Distribution and Molecular Characterization of β-Glucans from Hull-Less Barley Bran, Shorts and Flour
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Hull-Less Barley Samples
2.2. Yields and Chemical Compositions of Hull-Less Barley Roller-Milled Fractions
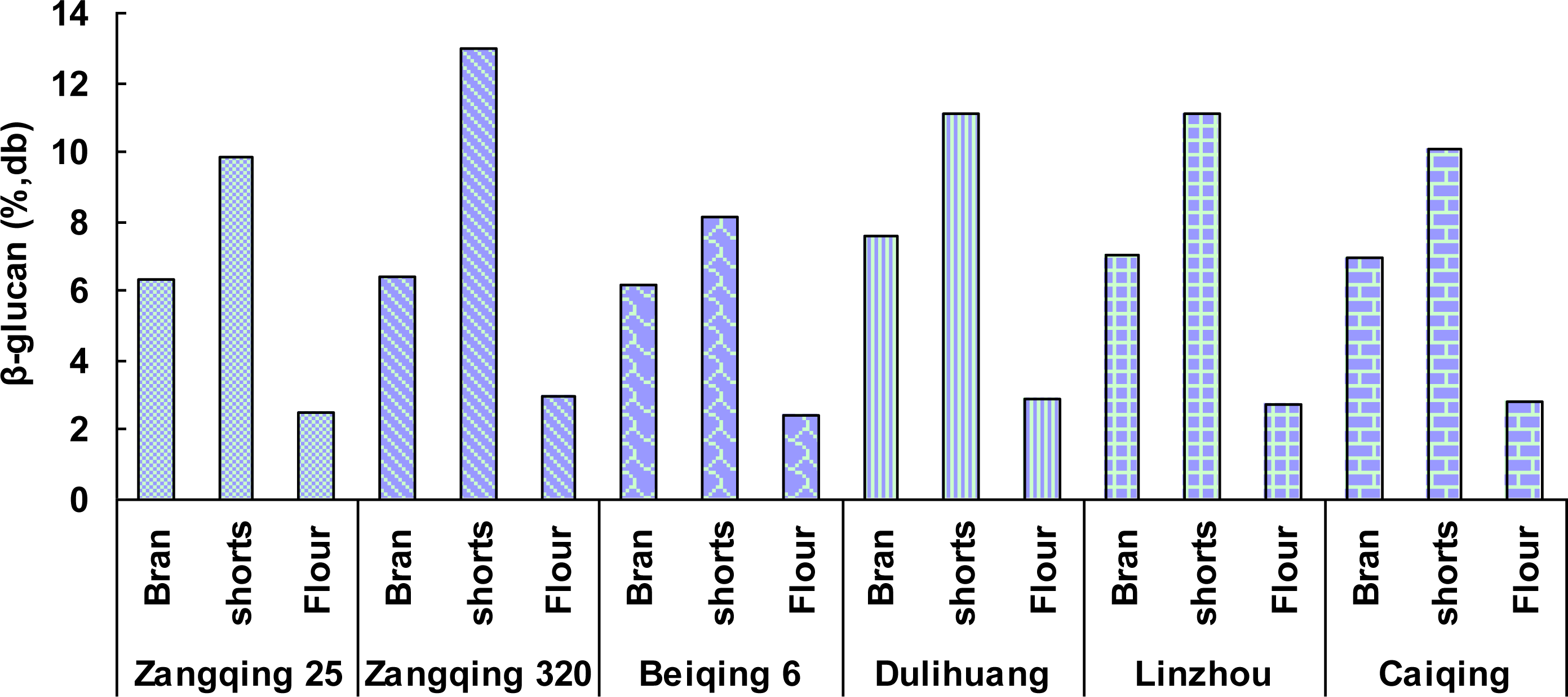
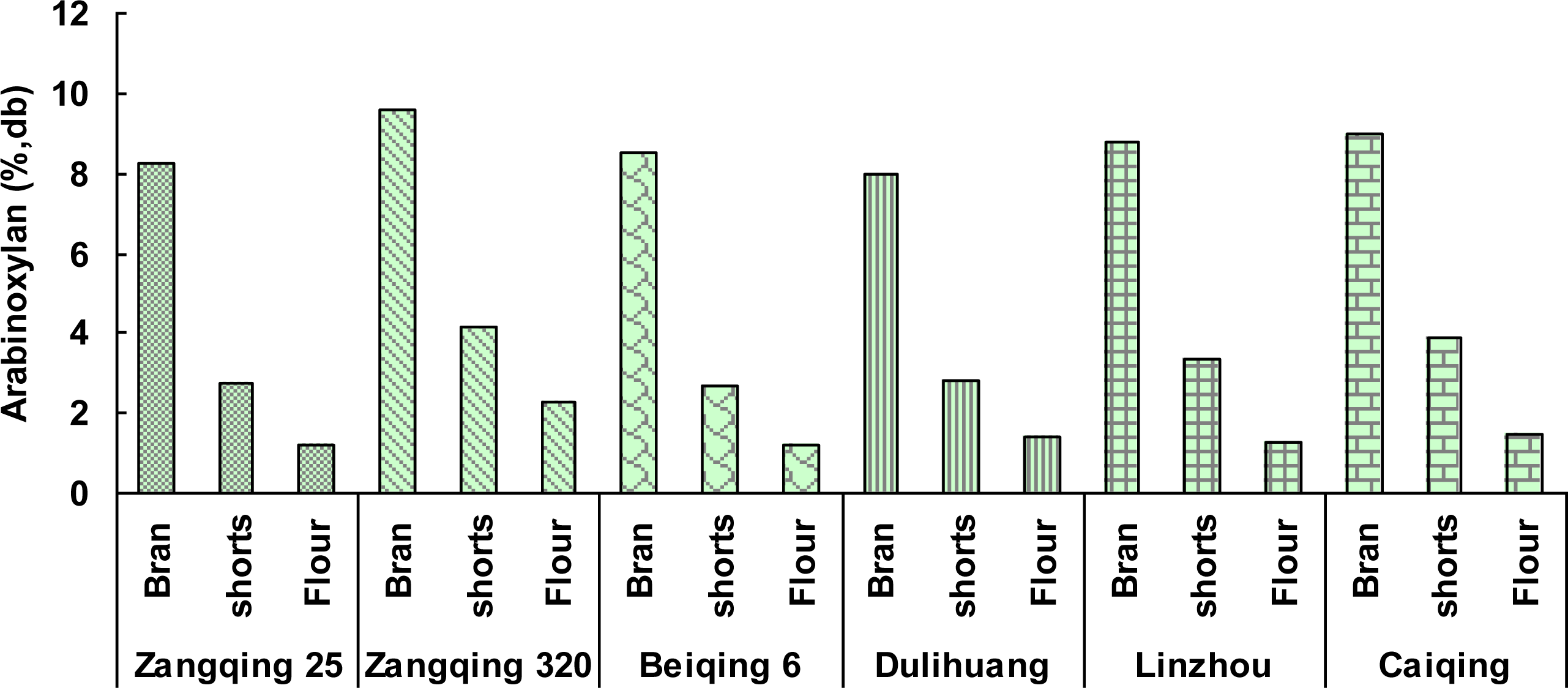
2.3. Yields and Compositions of β-Glucans from Hull-Less Barley Roller-Milled Fractions
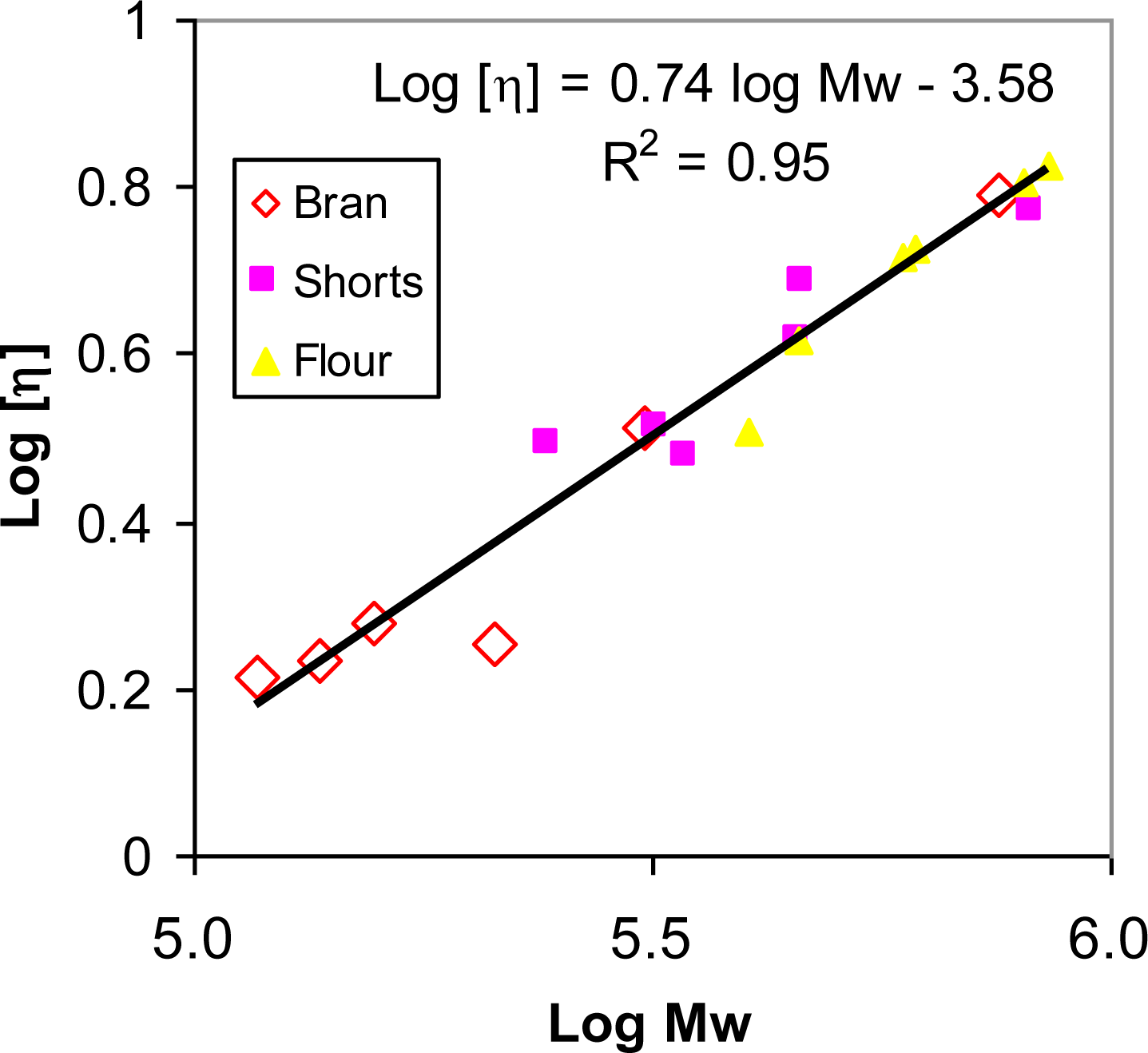
2.4. Molecular Properties of β-glucans from Roller-Milled Fractions of Hull-Less Barleys
3. Experimental Section
3.1. Materials
3.2. Hull-Less Barley Milling
3.3. Preparation and Purification of β-Glucans
3.4. Test Weight and 1000 Kernel Weight
3.5. Chemical Analyses
3.6. Characterization of β-glucan by Size Exclusion Chromatography (HPSEC)
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
References
- Bergund, PT; Fastnaught, CE; Holm, ET. Food uses of waxy hull-less barley. Cereal Food. World 1992, 37, 707–714. [Google Scholar]
- Bhatty, RS. Physiochemical and functional (breadmaking) properties of hull-less barley fractions. Cereal Chem 1986, 63, 31–35. [Google Scholar]
- Swanson, RB; Penfield, MP. Barley flour level and salt level selection for a whole-grain bread formula. J. Food Sci 1988, 53, 896–901. [Google Scholar]
- Newman, RK; Newman, CW. Barely as a food grain. Cereal Food. World 1991, 36, 800–805. [Google Scholar]
- Michael, UB; Arrigoni, E; Amado, R. Extraction of oat gum from oat bran: Effects of process on yield, molecular weight distribution, viscosity and β-d-glucan content of the gum. Cereal Chem 1996, 73, 58–62. [Google Scholar]
- Izydorczyk, MS; Jacobs, M; Dexter, JE. Distribution and structural variation of nonstarch polysaccharides in milling fractions of hull-less barley with variable amylose content. Cereal Chem 2003, 80, 645–653. [Google Scholar]
