Chaperoning Roles of Macromolecules Interacting with Proteins in Vivo
Abstract
:1. Introduction
2. Macromolecule-Mediated Chaperone Type Based on Their Surface Charges and Steric Hindrance
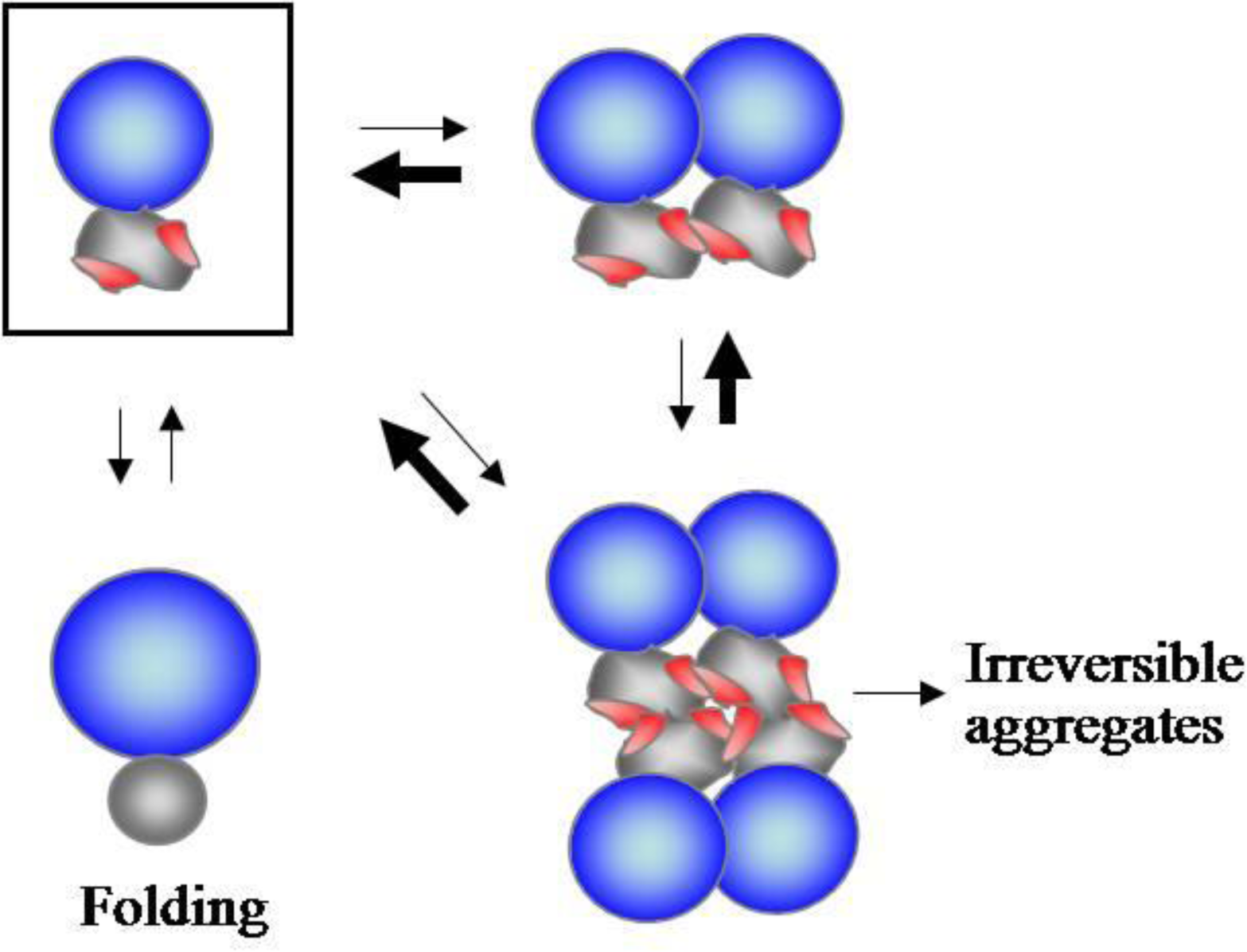
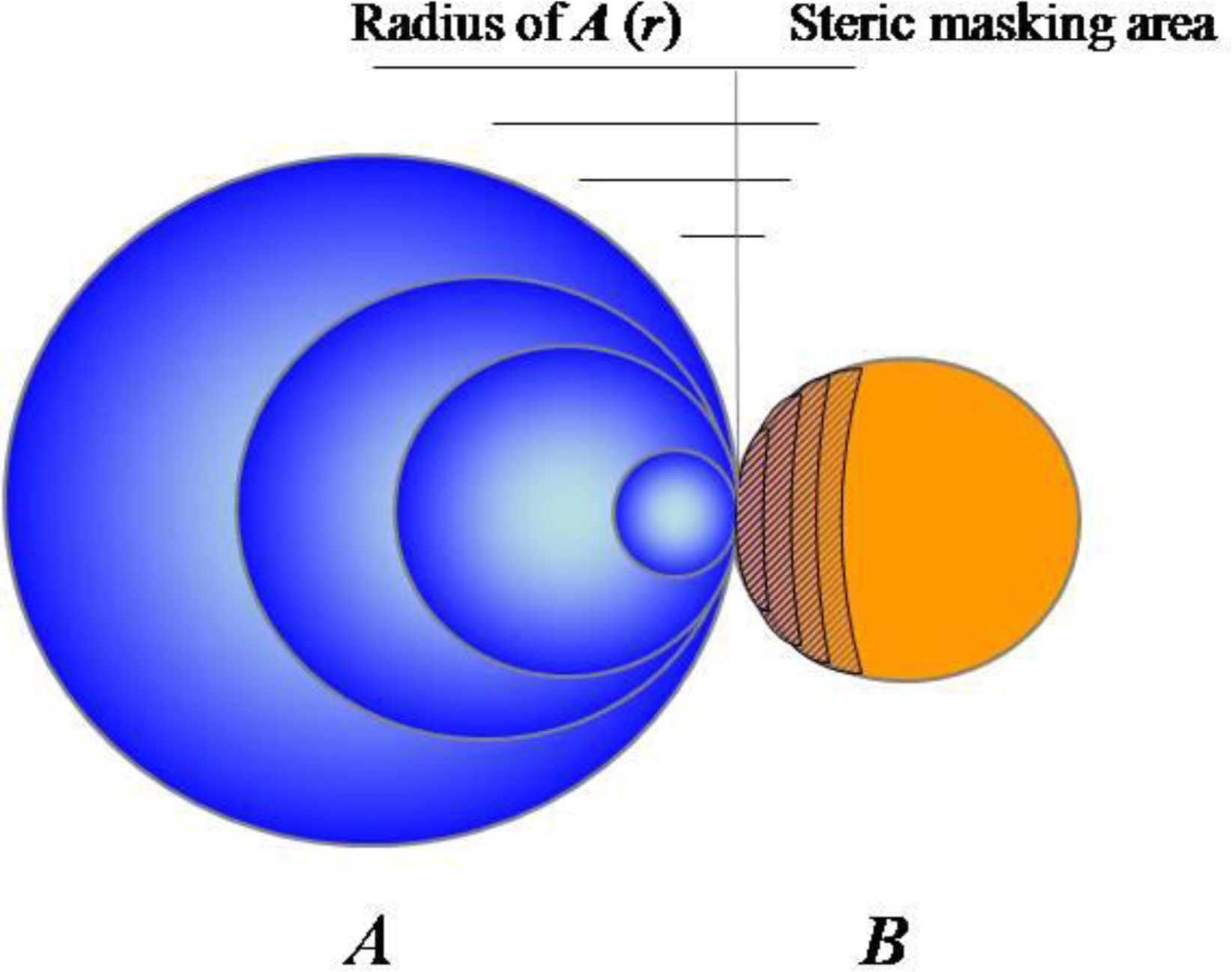
2.1. Accumulating Evidence for Charge and Steric Hindrance as Important Stabilizing Factors
2.2. N-Terminal Domains as Solubility Enhancers for Their Linked Domains
2.3. Substrate Stabilizing Factors of DnaK
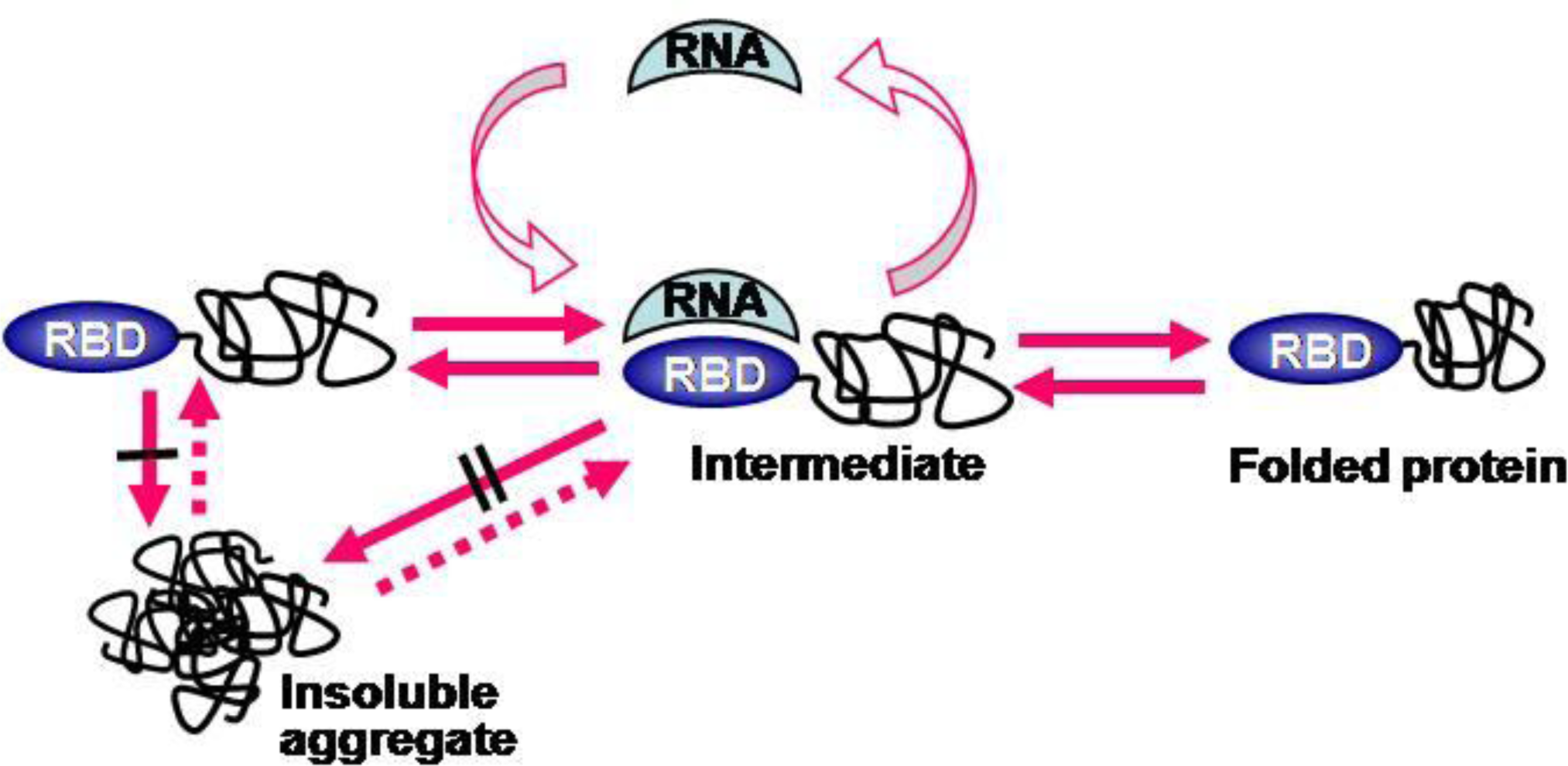
2.4. RNA-Mediated Chaperone Type
3. Perspectives
Acknowledgments
References
- Bukau, B; Horwich, AL. The Hsp70 and Hsp60 chaperone machines. Cell 1998, 92, 351–366. [Google Scholar]
- Hartl, FU; Hayer-Hartl, M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 2002, 295, 1852–1858. [Google Scholar]
- Anfinsen, CB. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar]
- Dunker, AK; Oldfield, CJ; Meng, J; Romero, P; Yang, JY; Chen, JW; Vacic, V; Obradovic, Z; Uversky, VN. The unfoldomics decade: an update on intrinsically disordered proteins. BMC Genomics 2008, 16, S1. [Google Scholar]
- Uversky, VN; Dunker, AK. Understanding protein non-folding. Biochim. Biophys. Acta 2010, 1804, 1231–1264. [Google Scholar]
- Xue, B; Williams, RW; Oldfield, CJ; Dunker, AK; Uversky, VN. Archaic chaos: intrinsically disordered proteins in Archaea. BMC Syst. Biol 2010, 4, S1. [Google Scholar]
- Chiti, F; Dobson, CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem 2006, 75, 333–366. [Google Scholar]
- Prusiner, SB. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar]
- Uversky, VN; Oldfield, CJ; Midic, U; Xie, H; Xue, B; Vucetic, S; Iakoucheva, LM; Obradovic, Z; Dunker, AK. Unfoldomics of human diseases: linking protein intrinsic disorder with diseases. BMC Genomics 2009, 10, S7. [Google Scholar]
- Hemmingsen, SM; Woolford, C; van der Vies, SM; Tilly, K; Dennis, DT; Georgopoulos, CP; Hendrix, RW; Ellis, RJ. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature 1988, 333, 330–334. [Google Scholar]
