Stabilization of the Single-Chain Fragment Variable by an Interdomain Disulfide Bond and Its Effect on Antibody Affinity
Abstract
:1. Introduction
2. Results
2.1. Modeling of scFv and Design of the Interdomain Disulfide Bond
2.2. Expression of Antibody Fragments by E. coli
2.3. Aggregation Behavior
2.4. Guanidinium Chloride (GdnHCl)-Induced Unfolding Profiles
2.5. Effect of Disulfide Bond on Binding Activity
3. Experimental Section
3.1. Bacterial Strains, Vectors, Enzymes, and Chemicals
3.2. Homology Modeling and Molecular Docking
3.3. Cloning and Construction of scFv
3.4. Expression and Purification of scFv
3.5. Determination of Free Sulfhydryl Groups
3.6. Analytical Gel Filtration
3.7. Fluorescence Measurement
3.8. Enzyme-Linked Immunosorbent Assay
4. Discussion
5. Conclusion
Acknowledgments
References
- Worn, A; Pluckthun, A. Different equilibrium stability behavior of ScFv fragments: identification, classification, and improvement by protein engineering. Biochemistry 1999, 38, 8739–8750. [Google Scholar]
- Pluckthun, A. Mono- and bivalent antibody fragments produced in Escherichia coli: engineering, folding and antigen binding. Immunol. Rev 1992, 130, 151–188. [Google Scholar]
- Rothlisberger, D; Honegger, A; Pluckthun, A. Domain interactions in the Fab fragment: a comparative evaluation of the single-chain Fv and Fab format engineered with variable domains of different stability. J. Mol. Biol 2005, 4, 773–789. [Google Scholar]
- Worn, A; Pluckthun, A. Stability engineering of antibody single-chain Fv fragments. J. Mol. Biol 2001, 305, 989–1010. [Google Scholar]
- Huang, BC; Davern, S; Kennel, SJ. Mono and bivalent binding of a scFv and covalent diabody to murine laminin-1 using radioiodinated proteins and SPR measurements: Effects on tissue retention in vivo. J. Immunol Methods 2006, 313, 149–160. [Google Scholar]
- Yang, L; Ding, HS; Gu, ZN; Zhao, JX; Chen, HQ; Tian, FW; Chen, YQ; Zhang, H; Wei, C. Selection of single chain fragment variables with directly coating of Aflatoxin B1 to enzyme-linked immnunosorbent assay plates. J. Agric. Food Chem 2009, 57, 8927–8932. [Google Scholar]