- Peterson, DM. Genotype and environment effects on oat β-glucan concentration. Crop Sci 1991, 31, 1517–1520. [Google Scholar]
- Lee, CJ; Horsley, RD; Manthey, FA; Schwarz, PB. Comparisons of β-glucan content of barley and oat. Cereal Chem 1997, 74, 571–575. [Google Scholar]
- Bhatty, RS. β-Glucan and flour yield of hull-less barley. Cereal Chem 1999, 76, 314–315. [Google Scholar]
- Knuckles, BE; Chiu, MM; Betschart, AA. β-Glucan-enriched fractions by laboratory-scale dry milling and sieving of barley and oats. Cereal Chem 1992, 69, 198–202. [Google Scholar]
- Wu, YU; Stringfellow, AC; Inglett, GE. Protein and β-glucan enriched fractions from high-protein, high β-glucan barleys by sieving and air classification. Cereal Chem 1994, 71, 220–223. [Google Scholar]
- Makoto, K; Charles, WB. Release of β-glucan from cell walls of starchy endosperm of barley. Cereal Chem 2001, 78, 121–124. [Google Scholar]
- Wood, PJ; Weisz, J; Mahn, W. Molecular characterization of cereal β-glucans. II. Size-exclusion chromatography for comparison of molecular weight. Cereal Chem 1991, 68, 530–531. [Google Scholar]
- Per Åman, LR; Roger, A. Molecular weight distribution of β-glucan in oat-based foods. Cereal Chem 2004, 81, 356–360. [Google Scholar]
- Wei, L; Steve, WC; Yukio, K. Extraction, fractionation, structural and physical characterization of wheat β-d-glucans. Carbohyd. Polym 2006, 63, 408–416. [Google Scholar]
- Bhatty, RS. β-Glucan content and viscosities of barleys and their roller-Milled Flour and bran products. Cereal Chem 1992, 69, 469–471. [Google Scholar]
- Holtekjolen, AK; Uhlen, AK; Brathen, SS. Contents of starch and non-starch polysaccharides in barley varieties of different origin. Food Chem 2006, 94, 348–358. [Google Scholar]