- Pelham, HR. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 1986, 46, 959–961. [Google Scholar]
- Ellis, RJ. Protein folding: importance of the Anfinsen cage. Curr. Biol 2003, 13, R881–R883. [Google Scholar]
- Brinker, A; Pfeifer, G; Kerner, MJ; Naylor, DJ; Hartl, FU; Hayer-Hartl, M. Dual function of protein confinement in chaperonin-assisted protein folding. Cell 2001, 107, 223–233. [Google Scholar]
- Apetri, AC; Horwich, AL. Chaperonin chamber accelerates protein folding through passive action of preventing aggregation. Proc. Natl. Acad. Sci. USA 2008, 105, 17351–17355. [Google Scholar]
- Chakraborty, K; Chatila, M; Sinha, J; Shi, Q; Poschner, BC; Sikor, M; Jiang, G; Lamb, DC; Hartl, FU; Hayer-Hartl, M. Chaperonin-catalyzed rescue of kinetically trapped states in protein folding. Cell 2010, 142, 112–122. [Google Scholar]
- Deuerling, E; Schulze-Specking, A; Tomoyasu, T; Mogk, A; Bukau, B. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 1999, 400, 693–696. [Google Scholar]
- Ullers, RS; Luirink, J; Harms, N; Schwager, F; Georgopoulos, C; Genevaux, P. SecB is a bona fide generalized chaperone in Escherichia coli. Proc. Natl. Acad. Sci. USA 2004, 101, 7583–7588. [Google Scholar]
- Vorderwülbecke, S; Kramer, G; Merz, F; Kurz, TA; Rauch, T; Zachmann-Brand, B; Bukau, B; Deuerling, E. Low temperature of GroEL/ES overproduction permits growth of Escherichia coli cells lacking trigger factor and DnaK. FEBS Lett 2004, 559, 181–187. [Google Scholar]
- Kerner, MJ; Naylor, DJ; Ishihama, Y; Maier, T; Chang, HC; Stines, AP; Georgopoulos, C; Frishman, D; Hayer-Hartl, M; Mann, M; Hartl, FU. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell 2005, 122, 209–220. [Google Scholar]
- Masters, M; Blakely, G; Coulson, A; McLennan, N; Yerko, V; Acord, J. Protein folding in Escherichia coli: the chaperonin GroE and its substrates. Res. Microbiol 2009, 160, 267–277. [Google Scholar]
- Wong, P; Houry, WA. Chaperone networks in bacteria: analysis of protein homeostasis in minimal cells. J. Struct. Biol 2004, 146, 79–89. [Google Scholar]
