Light-Emitting Devices with Conjugated Polymers
Abstract
:1. Introduction
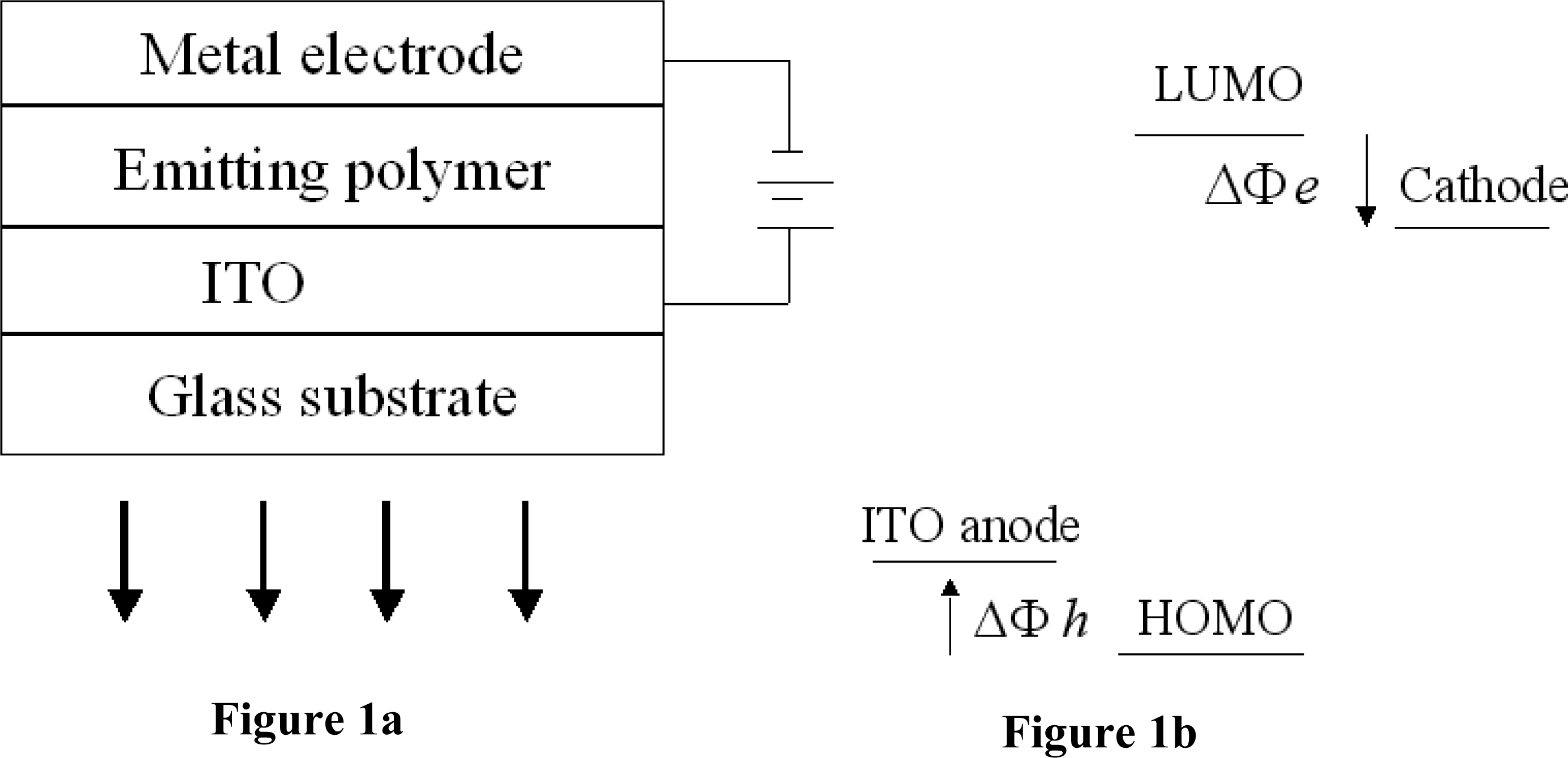
2. Charge Injection and Interfaces between the Polymer and the Electrodes
3. Electrical Conductivity and Charge Transport in Conjugated Polymers
4. Charge Carriers Recombination and Light Emission
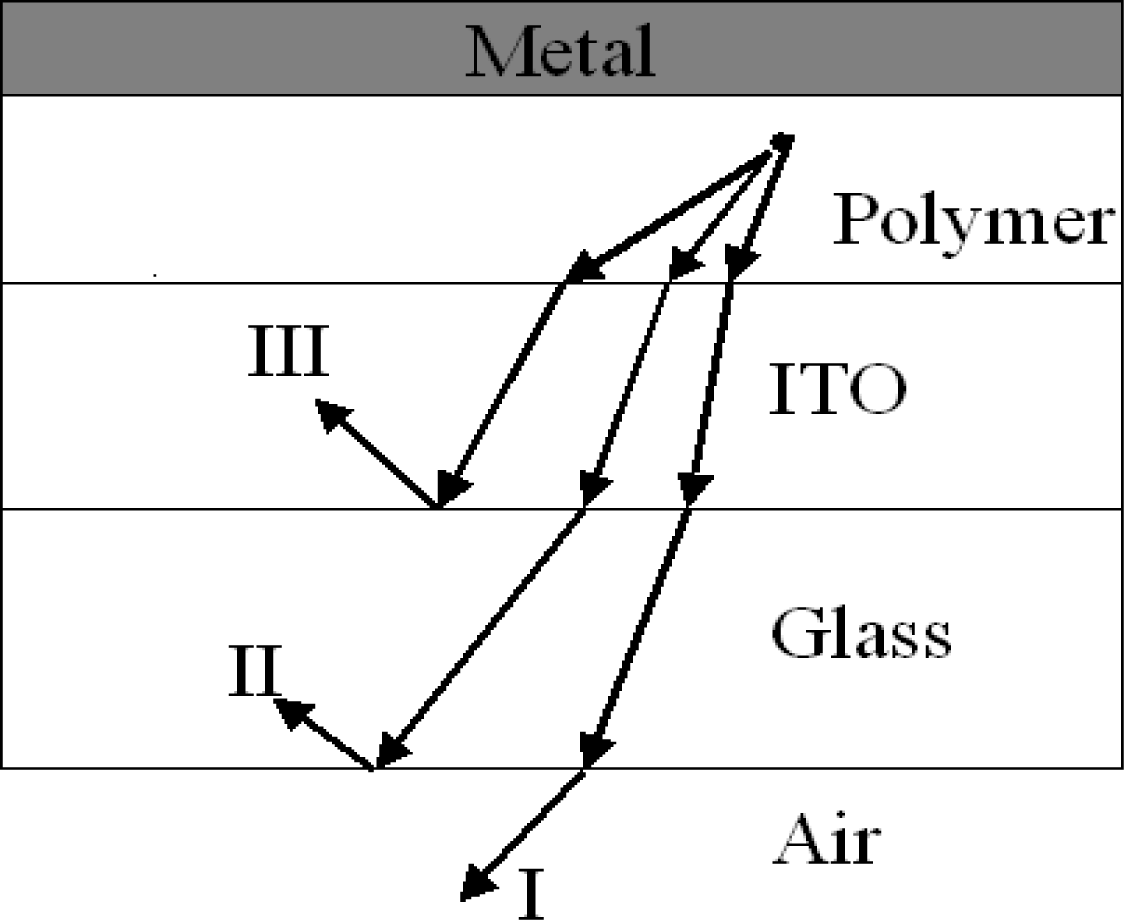
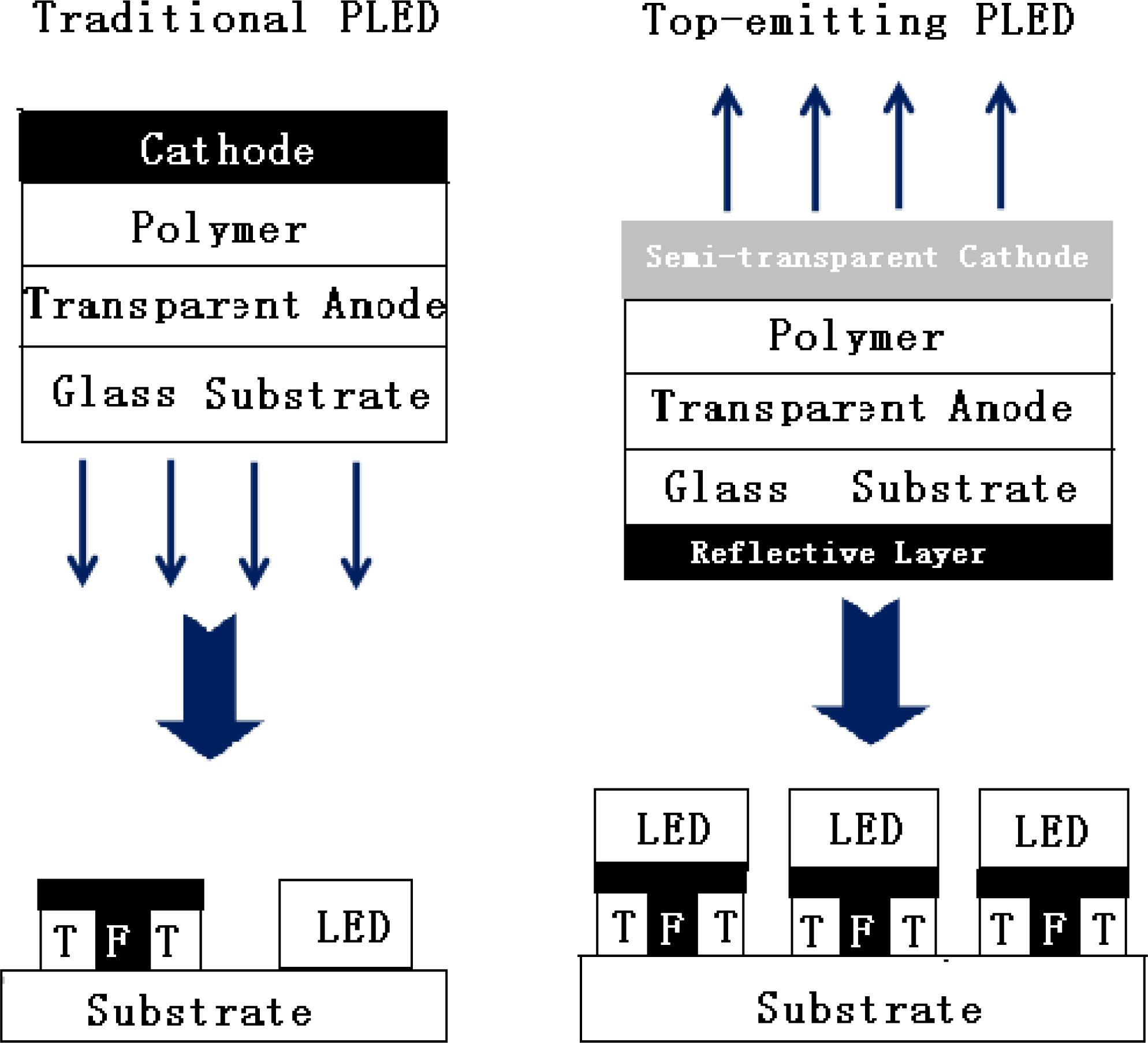
5. Pattering Methods for Full-Color PLED Displays
6. Conclusions and Outlook
Acknowledgments
References
- Pope, M; Kallmann, H; Magnante, P. Electroluminescence in organic crystals. J. Chem. Phys 1963, 38, 2042–2043. [Google Scholar]
- Helfrich, W; Schneider, WG. Recombination radiation in anthracene crystal. Phys. Rev. Lett 1965, 14, 229–231. [Google Scholar]
- Tang, CW; Vanslyke, SA. Organic electroluminescent diodes. Appl. Phys. Lett 1987, 51, 913–915. [Google Scholar]
- Adachi, C; Tsutsui, T; Saito, S. Organic electroluminescent device having a hole conductor as an emitting layer. Appl. Phys. Lett 1989, 55, 1489–1491. [Google Scholar]
- Burroughes, H; Bradley, DDC; Brown, AR; Marks, RN; Mackay, K; Friend, RH; Burns, PL; Holmes, AB. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar]
- Braun, D; Heeger, AJ. Visible light emission from semiconducting polymer diodes. Appl. Phys. Lett 1991, 58, 1982–1984. [Google Scholar]
- Friend, RH; Gymer, RW; Holmes, AB; Burroughes, JH; Marks, RN; Taliani, C; Bradley, DDC; Dos Santos, DA; Bre das, JL; LoÈ gdlund, M; Salaneck, WR. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar]
- Grüner, J; Cacialli, F; Friend, RH. Emission enhancement in single-layered conjugated polymer microcavities. J. Appl. Phys 1996, 80, 207–215. [Google Scholar]
- Almassio, MS; Sarimbalis, MN; Montani, RS; Garay, RO. Synthesis and characterization of poly(1,4-phenylene vinylene)-co-(2,5-pyridilene vinylene)’s. Polym. Bull 2005, 54, 163–172. [Google Scholar]
- Tang, RP; Zhan’ao, T; Cheng, CX; Li, YF; Xi, F. Synthesis, electroluminescence, and photovoltaic properties of dendronized poly(p-phenylene vinylene) derivatives. Polymer 2005, 46, 5341–5350. [Google Scholar]
- Mo, YQ; Huang, J; Jiang, JX; Deng, XY; Niu, YH; Cao, Y. Influence of traces of water on the synthesis and electroluminescence properties of poly(2-methoxy, 5-(2'-ethylhexyloxy)-1,4-phenylene vinylene). Chin. J. Polym. Sci 2002, 20, 461–465. [Google Scholar]
- Weinfurtner, KH; Fujikawa, H; Tokito, S; Taga, Y. Highly efficient pure blue electroluminescence from polyfluorene: Influence of the molecular weight distribution on the aggregation tendency. Appl. Phys. Lett 2000, 76, 2502–2504. [Google Scholar]
- Yu, WL; Cao, Y; Pei, JA; Huang, W; Heeger, AJ. Blue polymer light-emitting diodes from poly(9,9-dihexylfluorene-alt-co-2,5-didecyloxy-para-phenylene). Appl. Phys. Lett 1999, 75, 3270–3272. [Google Scholar]
- Hou, Q; Xu, YS; Yang, W; Yuan, M; Peng, JB; Cao, Y. Novel red-emitting fluorene-based copolymers. J. Mater. Chem 2002, 12, 2887–2892. [Google Scholar]
- Millard, IS. High-efficiency polyfluorene polymers suitable for RGB applications. Synth. Met 2000, 111, 119–123. [Google Scholar]