- Andersson, AAM; Courtin, CM; Delcour, JA; Fredriksson, H; Schofield, JD; Trogh, I; Tsiami, AA; Åman1, P. Milling performance of north European hull-less barleys and characterization of resultant millstreams. Cereal Chem 2003, 80, 667–673. [Google Scholar]
- Bhatty, RS. Milling of regular and waxy starch hull-less barleys for the production of bran and flour. Cereal Chem 1997, 74, 693–699. [Google Scholar]
- Izydorczyk, MS; Dexter, JE; Desjardins, RG. Roller milling of Canadian hull-less barley: Optimization of roller milling conditions and composition of mill streams. Cereal Chem 2003, 80, 637–644. [Google Scholar]
- Rolando, AF; Kevin, B; Hicks, DW. High-starch and high-β-glucan barley fractions milled with experimental mills. Cereal Chem 2005, 82, 727–733. [Google Scholar]
- Knuckles, BE; Chiu, MM. β-Glucan enrichment of barley fractions by air classification and sieving. J. Food Sci 1995, 60, 1070–1074. [Google Scholar]
- Knuckles, BE; Yokoyama, WH; Chiu, MM. Molecular characterization of barley β-glucans by size-exclusion chromatography with multiple-angle laser light scattering and other detectors. Cereal Chem 1997, 74, 599–604. [Google Scholar]
- Beer, MU; Wood, PJ; Weisz, J. Comparison of molecular weight distribution, structure and β-glucan content of consecutive extracts of various oat and barley cultivars. Cereal Food. World 1995, 40, 652–629. [Google Scholar]
- Manzanares, P; Navarro, A; Sendra, JM; Carbonell, JV. Determination of the average molecular weight of barley β-glucan within the range 30–100 k by the Calcofluor-FIA method. J. Cereal Sci 1993, 17, 211–223. [Google Scholar]
- Cui, WW; Peter, JW; John, W; Michael, UB. Nonstarch polysaccharides from preprocessed wheat bran: Carbohydrate analysis and novel rheological properties. Cereal Chem 1999, 76, 129–133. [Google Scholar]
- Douglas, SG. A rapid method for the determination of pentosans in wheat flour. Food Chem 1981, 7, 139–145. [Google Scholar]