- Parsell, DA; Kowal, AS; Singer, MA; Lindquist, S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature 1994, 372, 475–478. [Google Scholar]
- De Los Rios, P; Ben-Zvi, A; Slutsky, O; Azem, A; Goloubinoff, P. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc. Natl. Acad. Sci. USA 2006, 103, 6166–6171. [Google Scholar]
- Kandror, O; Busconi, L; Sherman, M; Goldberg, AL. Rapid degradation of an abnormal protein in Escherichia coli involves the chaperones GroEL and GroES. J. Biol. Chem 1994, 269, 23575–23582. [Google Scholar]
- Taipale, M; Jarosz, DF; Lindquist, S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol 2010, 11, 515–528. [Google Scholar]
- Tompa, P; Kovacs, D. Intrinsically disordered chaperones in plants and animals. Biochem. Cell Biol 2010, 88, 167–174. [Google Scholar]
- Speed, MA; Wang, DI; King, J. Specific aggregation of partially folded polypeptide chains: the molecular basis of inclusion body composition. Nat. Biotechnol 1996, 14, 1283–1287. [Google Scholar]
- Wright, CF; Teichmann, SA; Clarke, J; Dobson, CM. The importance of sequence diversity in the aggregation and evolution of proteins. Nature 2005, 438, 878–881. [Google Scholar]
- Baldwin, RL. Energetics of protein folding. J. Mol. Biol 2007, 10, 283–301. [Google Scholar]
- Hartl, FU; Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol 2009, 16, 574–581. [Google Scholar]
- Otzen, DE; Kristensen, O; Oliveberg, M. Designed protein tetramer zipped together with a hydrophobic Alzheimer homology: a structural clue to amyloid assembly. Proc. Natl. Acad. Sci. USA 2000, 97, 9907–9912. [Google Scholar]
- Uversky, VN; Gillespie, JR; Fink, AL. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins 2000, 41, 415–427. [Google Scholar]
- Chiti, F; Calamai, M; Taddei, N; Stefani, M; Ramponi, G; Dobson, CM. Studies of the aggregation of mutant proteins in vitro provide insights into the genetics of amyloid diseases. Proc. Natl. Acad. Sci. USA 2002, 99, 16419–16426. [Google Scholar]