- Fellouse, FA; Li, B; Compaan, DM; Peden, AA; Hymowitz, SG; Sidhu, SS. Molecular recognition by a binary code. J. Mol. Biol 2005, 5, 1153–1162. [Google Scholar]
- Kusharyoto, W; Pleiss, J; Bachmann, TT; Schmid, RD. Mapping of a hapten-binding site: molecular modeling and site-directed mutagenesis study of an anti-atrazine antibody. Protein Eng 2002, 15, 233–241. [Google Scholar]
- Mizuguchi, K; Parker, JS; Blundell, TL; Gay, NJ. Geting knotted: a model for the structure and activation of Spatzle. Trends Biochem. Sci 1998, 23, 239–242. [Google Scholar]
- Wiederstein, M; Sippl, MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 2007, 35, W407–W410. [Google Scholar]
- Frey, D; Huber, T; Pluckthun, A; Grutter, MG. Structure of the recombinant antibody Fab fragment f3p4. Acta Crystallogr D 2008, 64, 636–643. [Google Scholar] [Green Version]
- Li, VG; Galvano, F; Frigiola, A. Potential immunoregulatory role of heme oxygenase-1 in human milk: a combined biochemical and molecular modeling approach. J. Nutr. Biochem 2009, 154, 1843–1846. [Google Scholar]
- Loewen, MC; Klein-Seetharaman, J; Getmanova, EV; Reeves, PJ; Schwalbe, H; Khorana, HG. Solution 19F nuclear Overhauser effects in structural studies of the cytoplasmic domain of mammalian rhodopsin. Proc. Natl. Acad. Sci USA 2001, 98, 4888–4892. [Google Scholar]
- Reiter, Y; Brinkmann, U; Jung, SH; Pastan, I; Lee, B. Disulfide stabilization of antibody Fv: computer predictions and experimental evaluation. Protein Eng 1995, 8, 1323–1331. [Google Scholar]
- Chothia, C; Lesk, AM; Tramontano, A; Levitt, M; Smith-Gill, SJ; Air, G. Conformations of immunoglobulin hypervariable regions. Nature 1989, 342, 877–883. [Google Scholar]
- Harvey, BR; Georgiou, G; Hayhurst, A; Jeong, KJ; Iverson, BL; Rogers, GK. Anchored periplasmic expression, a versatile technology for the isolation of high-affinity antibodies from Escherichia coli-expressed libraries. Proc Natl. Acad. Sci USA 2004, 101, 9193–9198. [Google Scholar]