- Hou, Q; Zhang, Y; Yang, RQ; Yang, W; Cao, Y. Synthesis and electroluminescent properties of copolymers derived from fluorene and metal-free and Pt (II) tetraphenylporphyrin. Synth. Met 2005, 153, 193–196. [Google Scholar]
- Huang, F; Niu, YH; Liu, MS; Zhou, XH; Tian, YQ; Jen, AKY. Efficient ultraviolet-blue polymer light-emitting diodes based on a fluorene-based non-conjugated polymer. Appl Phys Lett 2006, 89, 081104:1–081104:3. [Google Scholar]
- Misaki, M; Chikamatsu, M; Yoshida, Y; Azumi, R; Tanigaki, N; Yase, K; Nagamatsu, S; Ueda, Y. Highly efficient polarized polymer light-emitting diodes utilizing oriented films of beta-phase poly(9,9-dioctylfluorene). Appl Phys Lett 2008, 93, 243503:1–243503:3. [Google Scholar]
- Mo, YQ; Deng, XY; Jiang, X; Cui, QH. Blue electroluminescence from 3,6-Silafluorene-based copolymers. J. Polym. Sci. Part A: Polym. Chem 2009, 47, 3286–3295. [Google Scholar]
- Jin, Y; Xu, YB; Qiao, Z; Peng, JB; Wang, BZ; Cao, DR. Enhancement of electroluminescence properties of red diketopyrrolopyrrole-doped copolymers by oxadiazole and carbazole units as pendants. Polymer 2010, 51, 5726–5733. [Google Scholar]
- Giovanella, U; Betti, P; Bolognesi, A; Destri, S; Melucci, M; Pasini, M; Porzio, W; Botta, C. Core-type polyfluorene-based copolymers for low-cost light-emitting technologies. Org. Electron 2010, 11, 2012–2018. [Google Scholar]
- Lee, PI; Hsu, SLC; Lin, PY. White-light-emitting diodes from single polymer systems based on polyfluorene copolymers with quinoxaline derivatives. Macromolecules 2010, 43, 8051–8057. [Google Scholar]
- Grimsdale, AC; Chan, KL; Martin, RE; Jokisz, PG; Holmes, AB. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev 2009, 109, 897–1091. [Google Scholar]
- Cao, Y; Yu, G; Zhang, C; Menon, R; Heeger, AJ. Polymer light-emitting diodes with polyethylene dioxythiophene-polystyrene sulfonate as the transparent anode. Synth. Met 1997, 87, 171–174. [Google Scholar]
- Robinson, MR; O'Regan, MB; Bazan, GC. Synthesis, morphology and optoelectronic properties of tris[(N-ethylcarbazolyl)(3′,5′-hexyloxybenzoyl)methane](phenanthroline)-europium. Chem. Commun 2000, 17, 1645–1646. [Google Scholar]
- Yu, WL; Pei, J; Cao, Y; Huang, W. Hole-injection enhancement by copper phthalocyanine (CuPc) in blue polymer light-emitting diodes. J. Appl. Phys 2001, 89, 2343–2350. [Google Scholar]
- Harding, MJ; Poplavskyy, D; Choong, VE; Campbell, AJ; So, F. Effects of solution-processed polymer interlayers on hole injection and device performance of polymer light-emitting diodes. Org. Electron 2008, 9, 183–190. [Google Scholar]
- Niu, YH; Liu, MS; Ka, JW; Jen, AKY. Thermally crosslinked hole-transporting layers for cascade hole-injection and effective electron-blocking/exciton-confinement in phosphorescent polymer light-emitting diodes. Appl Phys Lett 2006, 88, 093505:1–093505:3. [Google Scholar]
- Kim, JS; Friend, RH; Grizzi, I; Burroughes, JH. Spin-cast thin semiconducting polymer interlayer for improving device efficiency of polymer light-emitting diodes. Appl Phys Lett 2005, 87, 023506:1–023506:3. [Google Scholar]
- Niu, YH; Huang, J; Cao, Y. High-efficiency polymer light-emitting diodes with stable saturated red emission: Use of carbazole-based copolymer blends in a poly(p-phenylenevinylene) derivative. Adv. Mater 2003, 15, 807–811. [Google Scholar]