- Dosztányi, Z; Csizmók, V; Tompa, P; Simon, I. The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J. Mol. Biol 2005, 347, 827–839. [Google Scholar]
- Lawrence, MS; Phillips, KJ; Liu, DR. Supercharging proteins can impart unusual resilience. J. Am. Chem. Soc 2007, 129, 10110–10112. [Google Scholar]
- Chiti, F; Stefani, M; Taddei, N; Ramponi, G; Dobson, CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 2003, 424, 805–808. [Google Scholar]
- Tompa, P; Csermely, P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J 2004, 18, 1169–1175. [Google Scholar]
- Chen, J; Skehel, JJ; Wiley, DC. A polar octapeptide fused to the N-terminal fusion peptide solubilizes the influenza virus HA2 subunit ectodomain. Biochemistry 1998, 37, 13643–13649. [Google Scholar]
- Zhang, YB; Howitt, J; McCorkle, S; Lawrence, P; Springer, K; Freimuth, P. Protein aggregation during overexpression limited by peptide extensions with large net negative charge. Protein Expr. Purif 2004, 36, 207–216. [Google Scholar]
- Kvam, E; Sierks, MR; Shoemaker, CB; Messer, A. Physico-chemical determinants of soluble intrabody expression in mammalian cell cytoplasm. Protein Eng. Des. Sel 2010, 23, 489–498. [Google Scholar]
- Høiberg-Nielsen, R; Fuglsang, CC; Arleth, L; Westh, P. Interrelationships of glycosylation and aggregation kinetics for Peniophora lycii phytase. Biochemistry 2006, 45, 5057–5066. [Google Scholar]
- Rajan, RS; Li, T; Aras, M; Sloey, C; Sutherland, W; Arai, H; Briddell, R; Kinstler, O; Lueras, AM; Zhang, Y; Yeghnazar, H; Treuheit, M; Brems, DN. Modulation of protein aggregation by polyethylene glycol conjugation: GCSF as a case study. Protein Sci 2006, 15, 1063–1075. [Google Scholar]
- Olsen, SN; Andersen, KB; Randolph, TW; Carpenter, JF; Westh, P. Role of electrostatic repulsion on colloidal stability of Bacillus halmapalus alpha-amylase. Biochim. Biophys. Acta 2009, 1794, 1058–1065. [Google Scholar]