- McCleary, BV; Glennie, HM. Enzymatic quantification of (1→3) (1→4)-β-d-glucan in barley and malt. J. Inst. Brew 1985, 91, 285–295. [Google Scholar]
| Sample | Test weight (g/L) | 1000 Kernel Wt (g) | Ash (%, db) | Starch (%, db) | Crude protein (%, db) | Arabinoxylan (%, db) | β-glucan (%, db) |
|---|---|---|---|---|---|---|---|
| Zangqing 25 | 786 ± 6 | 51.3 ± 1.4 | 1.79 ± 0.01 | 55.4 ± 1.1 | 9.4 ± 0.4 | 4.73 ± 0.02 | 7.62 ± 0.01 |
| Zangqing32 | 727 ± 7 | 39.9 ± 0.3 | 1.80 ± 0.00 | 56.9 ± 0.9 | 9.2 ± 0.1 | 3.93 ± 0.01 | 4.96 ± 0.03 |
| Beiqing 6 | 769 ± 4 | 47.3 ± 1.3 | 1.65 ± 0.02 | 56.2 ± 1.4 | 10.1 ± 0.1 | 2.97 ± 0.01 | 6.08 ± 0.02 |
| Dulihuan | 742 ± 6 | 41.4 ± 0.9 | 1.93 ± 0.01 | 55.6 ± 0.7 | 10.1 ± 0.3 | 3.25 ± 0.04 | 5.61 ± 0.02 |
| Lizhou | 756 ± 9 | 43.3 ± 0.7 | 1.81 ± 0.00 | 55.9 ± 0.6 | 10.2 ± 0.3 | 3.16 ± 0.02 | 5.42 ± 0.01 |
| Caiqing | 737 ± 5 | 40.8 ± 1.0 | 1.87 ± 0.01 | 55.6 ± 1.2 | 10.2 ± 0.2 | 3.63 ± 0.03 | 5.29 ± 0.02 |
| Sample | Yield | Ash | Starch | Protein | Arabinoxylan | β-glucan | |
|---|---|---|---|---|---|---|---|
| Zangqing 25 | Bran | 16.7 | 3.43 ± 0.01 | 31.1 ± 0.4 | 19.3 ± 0.2 | 8.24 ± 0.01 | 6.34 ± 0.02 |
| shorts | 11.1 | 1.80 ± 0.00 | 43.8 ± 0.3 | 12.7 ± 0.2 | 2.75 ± 0.02 | 9.85 ± 0.03 | |
| Flour | 71.3 | 0.81 ± 0.01 | 68.8 ± 1.0 | 11.8 ± 0.1 | 1.20 ± 0.01 | 2.48 ± 0.01 | |
| Zangqing320 | Bran | 16.3 | 3.50 ± 0.01 | 28.5 ± 0.2 | 17.4 ± 0.2 | 9.59 ± 0.01 | 6.44 ± 0.01 |
| shorts | 12.5 | 1.77 ± 0.00 | 41.6 ± 0.4 | 10.9 ± 0.1 | 4.18 ± 0.01 | 13.01 ± 0.02 | |
| Flour | 69.5 | 0.66 ± 0.01 | 67.9 ± 0.9 | 11.7 ± 0.1 | 2.29 ± 0.01 | 2.95 ± 0.00 | |
| Beiqing6 | Bran | 17.0 | 3.19 ± 0.00 | 35.8 ± 0.5 | 18.6 ± 0.1 | 8.51 ± 0.02 | 6.15 ± 0.00 |
| shorts | 12.4 | 1.78 ± 0.01 | 49.8 ± 0.8 | 11.4 ± 0.3 | 2.70 ± 0.03 | 8.12 ± 0.01 | |