- Niu, YH; Yang, W; Cao, Y. High-efficiency blue-light-emitting diodes with narrow linewidth based on blends of poly[2-(2′-ethylhexyloxy)-1,4-phenylene] and poly(dialkylfluorene-co-dibenzothiophene). Appl. Phys. Lett 2002, 81, 2884–2886. [Google Scholar]
- Ma, WL; Iyer, PK; Gong, X; Liu, B; Moses, D; Bazan, GC; Heeger, AJ. Water/methanol-soluble conjugated copolymer as an electron-transport layer in polymer light-emitting diodes. Adv. Mater 2005, 17, 274–277. [Google Scholar]
- Pope, M; Swenberg, CE. Electronic Processes in Organic Crystals and Polymers, 1st ed; Oxford University Press: New York, NY, USA, 1982. [Google Scholar]
- Hayashi, S; Etoh, H; Saito, S. Electroluminescence of perylene films with a conducting polymer as an anode. Jpn. J. Appl. Phys 1986, 25, 773–775. [Google Scholar]
- Mu, H; Li, W; Jones, R; Steckl, A; Klotzkin, D. A comparative study of electrode effects on the electrical and luminescent characteristics of Alq(3)/TPD OLED: Improvements due to conductive polymer (PEDOT) anode. J. Lumin 2007, 126, 225–229. [Google Scholar]
- Yang, Y; Heeger, AJ. Polyaniline as a transparent electrode for polymer light-emitting diodes: Lower operating voltage and higher efficiency. Appl. Phys. Lett 1994, 64, 1245–1247. [Google Scholar]
- Hung, LS; Tang, CW; Mason, MG. Enhanced electron injection in organic electroluminescence devices using an Al/LiF electrode. Appl. Phys. Lett 1997, 70, 152–154. [Google Scholar]
- Yoon, J; Kim, JJ; Lee, TW; Park, OO. Evidence of band bending observed by electroabsorption studies in polymer light emitting device with ionomer/Al or LiF/Al cathode. Appl. Phys. Lett 2000, 76, 2152–2154. [Google Scholar]
- Brown, TM; Friend, RH; Millard, IS; Lacey, DJ; Burroughes, JH; Cacialli, F. LiF/Al cathodes and the effect of LiF thickness on the device characteristics and built-in potential of polymer light-emitting diodes. Appl. Phys. Lett 2000, 77, 3096–3098. [Google Scholar]
- Yang, X; Mo, Y; Yang, W; Yu, G; Cao, Y. Efficient polymer light emitting diodes with metal fluoride Al cathodes. Appl. Phys. Lett 2001, 79, 563–565. [Google Scholar]
- Deng, XY; Tong, SW; Hung, LS; Mo, YQ; Cao, Y. Role of ultrathin Alq(3) and LiF layers in conjugated polymer light-emitting diodes. Appl. Phys. Lett 2003, 82, 3104–3106. [Google Scholar]
- Xu, QF; Ouyang, JY; Yang, Y. Ultrahigh efficiency green polymer light-emitting diodes by nanoscale interface modification. Appl. Phys. Lett 2003, 83, 4695–4697. [Google Scholar]
- Lee, TW; Park, OO; Lee, MD; Zyung, T; Ahn, T; Shim, HK. Polymer light-emitting devices using ionomers as an electron injecting and hole blocking layer. J. Appl. Phys 2001, 90, 2128–2134. [Google Scholar]
- Cao, Y; Yu, G; Heeger, AJ. Efficient, low operating voltage polymer light-emitting diodes with aluminum as the cathode material. Adv Mater 1998, 10, 917–921. [Google Scholar]
- Deng, XY; Lau, WM; Wong, KY; Low, KH; Chow, HF; Cao, Y. High efficiency low operating voltage polymer light-emitting diodes with aluminum cathode. Appl. Phys. Lett 2004, 84, 3522–3524. [Google Scholar]
