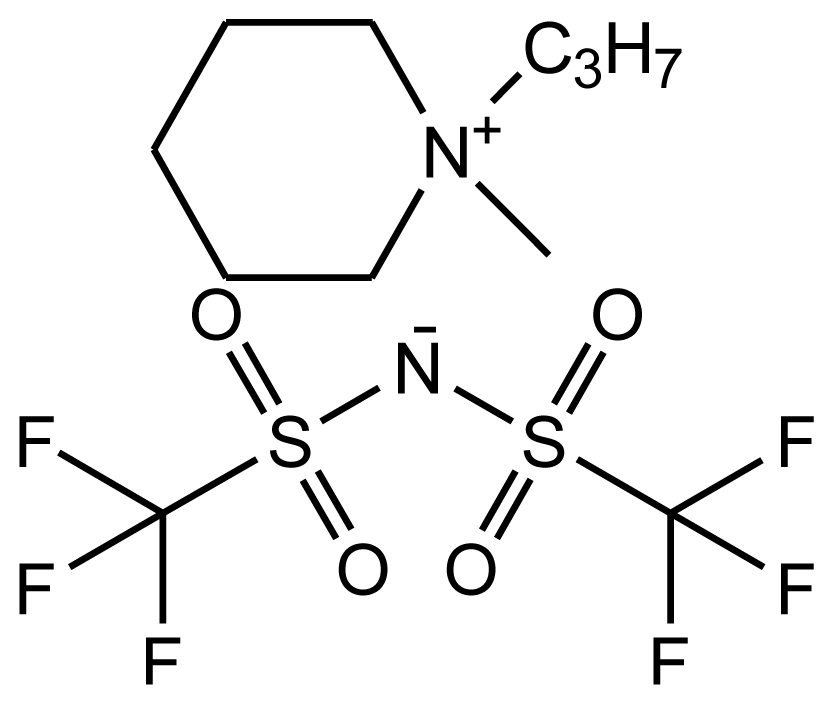 | [22] |
| abbreviation: [bmPIP][NTf2] name: 1-butyl-1-methylpiperidinium bis(trifluoromethylsulfonyl)-amide source: IoLiTec purity > 0.99 mass fraction water content: < 250 ppm halide content < 100 ppm | 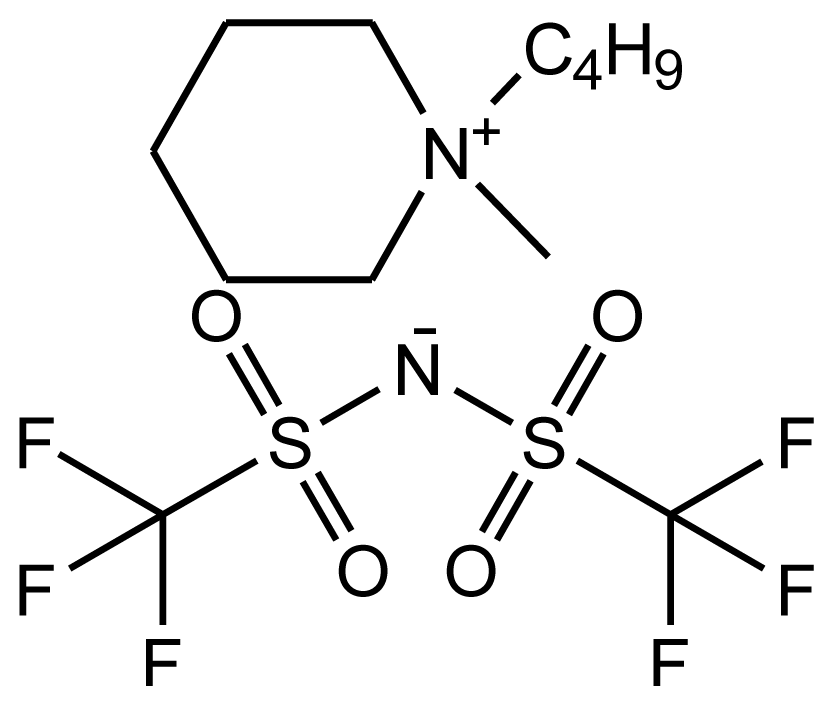 | [23] |
| abbreviation: [OiQuin][NTf2] name: N-octyl-isoquinolinium bis(trifluoromethylsulfonyl)-amide source: synthesized purity > 0.99 mass fraction water content: < 180 ppm halide content < 100 ppm |  | [24] |
| Ionic Liquid | T/K | δ2/MPa0.5 | ΔvapH/kJ·mol−1 |
|---|---|---|---|
| [N-C3OHPY][FAP] | 298.15 | 25.0a | 212.3 |
| 308.15 | 24.7 | 209.6 | |
| 318.15 | 24.5 | 206.6 | |
| 328.15 | 24.2 | 203.3 | |
| 338.15 | 23.9 | 199.6 | |
| 348.15 | 23.6 | 196.2 | |
| 358.15 | 23.3 | 192.1 | |
| [N-C3OHPY][NTf2] | 298.15 | 26.0a | 186.1 |
| 318.15 | 25.6 | 182.0 | |
| 328.15 | 25.3 | 179.5 | |
| 338.15 | 25.1 | 176.9 | |
| 348.15 | 24.8 | 174.2 | |
| 358.15 | 24.5 | 171.2 | |
| [emim][TCB] | 298.15 | 25.9 | 149.5 |
| 308.15 | 25.7 | 149.0 | |
| 318.15 | 25.5 | 147.9 | |
| 328.15 | 25.3 | 146.8 | |
| 338.15 | 25.1 | 145.6 | |
| 348.15 | 24.9 | 144.4 | |
| 358.15 | 24.6 | 142.6 | |
| [dmim][TCB] | 298.15 | 24.0a | 205.6 |
| 328.15 | 23.6 | 201.9 | |
| 338.15 | 23.3 | 199.4 | |
| 348.15 | 23.1 | 197.1 | |
| 358.15 | 22.8 | 194.2 | |
| 368.15 | 22.5 | 190.5 | |
| [bmPIP][SCN] | 298.15 | 30.7a | 198.9 |
| 318.15 | 30.1 | 193.4 | |
| 328.15 | 29.8 | 190.4 | |
| 338.15 | 29.5 | 187.2 | |
| 348.15 | 29.1 | 183.9 | |
| 358.15 | 28.8 | 180.5 | |
| [pmPIP][NTf2] | 298.15 | 23.8b | 172.4 |
| 308.15 | 23.6 | 170.9 | |
| 318.15 | 23.3 | 167.9 | |
| 328.15 | 23.2 | 166.5 | |
| 338.15 | 22.9 | 164.2 | |
| 348.15 | 22.7 | 162.6 | |
| 358.15 | 22.5 | 160.7 | |
| [bmPIP][NTf2] | 298.15 | 23.4b | 175.1 |
| 308.15 | 23.2 | 173.4 | |
| 318.15 | 23.0 | 171.7 | |