- Cho, YH; Decker, EA; McClements, DJ. Competitive adsorption of mixed anionic polysaccharides at the surfaces of protein-coated lipid droplets. Langmuir 2009, 25, 2654–2660. [Google Scholar]
- Kapust, RB; Waugh, DS. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci 1999, 8, 1668–1674. [Google Scholar]
- Braun, P; LaBaer, J. High throughput protein production for functional proteomics. Trends Biotechnol 2003, 21, 383–388. [Google Scholar]
- Esposito, D; Chatterjee, DK. Enhancement of soluble protein expression through the use of fusion tags. Curr. Opin. Biotechnol 2006, 17, 353–358. [Google Scholar]
- Wall, JG; Plückthun, A. Effects of overexpressing folding modulators on the in vivo folding of heterologous proteins in Escherichia coli. Curr. Opin. Biotechnol 1995, 6, 507–516. [Google Scholar]
- Kim, CW; Han, KS; Ryu, KS; Kim, BH; Kim, KH; Choi, SI; Seong, BL. N-terminal domains of native multidomain proteins have the potential to assist de novo folding of their downstream domains in vivo by acting as solubility enhancers. Protein Sci 2007, 16, 635–643. [Google Scholar]
- Mogk, A; Tomoyasu, T; Goloubinoff, P; Rüdiger, S; Röder, D; Langen, H; Bukau, B. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J 1999, 18, 6934–6949. [Google Scholar]
- Rüdiger, S; Germeroth, L; Schneider-Mergener, J; Bukau, B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J 1997, 16, 1501–1507. [Google Scholar]
- Fenton, WA; Kashi, Y; Furtak, K; Horwich, AL. Residues in chaperonin GroEL required for polypeptide binding and release. Nature 1994, 371, 614–619. [Google Scholar]
- Zhu, X; Zhao, X; Burkholder, WF; Gragerov, A; Ogata, CM; Gottesman, ME; Hendrickson, WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 1996, 272, 1606–1614. [Google Scholar]
- Misselwitz, B; Staeck, O; Rapoport, TA. J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences. Mol. Cell 1998, 2, 593–603. [Google Scholar]
- Aoki, K; Taguchi, H; Shindo, Y; Yoshida, M; Ogasahara, K; Yutani, K; Tanaka, N. Calorimetric observation of a GroEL-protein binding reaction with little contribution of hydrophobic interaction. J. Biol. Chem 1997, 272, 32158–32162. [Google Scholar]