| Flour | 69.4 | 0.74 ± 0.02 | 68.0 ± 1.1 | 11.4 ± 0.2 | 1.20 ± 0.02 | 2.40 ± 0.04 | |
| Dulihuang | Bran | 18.1 | 2.82 ± 0.01 | 34.6 ± 0.3 | 16.9 ± 0.1 | 7.99 ± 0.01 | 7.58 ± 0.01 |
| shorts | 11.7 | 1.44 ± 0.00 | 44.0 ± 0.7 | 12.5 ± 0.3 | 2.82 ± 0.00 | 11.13 ± 0.02 | |
| Flour | 69.0 | 0.85 ± 0.00 | 69.6 ± 0.7 | 11.9 ± 0.4 | 1.44 ± 0.02 | 2.92 ± 0.01 | |
| Linzhou | Bran | 18.0 | 3.24 ± 0.01 | 27.8 ± 0.4 | 16.4 ± 0.3 | 8.76 ± 0.03 | 7.05 ± 0.01 |
| shorts | 10.2 | 1.52 ± 0.00 | 48.5 ± 0.6 | 9.7 ± 0.0 | 3.34 ± 0.03 | 11.12 ± 0.03 | |
| Flour | 70.7 | 0.99 ± 0.01 | 68.8 ± 1.2 | 12.5 ± 0.1 | 1.26 ± 0.02 | 2.72 ± 0.01 | |
| Caiqing | Bran | 16.4 | 2.51 ± 0.02 | 31.5 ± 0.3 | 15.8 ± 0.3 | 9.01 ± 0.01 | 6.95 ± 0.02 |
| shorts | 11.3 | 1.15 ± 0.02 | 48.1 ± 0.6 | 10.6 ± 0.3 | 3.86 ± 0.01 | 10.08 ± 0.01 | |
| Flour | 71.1 | 0.76 ± 0.01 | 69.1 ± 1.0 | 11.5 ± 0.2 | 1.49 ± 0.02 | 2.80 ± 0.01 | |
| Sample | Yield (%) | Ash (%, db) | Starch (%, db) | Protein (%, db) | β-glucan (%, db) | Arabinoxylan (%, db) | |
|---|---|---|---|---|---|---|---|
| Zangqing 25 | Bran | 7.68 ± 0.51 | 9.15 ± 0.11 | 2.7 ± 0.5 | 5.6 ± 0.1 | 58.03 ± 0.92 | 16.79 ± 0.42 |
| shorts | 10.23 ± 0.72 | 8.37 ± 0.07 | 4.3 ± 0.1 | 3.1 ± 0.0 | 65.73 ± 0.83 | 11.84 ± 0.53 | |
| Flour | 3.06 ± 0.14 | 5.23 ± 0.08 | 5.8 ± 0.3 | 3.4 ± 0.1 | 70.4 ± 0.81 | 12.86 ± 0.32 | |
| Zangqing320 | Bran | 7.24 ± 0.66 | 8.64 ± 0.12 | 5.3 ± 0.6 | 5.5 ± 0.0 | 45.38 ± 0.74 | 17.26 ± 0.23 |
| shorts | 13.77 ± 0.77 | 7.79 ± 0.09 | 7.3 ± 0.5 | 2.8 ± 0.0 | 61.26 ± 0.62 | 13.59 ± 0.33 | |
| Flour | 3.18 ± 0.21 | 6.29 ± 0.06 | 2.8 ± 0.5 | 5.3 ± 0.1 | 70.04 ± 0.82 | 14.28 ± 0.28 | |
| Beiqing6 | Bran | 7.33 ± 0.33 | 9.32 ± 0.10 | 2.7 ± 0.3 | 7.2 ± 0.1 | 60.7 ± 0.88 | 15.76 ± 0.30 |
| shorts | 9.47 ± 0.57 | 9.54 ± 0.07 | 5.3 ± 0.2 | 3.6 ± 0.2 | 65.82 ± 0.63 | 11.08 ± 0.09 | |
| Flour | 2.95 ± 0.30 | 7.71 ± 0.06 | 4.7 ± 0.2 | 9.6 ± 0.0 | 66.29 ± 0.82 | 10.81 ± 0.14 | |