| 328.15 | 22.8 | 169.7 | |
| 338.15 | 22.6 | 168.0 | |
| 348.15 | 22.4 | 166.4 | |
| 358.15 | 22.2 | 164.6 | |
| [OiQuin][NTf2] | 298.15 | 22.5b | 201.3 |
| 328.15 | 21.9 | 195.5 | |
| 338.15 | 21.7 | 193.2 | |
| 348.15 | 21.6 | 192.1 | |
| 358.15 | 21.4 | 189.7 | |
| 368.15 | 21.2 | 187.6 | |
| Ionic Liquid | δ2/MPa0.5 | Method, Reference |
|---|---|---|
| [emim][NTf2] | 16.2 | melting temperature [9] |
| 19.3 | activation energy of viscosity [12] | |
| 21.3b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 120.6) [25] | |
| 22.3 | IGC [4] | |
| 22.6b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 134) [26] | |
| 22.7b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 136) [27] | |
| 27.5a | activation energy of viscosity [11] | |
| 27.6 | intrinsic viscosity [10] | |
| 38.4 | lattice energy density [16] | |
| [bmim][NTf2] | 19.8b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 118.5) [25] |
| 20.9 | activation energy of viscosity [12] | |
| 21.2b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 134) [26] | |
| 21.3 | surface tension [13] | |
| 22.9b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 155) [27] | |
| 25.5 | Kamlet-Taft Equation [14] | |
| 26.5a | activation energy of viscosity [11] | |
| 26.7 | intrinsic viscosity [10] | |
| [hmim][NTf2] | 19.0b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 124.1) [25] |
| 19.5 | activation energy of viscosity [12] | |
| 20.3 | IGC [2] | |
| 20.5b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 139) [26] | |
| 22.9b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 173) [27] | |
| 25.2a | activation energy of viscosity [11] | |
| 25.6 | intrinsic viscosity [10] | |
| [omim][NTf2] | 18.9b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 132.3) [25] |
| 20.2b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 149) [28] | |