- Niu, YH; Ma, H; Xu, QM; Jen, AKY. High-efficiency light-emitting diodes using neutral surfactants and aluminum cathode. Appl Phys Lett 2005, 86, 083504:1–083504:3. [Google Scholar]
- Guo, TF; Yang, FS; Tsai, ZJ. High-performance polymer light-emitting diodes utilizing modified Al cathode. Appl Phys Lett 2005, 87, 013504:1–013504:3. [Google Scholar]
- Niu, YH; Jen, AKY; Shu, CF. High-efficiency polymer light-emitting diodes using neutral surfactant modified aluminum cathode. J. Phys. Chem. B 2006, 110, 6010–6014. [Google Scholar]
- Huang, F; Niu, YH; Zhang, Y; Ka, JW; Liu, MS; Jen, AKY. A conjugated, neutral surfactant as electron-injection material for high-efficiency polymer light-emitting diodes. Adv. Mater 2007, 19, 2010–2014. [Google Scholar]
- Huang, F; Zhang, Y; Liu, MS; Jen, AKY. Electron-Rich Alcohol-Soluble Neutral Conjugated Polymers as Highly Efficient Electron-Injecting Materials for Polymer Light-Emitting Diodes. Adv. Funct. Mater 2009, 19, 2457–2466. [Google Scholar]
- Yang, RQ; Xu, YH; Dang, XD; Nguyen, TQ; Cao, Y; Bazan, GC. Conjugated oligoelectrolyte electron transport/injection layers for organic optoelectronic devices. J. Am. Chem. Soc 2008, 130, 3282–3283. [Google Scholar]
- Huang, F; Niu, YH; Zhang, Y; Ka, JW; Liu, MS; Jen, AKY. A conjugated, neutral surfactant as electron-injection material for high-efficiency polymer light-emitting diodes. Adv. Mater 2007, 9, 2010–2014. [Google Scholar]
- Zeng, WJ; Wu, HB; Zhang, C; Huang, F; Peng, JB; Yang, W; Cao, Y. Polymer light-emitting diodes with cathodes printed from conducting Ag paste. Adv. Mater 2007, 19, 810–814. [Google Scholar]
- Wu, HB; Huang, F; Mo, YQ; Yang, W; Wang, DL; Peng, JB; Cao, Y. Efficient electron injection from a bilayer cathode consisting of aluminum and alcohol-/water-soluble conjugated polymers. Adv. Mater 2004, 16, 1826–1830. [Google Scholar]
- Sze, SM. Physics of Semiconductor Devices, 2nd ed; Wiley-Interscience: New York, NY, USA, 1981. [Google Scholar]
- Kang, ET; Ehrlich, P; Bhatt, AP; Anderson, WA. Charge carrier generation, transport, and trapping in a photoconductive conjugated polymer: Polyphenylacetylene. Appl. Phys. Lett 1982, 41, 1136–1138. [Google Scholar]
- Emin, D. Semiclassical small-polaron hopping in a generalized molecular-crystal model. Phys. Rev. B 1991, 43, 11720–11724. [Google Scholar]
- Bussac, MN; Zuppiroli, L. High-field mobility in an assembly of conjugated polymer segments. Phys. Rev. B 1996, 54, 4674–4679. [Google Scholar]
- Wang, JF; Kawabe, Y; Shaheen, SE; Morrell, MM; Jabbour, GE; Lee, PA; Anderson, J; Armstrong, NR; Kippelen, B; Mash, EA; Peyhambarian, N. Exciplex electroluminescence from organic bilayer devices composed of triphenyldiamine and quinoxaline derivatives. Adv. Mater 1998, 10, 230–233. [Google Scholar]
- Morteani, AC; Dhoot, AS; Kim, JS; Silva, C; Greenham, NC; Murphy, C; Moons, E; Cina, C; Burroughes, JH; Friend, RH. Barrier-free electron-hole capture in polymer blend heterojunction light-emitting diodes. Adv. Mater 2003, 15, 1708–1712. [Google Scholar]
- Bassler, H. Injection, transport and recombination of charge carriers in organic light-emitting diodes. Polym. Adv. Technol 1998, 9, 402–418. [Google Scholar]