| Dulihuang | Bran | 8.28 ± 0.61 | 8.11 ± 0.10 | 3.3 ± 0.1 | 4.8 ± 0.1 | 56.15 ± 0.64 | 16.13 ± 0.19 |
| shorts | 11.73 ± 0.69 | 9.64 ± 0.09 | 5.4 ± 0.1 | 4.0 ± 0.1 | 58.77 ± 0.53 | 13.41 ± 0.22 | |
| Flour | 3.40 ± 0.24 | 7.98 ± 0.07 | 6.2 ± 0.0 | 5.5 ± 0.1 | 61.65 ± 0.91 | 13.3 ± 0.25 | |
| Linzhou | Bran | 8.26 ± 0.58 | 9.39 ± 0.08 | 2.9 ± 0.2 | 4.8 ± 0.0 | 58.05 ± 0.76 | 16.21 ± 0.13 |
| shorts | 12.01 ± 0.97 | 7.47 ± 0.10 | 9.0 ± 0.1 | 3.1 ± 0.2 | 58.45 ± 0.82 | 13.03 ± 0.25 | |
| Flour | 3.44 ± 0.22 | 8.24 ± 0.06 | 3.2 ± 0.1 | 4.3 ± 0.0 | 66.57 ± 0.67 | 14.49 ± 0.46 | |
| Caiqing | Bran | 7.91 ± 0.45 | 9.68 ± 0.05 | 3.5 ± 0.8 | 5.3 ± 0.1 | 51.61 ± 0.81 | 16.48 ± 0.37 |
| shorts | 11.03 ± 0.69 | 8.19 ± 0.07 | 7.8 ± 0.4 | 2.6 ± 0.1 | 60.52 ± 0.77 | 12.76 ± 0.26 | |
| Flour | 2.83 ± 0.23 | 7.76 ± 0.09 | 5.1 ± 0.0 | 4.4 ± 0.1 | 71.41 ± 0.32 | 11.69 ± 0.22 | |
| Samples | Yield (%) | Ash (%, db) | Starch (%, db) | Protein (%, db) | β-glucan (%, db) | Arabinoxylan (%, db) | |
|---|---|---|---|---|---|---|---|
| Zangqing 25 | Bran | 4.25 ± 0.36 | 0.34 ± 0.02 | 2.3 ± 0.66 | 1.49 ± 0.66 | 91.38 ± 0.66 | 2.24 ± 0.66 |
| shorts | 7.47 ± 0.42 | 0.49 ± 0.03 | 2.1 ± 0.66 | 2.14 ± 0.66 | 92.04 ± 0.66 | 2.47 ± 0.66 | |
| Flour | 1.84 ± 0.06 | 0.39 ± 0.01 | 2.7 ± 0.66 | 1.81 ± 0.66 | 90.05 ± 0.66 | 2.32 ± 0.66 | |
| Beiqing6 | Bran | 3.88 ± 0.28 | 0.64 ± 0.04 | 2.4 ± 0.66 | 1.57 ± 0.66 | 94.57 ± 0.66 | 2.38 ± 0.66 |
| shorts | 10.32 ± 0.56 | 0.51 ± 0.03 | 1.8 ± 0.66 | 1.79 ± 0.66 | 93.38 ± 0.66 | 2.74 ± 0.66 | |
| Flour | 1.72 ± 0.08 | 0.49 ± 0.01 | 2.1 ± 0.66 | 2.14 ± 0.66 | 90.88 ± 0.66 | 2.17 ± 0.66 | |
| Dulihuang | Bran | 4.23 ± 0.32 | 0.34 ± 0.02 | 1.3 ± 0.66 | 2.19 ± 0.66 | 94.38 ± 0.66 | 1.24 ± 0.66 |
| shorts | 6.58 ± 0.44 | 0.29 ± 0.01 | 2.2 ± 0.66 | 1.04 ± 0.66 | 95.04 ± 0.66 | 2.47 ± 0.66 | |