| 20.2b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 149) [26] | |
| 23.0b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 192) [27] | |
| 25.0 | intrinsic viscosity [10] | |
| [bmPY][NTf2] | 20.6 | IGC [2] |
| 21.2 | activation energy of viscosity [12] | |
| [bmPYR][NTf2] | 21.1 | from surface tension [13] |
| 22.2b | enthalpy of vaporization (ΔvapH298.15/kJ·mol−1 = 152) [29] | |
| [N-C3OHPY][NTf2] | 25.6c | IGC [this work] |
| 23.0c | activation energy of viscosity [this work] | |
| [pmPIP][NTf2] | 23.6c | IGC [this work] |
| 23.5c | NRHB [15] | |
| 23.4c | PC-SAFT [15] | |
| 22.2c | activation energy of viscosity [this work] | |
| [bmPIP][NTf2] | 23.2c | IGC [this work] |
| 21.8c | activation energy of viscosity [this work] | |
| Ionic Liquid | T/K | IGC | Activation Energy of Viscosity |
|---|---|---|---|
| [N-C3OHPY][NTf2] | 308.15 | 25.6 | 23.0 |
| 318.15 | 25.3 | 22.8 | |
| 328.15 | 25.1 | 22.7 | |
| 338.15 | 24.8 | 22.6 | |
| 348.15 | 24.5 | 22.6 | |
| [pmPIP][NTf2] | 308.15 | 23.6 | 22.2 |
| 318.15 | 23.3 | 22.0 | |
| 328.15 | 23.2 | 21.9 | |
| 338.15 | 22.9 | 21.8 | |
| 348.15 | 22.7 | 21.8 | |
| [bmPIP][NTf2] | 308.15 | 23.2 | 21.8 |
| 318.15 | 23.0 | 21.6 | |
| 328.15 | 22.8 | 21.5 | |
| 338.15 | 22.6 | 21.4 | |
| 348.15 | 22.4 | 21.3 | |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marciniak, A. The Hildebrand Solubility Parameters of Ionic Liquids—Part 2. Int. J. Mol. Sci. 2011, 12, 3553-3575. https://doi.org/10.3390/ijms12063553
Marciniak A. The Hildebrand Solubility Parameters of Ionic Liquids—Part 2. International Journal of Molecular Sciences. 2011; 12(6):3553-3575. https://doi.org/10.3390/ijms12063553
Chicago/Turabian StyleMarciniak, Andrzej. 2011. "The Hildebrand Solubility Parameters of Ionic Liquids—Part 2" International Journal of Molecular Sciences 12, no. 6: 3553-3575. https://doi.org/10.3390/ijms12063553