- Martinez-Hackert, E; Hendrickson, WA. Promiscuous substrate recognition in folding and assembly activities of the trigger factor chaperone. Cell 2009, 138, 923–934. [Google Scholar]
- Trombetta, ES; Helenius, A. Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol 1998, 8, 587–592. [Google Scholar]
- Ryu, K; Kim, CW; Kim, BH; Han, KS; Kim, KH; Choi, SI; Seong, BL. Assessment of substrate-stabilizing factors for DnaK on the folding of aggregation-prone proteins. Biochem. Biophys. Res. Commun 2008, 373, 74–79. [Google Scholar]
- Ellis, RJ; Hartl, FU. Principles of protein folding in the cellular environment. Curr. Opin. Struct. Biol 1999, 9, 102–110. [Google Scholar]
- Feldman, DE; Frydman, J. Protein folding in vivo: the importance of molecular chaperones. Curr. Opin. Struct. Biol 2000, 10, 26–33. [Google Scholar]
- Ferbitz, L; Maier, T; Patzelt, H; Bukau, B; Deuerling, E; Ban, N. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature 2004, 431, 590–596. [Google Scholar]
- Craig, EA; Eisenman, HC; Hundley, HA. Ribosome-tethered molecular chaperones: the first line of defense against protein misfolding? Curr. Opin. Microbiol 2003, 6, 157–162. [Google Scholar]
- Albanèse, V; Reissmann, S; Frydman, J. A ribosome-anchored chaperone network that facilitates eukaryotic ribosome biogenesis. J. Cell. Biol 2010, 189, 69–81. [Google Scholar]
- Koplin, A; Preissler, S; Ilina, Y; Koch, M; Scior, A; Erhardt, M; Deuerling, E. A dual function for chaperones SSB-RAC and the NAC nascent polypeptide-associated complex on ribosomes. J. Cell Biol 2010, 189, 57–68. [Google Scholar]
- Das, B; Chattopadhyay, S; Bera, AK; Dasgupta, C. In vitro protein folding by ribosomes from Escherichia coli, wheat germ and rat liver: the role of the 50S particle and its 23S rRNA. Eur. J. Biochem 1996, 235, 613–621. [Google Scholar]