| Flour | 1.32 ± 0.04 | 0.74 ± 0.04 | 2.1 ± 0.66 | 1.69 ± 0.66 | 92.38 ± 0.66 | 3.14 ± 0.66 | |
| Linzhou | Bran | 5.30 ± 0.66 | 0.59 ± 0.05 | 2.0 ± 0.66 | 2.04 ± 0.66 | 94.29 ± 0.66 | 2.07 ± 0.66 |
| shorts | 8.79 ± 0.62 | 0.34 ± 0.02 | 2.0 ± 0.66 | 1.19 ± 0.66 | 92.38 ± 0.66 | 2.24 ± 0.66 | |
| Flour | 1.72 ± 0.02 | 0.33 ± 0.01 | 2.2 ± 0.66 | 1.34 ± 0.66 | 90.04 ± 0.66 | 1.40 ± 0.66 | |
| Zangqing320 | Bran | 5.22 ± 0.38 | 0.24 ± 0.02 | 2.3 ± 0.66 | 1.49 ± 0.66 | 95.38 ± 0.66 | 2.24 ± 0.66 |
| shorts | 8.88 ± 0.64 | 0.49 ± 0.03 | 2.1 ± 0.66 | 1.14 ± 0.66 | 93.04 ± 0.66 | 1.47 ± 0.66 | |
| Flour | 1.58 ± 0.06 | 0.39 ± 0.03 | 2.7 ± 0.66 | 2.81 ± 0.66 | 92.05 ± 0.66 | 2.30 ± 0.66 | |
| Caiqing | Bran | 4.49 ± 0.38 | 0.34 ± 0.02 | 2.4 ± 0.66 | 2.57 ± 0.66 | 92.57 ± 0.66 | 2.38 ± 0.66 |
| shorts | 8.62 ± 0.60 | 0.52 ± 0.04 | 1.8 ± 0.66 | 2.79 ± 0.66 | 93.38 ± 0.66 | 2.74 ± 0.66 | |
| Flour | 1.73 ± 0.04 | 0.44 ± 0.02 | 2.1 ± 0.66 | 1.14 ± 0.66 | 94.88 ± 0.66 | 2.17 ± 0.66 | |
| Standard 1 | 0.21 ± 0.01 | 1.1 ± 0.66 | 0.54 ± 0.66 | 97.04 ± 0.66 | 0.87 ± 0.66 | ||
| Standard 2 | 0.16 ± 0.01 | 0.9 ± 0.66 | 0.32 ± 0.66 | 98.04 ± 0.66 | 0.62 ± 0.66 | ||
| Standard 3 | 0.25 ± 0.03 | 0.7 ± 0.66 | 0.27 ± 0.66 | 98.04 ± 0.66 | 0.47 ± 0.66 | ||
| Samples | Mw(×105) (g/mol) | [η] (dl/g) | Rg (nm) | Pd (Mn/Mw) | |
|---|---|---|---|---|---|
| Zangqing 25 | Bran | 2.12 ± 0.02 | 1.80 ± 0.06 | 22.06 ± 0.42 | 2.91 ± 0.03 |
| shorts | 3.44 ± 0.06 | 2.99 ± 0.08 | 31.25 ± 0.53 | 2.04 ± 0.02 | |
| Flour | 4.03 ± 0.03 | 3.23 ± 0.06 | 33.32 ± 0.38 | 1.27 ± 0.01 | |
| Beiqing6 | Bran | 1.57 ± 0.02 | 1.91 ± 0.02 | 20.25 ± 0.22 | 1.19 ± 0.03 |
| shorts | 3.36 ± 0.07 | 3.80 ± 0.07 | 33.59 ± 0.65 | 2.17 ± 0.04 | |
| Flour | 4.55 ± 0.07 | 4.14 ± 0.10 | 39.65 ± 0.72 | 1.70 ± 0.01 | |
| Dulihuang | Bran | 7.55 ± 0.09 | 6.19 ± 0.08 | 51.47 ± 0.68 | 2.10 ± 0.04 |