- Blom, PWM; de Jong, MJM; Breedijk, S. Temperature dependent electron-hole recombination in polymer light-emitting diodes. Appl. Phys. Lett 1997, 71, 930–932. [Google Scholar]
- Scott, JC; Karg, S; Carter, SA. Bipolar charge and current distributions in organic light-emitting diodes. J. Appl. Phys 1997, 82, 1454–1460. [Google Scholar]
- Cornil, J; dos Santos, DA; Crispin, X; Silbey, R; Brédas, JL. Influence of Interchain Interactions on the Absorption and Luminescence of Conjugated Oligomers and Polymers: A Quantum-Chemical Characterization. J. Am. Chem. Soc 1998, 120, 1289–1299. [Google Scholar]
- Lemmerc, U; Ochsea, A; Deussena, M; Mahrta, RF; Göbela, EO; Bässlera, H; Haring Bolivarb, P; Wegmannb, G; Kurzb, H. Energy transfer in molecularly doped conjugated polymers. Synth. Met 1996, 78, 289–293. [Google Scholar]
- Ruseckasa, A; Theanderc, M; Valkunasb, L; Anderssond, MR; Inganäsc, O; Sundström, V. Energy transfer in a conjugated polymer with reduced inter-chain coupling. J. Lumin 1998, 76, 474–477. [Google Scholar]
- Cao, Y; Parker, ID; Yu, G; Zhang, C; Heeger, AJ. Improved quantum efficiency for electroluminescence in semiconducting polymers. Nature 1999, 397, 414–417. [Google Scholar]
- Shuai1, Z; Beljonne1, D; Silbey, RJ; Brédas, JL. Singlet and Triplet Exciton Formation Rates in Conjugated Polymer Light-Emitting Diodes. Phys. Rev. Lett 2000, 84, 131–134. [Google Scholar]
- Baldo, MA; Obrien, DF; You, Y; Shoustikov, A; Sibley, S; Thompson, ME; Forrest, SR. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar]
- Cleave, VM; Yahioglu, G; Le Barny, P; Friend, RH; Tessler, N. Harvesting of singlet and triplet energy in polymer LEDs. Adv. Mater 1999, 11, 285–288. [Google Scholar]
- Gong, X; Robinson, MR; Ostrowski, JC; Moses, D; Bazan, GC; Heeger, AJ. High-Efficiency Polymer-Based Electrophosphorescent Devices. Adv. Mater 2002, 14, 581–585. [Google Scholar]
- Zhu, WG; Mo, YQ; Yuan, M; Yang, W; Cao, Y. Highly efficient electrophosphorescent devices based on conjugated polymers doped with iridium complexes. Appl. Phys. Lett 2002, 80, 2045–2047. [Google Scholar]
- Cheng, G; Fei, T; Duan, Y; Zhao, Y; Ma, YG; Liu, SY. Highly efficient white polymer light-emitting devices based on wide bandgap polymer doped with blue and yellow phosphorescent dyes. Opt. Lett 2010, 35, 2436–2438. [Google Scholar]
- Li, AY; Li, YY; Cai, WZ; Zhou, GJ; Chen, Z; Wu, HB; Wong, WY; Yang, W; Peng, JB; Cao, Y. Realization of highly efficient white polymer light-emitting devices via interfacial energy transfer from poly(N-vinylcarbazole). Org. Electron 2010, 11, 529–534. [Google Scholar]
- Liang, B; Xu, YH; Chen, Z; Peng, JB; Cao, Y. White polymer phosphorescent light-emitting devices with a new yellow-emitting iridium complex doped into polyfluorene. Synth. Met 2009, 159, 1876–1879. [Google Scholar]
- Chen, FC; Chien, SC; Chen, YS. Single-layer triplet white polymer light-emitting diodes incorporating polymer oxides: Effect of charge trapping at phosphorescent dopants. Appl Phys Lett 2009, 94, 043306:1–043306:3. [Google Scholar]