- Chattopadhyay, S; Das, B; Dasgupta, C. Reactivation of denatured proteins by 23S ribosomal RNA: role of domain V. Proc. Natl. Acad. Sci. USA 1996, 93, 8284–8287. [Google Scholar]
- Choi, SI; Han, KS; Kim, CW; Ryu, KS; Kim, BH; Kim, KH; Kim, SI; Kang, TH; Shin, HC; Lim, KH; Kim, HK; Hyun, JM; Seong, BL. Protein solubility and folding enhancement by interaction with RNA. PLoS One 2008, 3, e2677. [Google Scholar]
- Sørensen, HP; Kristensen, JE; Sperling-Petersen, HU; Mortensen, KK. Soluble expression of aggregating proteins by covalent coupling to the ribosome. Biochem. Biophys. Res. Commun 2004, 319, 715–719. [Google Scholar]
- Schimmele, B; Gräfe, N; Plückthun, A. Ribosome display of mammalian receptor domains. Protein Eng. Des. Sel 2005, 18, 285–94. [Google Scholar]
- Choi, SI; Ryu, K; Seong, BL. RNA-mediated chaperone type for de novo protein folding. RNA Biol 2009, 6, 21–24. [Google Scholar]
- Brandt, F; Etchells, SA; Ortiz, JO; Elcock, AH; Hartl, FU; Baumeister, W. The native 3D organization of bacterial polysomes. Cell 2009, 136, 261–271. [Google Scholar]
- Dedmon, MM; Christodoulou, J; Wilson, MR; Dobson, CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J. Biol. Chem 2005, 280, 14733–14740. [Google Scholar]
- Ivanovic, T; Agosto, MA; Chandran, K; Nibert, ML. A role for molecular chaperone Hsc70 in reovirus outer capsid disassembly. J. Biol. Chem 2007, 282, 12210–12219. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Choi, S.I.; Lim, K.-H.; Seong, B.L. Chaperoning Roles of Macromolecules Interacting with Proteins in Vivo. Int. J. Mol. Sci. 2011, 12, 1979-1990. https://doi.org/10.3390/ijms12031979
Choi SI, Lim K-H, Seong BL. Chaperoning Roles of Macromolecules Interacting with Proteins in Vivo. International Journal of Molecular Sciences. 2011; 12(3):1979-1990. https://doi.org/10.3390/ijms12031979
Chicago/Turabian StyleChoi, Seong Il, Keo-Heun Lim, and Baik L. Seong. 2011. "Chaperoning Roles of Macromolecules Interacting with Proteins in Vivo" International Journal of Molecular Sciences 12, no. 3: 1979-1990. https://doi.org/10.3390/ijms12031979