| shorts | 8.23 ± 0.06 | 5.90 ± 0.06 | 53.15 ± 0.83 | 2.39 ± 0.03 | |
| Flour | 8.52 ± 0.07 | 6.73 ± 0.07 | 56.33 ± 1.02 | 1.07 ± 0.03 | |
| Linzhou | Bran | 3.10 ± 0.05 | 3.24 ± 0.03 | 30.90 ± 0.77 | 1.91 ± 0.01 |
| shorts | 4.62 ± 0.04 | 4.86 ± 0.05 | 41.41 ± 0.92 | 2.77 ± 0.02 | |
| Flour | 6.10 ± 0.06 | 5.32 ± 0.04 | 48.69 ± 1.02 | 1.08 ± 0.02 | |
| Zangqing320 | Bran | 1.37 ± 0.02 | 1.71 ± 0.01 | 19.20 ± 0.38 | 1.83 ± 0.05 |
| shorts | 2.43 ± 0.03 | 3.11 ± 0.03 | 28.34 ± 0.42 | 1.74 ± 0.04 | |
| Flour | 5.91 ± 0.07 | 5.19 ± 0.07 | 45.42 ± 0.76 | 1.37 ± 0.03 | |
| Caiqing | Bran | 1.18 ± 0.02 | 1.63 ± 0.03 | 17.85 ± 0.23 | 2.03 ± 0.03 |
| shorts | 3.19 ± 0.05 | 3.26 ± 0.04 | 33.44 ± 0.54 | 1.89 ± 0.05 | |
| Flour | 8.05 ± 0.09 | 6.43 ± 0.09 | 54.63 ± 0.68 | 1.78 ± 0.06 | |
| Standard 1 | High viscosity | 3.71 ± 0.03 | 4.15 ± 0.05 | 37.16 ± 0.62 | 1.26 ± 0.02 |
| Standard 2 | Medium viscosity | 2.49 ± 0.05 | 2.99 ± 0.03 | 29.10 ± 0.32 | 1.34 ± 0.02 |
| Standard 3 | Low viscosity | 1.58 ± 0.01 | 2.53 ± 0.03 | 23.86 ± 0.44 | 1.19 ± 0.04 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zheng, X.; Li, L.; Wang, Q. Distribution and Molecular Characterization of β-Glucans from Hull-Less Barley Bran, Shorts and Flour. Int. J. Mol. Sci. 2011, 12, 1563-1574. https://doi.org/10.3390/ijms12031563
Zheng X, Li L, Wang Q. Distribution and Molecular Characterization of β-Glucans from Hull-Less Barley Bran, Shorts and Flour. International Journal of Molecular Sciences. 2011; 12(3):1563-1574. https://doi.org/10.3390/ijms12031563
Chicago/Turabian StyleZheng, Xueling, Limin Li, and Qi Wang. 2011. "Distribution and Molecular Characterization of β-Glucans from Hull-Less Barley Bran, Shorts and Flour" International Journal of Molecular Sciences 12, no. 3: 1563-1574. https://doi.org/10.3390/ijms12031563