- Kim, TH; Lee, HK; Park, OO; Chin, BD; Lee, SH; Kim, JK. White-Light-Emitting Diodes Based on Iridium Complexes via Efficient Energy Transfer from a Conjugated Polymer. Adv. Funct. Mater 2006, 16, 611–617. [Google Scholar]
- Xu, YH; Peng, JB; Jiang, JX; Xu, W; Yang, W; Cao, Y. Efficient white-light-emitting diodes based on polymer codoped with two phosphorescent dyes. Appl Phys Lett 2005, 87, 193502:1–193502:3. [Google Scholar]
- Chance, RR; Prock, A; Silbey, R. Advances in Chemical Physics; Prigogine, I, Rice, SA, Eds.; Wiley-Interscience: New York, NY, USA, 1978; pp. 1–65. [Google Scholar]
- Becker, H; Burns, SE; Friend, RH. Effect of metal films on the photoluminescence and electroluminescence of conjugated polymers. Phys. Rev. B 1997, 56, 1893–1905. [Google Scholar]
- Obrien, D; Bleyer, A; Lidzey, DG; Bradley, DDC; Tsutsui, T. Efficient multilayer electroluminescence devices with poly(m-phenylenevinylene-co-2,5-dioctyloxy-p-phenylenevinylene) as the emissive layer. J. Appl. Phys 1997, 82, 2662–2670. [Google Scholar]
- Lacey, D. High-efficiency polymer light-emitting diodes. abstracts of the 9th International Workshop on Inorganic and Organic Electroluminesence, Bend, Oregon, USA, September; 13–171998. [Google Scholar]
- Tsutsui, T; Takada, N; Saito, S; Ogino, E. Sharply directed emission in organic electroluminescent diodes with an optical-microcavity structure. Appl. Phys. Lett 1994, 65, 1868–1870. [Google Scholar]
- Jordan, RH; Rothberg, LJ; Dodabalapur, A; Slusher, RE. Efficiency enhancement of microcavity organic light emitting diodes. Appl. Phys. Lett 1996, 69, 1997–1999. [Google Scholar]
- Grüner, J; Cacialli, F; Friend, RH. Emission enhancement in single-layer conjugated polymer microcavities. J. Appl. Phys 1996, 80, 207–215. [Google Scholar]
- Fisher, TA; Pate, DGMA; Weaver, MS; Whittaker, DM; Skolnick, MS; Bradley, DDC. Electroluminescence from a conjugated polymer microcavity structure. Appl. Phys. Lett 1995, 67, 1355–1357. [Google Scholar]
- Lemmer, U; Hennig, R; Guss, W; Ochse, A; Pommerehne, J; Sander, R; Greiner, A; Mahrt, RF; Bässler, H; Feldmann, J; Göbel, EO. Microcavity effects in a spin-coated polymer two-layer system. Appl. Phys. Lett 1995, 66, 1301–1303. [Google Scholar]
- Tessler, N; Denton, GJ; Friend, RH. Lasing from conjugated-polymer microcavities. Nature 1996, 382, 695–697. [Google Scholar]
- Tessler, N; Harrison, NT; Friend, RH. High peak brightness polymer light-emitting diodes. Adv. Mater 1998, 10, 64–68. [Google Scholar]
- Lidzey, DG; Bradley, DDC; Alvarado, SF; Seidler, PF. Electroluminescence in polymer films. Nature 1997, 386, 135–135. [Google Scholar]
- Lu, MH; Sturm, JC. External coupling efficiency in planar organic light-emitting devices. Appl. Phys. Lett 2001, 78, 1927–1929. [Google Scholar]
- Gu, G; Bulovic, V; Burrows, PE; Forrest, SR; Thompson, ME. Transparent organic light emitting devices. Appl. Phys. Lett 1996, 68, 2606–2608. [Google Scholar]
- Hung, LS; Tang, CW. Interface engineering in preparation of organic surface-emitting diodes. Appl. Phys. Lett 1999, 74, 3209–3211. [Google Scholar]
- Hung, LS; Tang, CW; Mason, MG; Raychaudhuri, P; Madathil, J. Application of an ultrathin LiF/Al bilayer in organic surface-emitting diodes. Appl. Phys. Lett 2001, 78, 544–546. [Google Scholar]
- Lu, MH; Weaver, MS; Zhou, TX; Rothman, M; Kwong, RC; Hack, M; Brown, JJ. High-efficiency top-emitting organic light-emitting devices. Appl. Phys. Lett 2002, 81, 3921–3923. [Google Scholar]
- Chong, LW; Chou, YN; Lee, YL; Wen, TC; Guo, TF. Hole-injection enhancement of top-emissive polymer light-emitting diodes by P3HT/FNAB modification of Ag anode. Org. Electron 2009, 10, 1141–1145. [Google Scholar]
- Hsieh, SN; Kuo, TY; Chong, LW; Wen, TC; Yang, FS; Guo, TF; Chung, CT. A Study of Semitransparent Cathodes on the Performance of Top-Emitting Polymer Light-Emitting Diodes. Photonics Technol. Lett 2009, 21, 109–111. [Google Scholar]
- Chuang, TK; Troccoli, M; Kuo, PC; Jamshidi-Roudbari, A; Hatalis, MK; Biaggio, I; Voutsas, AT. Top-emitting 230 dots/in. active-matrix polymer light-emitting diode displays on flexible metal foil substrates. Appl Phys Lett 2007, 90, 151114:1–151114:3. [Google Scholar]
- Deng, XY; Ho, MK; Wong, KY. Top-emitting polymer light-emitting diodes with environmentally stable cathodes. J Appl Phys 2006, 99, 016103:1–016103:3. [Google Scholar]
- Guo, TF; Yang, FS; Tsai, ZJ; Wen, TC; Hsieh, SN; Fu, YS; Chung, CT. Organic oxide/Al composite cathode in efficient polymer light-emitting diodes. Appl Phys Lett 2006, 88, 113501:1–113501:3. [Google Scholar]
- Hou, LT; Huang, F; Zeng, WJ; Peng, JB; Cao, Y. High-efficiency inverted top-emitting polymer light-emitting diodes. Appl Phys Lett 2005, 87, 153509:1–153509:3. [Google Scholar]
- Kido, J; Kimura, M; Nagai, K. Multilayer White Light-Emitting Organic Electroluminescent Device. Science 1995, 267, 1332–1334. [Google Scholar]
- Tasch, S; Brandstatter, C; Meghdadi, F; Leising, G; Froyer, G; Athouel, L. Red-green-blue light emission from a thin film electroluminescence device based on parahexaphenyl. Adv. Mater 1997, 9, 33–36. [Google Scholar]
- Dodabalapur, A; Rothberg, LJ; Miller, TM. Electroluminescence from organic semiconductors in patterned microcavities. Electron. Lett 1994, 30, 1000–1002. [Google Scholar]
- Nakamura, A; Tada, T; Mizukami, M; Hirose, S; Yagyu, S. Three-color polymer light-emitting diodes by stamped dye diffusion. Appl. Phys. Lett 2002, 80, 2189–2191. [Google Scholar]
- Tada, K; Onoda, M. Three-color polymer light-emitting devices patterned by maskless dye diffusion onto prepatterned electrode. Jpn. J. Appl. Phys. Lett 1999, 38, L1143–L1145. [Google Scholar]
- Pschenitzka, F; Sturm, JC. Three-color organic light-emitting diodes patterned by masked dye diffusion. Appl. Phys. Lett 1999, 74, 1913–1915. [Google Scholar]
- Niu, QL; Shao, YX; Xu, W; Wang, L; Han, SH; Liu, NH; Peng, JB; Cao, Y; Wang, J. Full color and monochrome passive-matrix polymer light-emitting diodes flat panel displays made with solution processes. Org. Electron 2008, 9, 95–100. [Google Scholar]
- Bharathan, J; Yang, Y. Polymer electroluminescent devices processed by inkjet printing: I. Polymer light-emitting logo. Appl. Phys. Lett 1998, 72, 2660–2662. [Google Scholar]
- Carter, J; Lyon, P; Creighton, C; Bale, M; Gregory, H. Developing a Scalable and Adaptable Ink Jet Printing Process for OLED Displays. SID Symp. Dig 2005, 36, 523. [Google Scholar]
- Birnstock, J; Blassing, J; Hunze, A; Scheffel, M; Stossel, M; Heuser, K; Wittmann, G; Worle, J; Winnacker, A. Screen-printed passive matrix displays based on light-emitting polymers. Appl. Phys. Lett 2001, 78, 3905–3907. [Google Scholar]
- Pardo, DA; Jabbour, GE; Peyghambarian, N. Application of screen printing in the fabrication of organic light-emitting devices. Adv. Mater 2000, 12, 1249–1255. [Google Scholar]
- Wang, PH; Ho, MS; Yang, SH; Chen, KB; Hsu, CS. Synthesis of thermal-stable and photo-crosslinkable polyfluorenes for the applications of polymer light-emitting diodes. J. Polym. Sci. Part A: Polym. Chem 2010, 48, 516–524. [Google Scholar]
- Muller, CD; Falcou, A; Reckefuss, N; Rojahn, M; Wiederhirn, V; Rudati, P; Frohne, H; Nuyken, O; Becker, H; Meerholz, K. Multi-colour organic light-emitting displays by solution processing. Nature 2003, 421, 829–833. [Google Scholar]
- Gather, MC; Kohnen, A; Falcou, A; Becker, H; Meerholz, K. Solution-processed full-color polymer organic light-emitting diode displays fabricated by direct photolithography. Adv. Funct. Mater 2007, 17, 191–200. [Google Scholar]
- Shirai, S; Kido, J. Fabrication of multi color polymer EL devices using the photo-bleaching method. J. Photopolym. Sci. Technol 2001, 14, 317–322. [Google Scholar]
- Pogantsch, A; Trattnig, G; Langer, G; Kern, W; Scherf, U; Tillmann, H; Horhold, HH; Zojer, E. Multicolor organic electroluminescent devices fabricated by a reductive photo-patterning method. Adv. Mater 2002, 14, 1722–1725. [Google Scholar]
- Deng, XY; Wong, KY. Cross-Linked Conjugated Polymers for Achieving Patterned Three-Color and Blue Polymer Light-Emitting Diodes with Multi-Layer Structures. Macromol. Rapid Commun 2009, 30, 1570–1576. [Google Scholar]
- Deng, XY; Wong, KY; Mo, YQ. Three-color polymeric light-emitting devices using selective photo-oxidation of multilayered conjugated polymers. Appl Phys Lett 2007, 90, 063505:1–063505:3. [Google Scholar]
- Cambridge Display Technology Home Page. Available online: http://www.cdtltd.co.uk (accessed on 10 Febuary 2011).
- Cambridge Display Technology Status. Available online: http://www.cdtltd.co.uk/technology/status/ (accessed on 10 February 2011).



| Spin/BE data@1000 cd/m2 | Red | Green | Blue | |||
|---|---|---|---|---|---|---|
| Efficiency [cd/A] | 11 | 31 | 28 | 50 | 9.0 | 6.0 |
| Color (C.I.E.) | x = 0.67 | x = 0.63 | x = 0.35 | x = 0.30 | x = 0.14 | x = 0.15 |
| y = 0.32 | y = 0.37 | y = 0.60 | y = 0.63 | y =0.22 | y = 0.14 | |
| Lifetime [h] | 200 k | 350 k | 200 k | 140 k | 34 k | 21 k |
| Vd [V] | 6.0 | 5.7 | 4.4 | 6.0 | 5.0 | ∼5.0 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Deng, X.-Y. Light-Emitting Devices with Conjugated Polymers. Int. J. Mol. Sci. 2011, 12, 1575-1594. https://doi.org/10.3390/ijms12031575
Deng X-Y. Light-Emitting Devices with Conjugated Polymers. International Journal of Molecular Sciences. 2011; 12(3):1575-1594. https://doi.org/10.3390/ijms12031575
Chicago/Turabian StyleDeng, Xian-Yu. 2011. "Light-Emitting Devices with Conjugated Polymers" International Journal of Molecular Sciences 12, no. 3: 1575-1594. https://doi.org/10.3390/ijms12031575