- Pedroso, I; Irun, MP; Machicado, C; Sancho, J. Four-state equilibrium unfolding of a scFv antibody fragment. Biochemistry 2002, 41, 9873–9884. [Google Scholar]
- Monsellier, E; Bedouelle, H. Quantitative measurement of protein stability from unfolding equilibria monitored with the fluorescence maximum wavelength. Protein Eng. Des. Sel 2005, 18, 445–456. [Google Scholar]
- Whitelegg, N; Rees, AR. Antibody variable regions: toward a unified modeling method. Methods Mol. Biol 2004, 248, 51–91. [Google Scholar]
- Lauer, B; Ottleben, I; Jacobsen, HJ; Reinard, T. Production of a single-chain variable fragment antibody against fumonisin B1. J. Agric. Food Chem 2005, 53, 899–904. [Google Scholar]
- Whitelegg, N; Rees, AR. Antibody variable regions: toward a unified modeling method. Methods Mol. Biol 2004, 248, 51–91. [Google Scholar]
- Thannhauser, TW; Konishi, Y; Scheraga, HA. Sensitive quantitative analysis of disulfide bonds in polypeptides and proteins. Anal. Biochem 1984, 138, 181–188. [Google Scholar]
- Atwell, JL; Breheney, KA; Lawrence, LJ; McCoy, AJ; Kortt, AA; Hudson, PJ. scFv multimers of the anti-neuraminidase antibody NC10: length of the linker between VHand VL domains dictates precisely the transition between diabodies and triabodies. Protein Eng 1999, 12, 597–604. [Google Scholar]
- Dolezal, O; De Gori, R; Walter, M; Doughty, L; Hattarki, M; Hudson, PJ; Kortt, AA. Single-chain Fv multimers of the anti-neuraminidase antibody NC10: the residue at position 15 in the VL domain of the scFv-o(VL-VH) molecule is primarily responsible for formation of a tetramer-trimer equilibrium. Protein Eng 2003, 16, 47–56. [Google Scholar]
- Brandts, JF; Hu, CQ; Lin, LN; Mos, MT. A simple model for proteins with interacting domains. Applications to scanning calorimetry data. Biochemistry 1989, 28, 8588–8596. [Google Scholar]
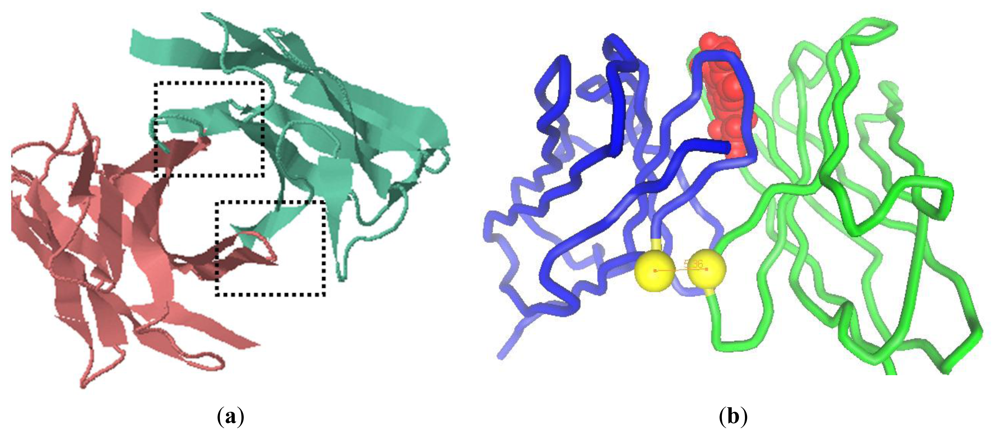
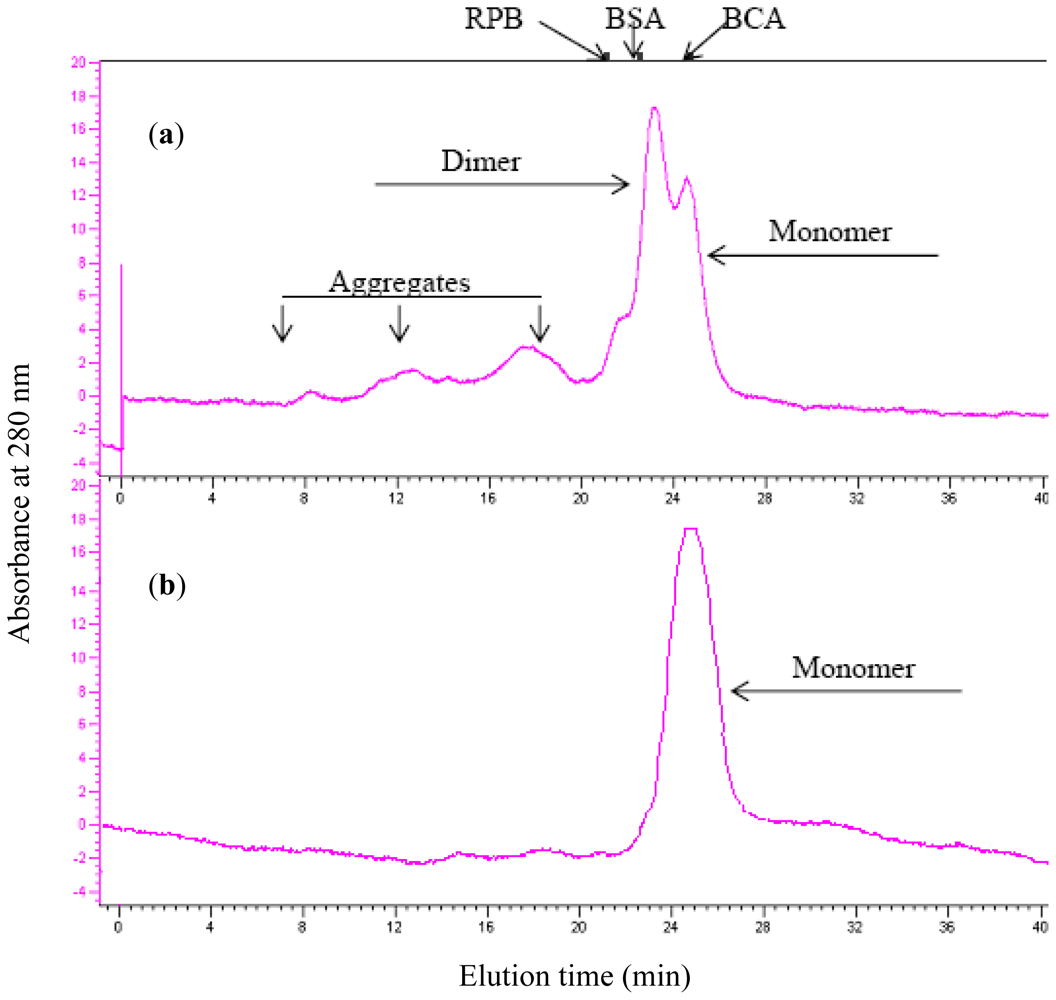
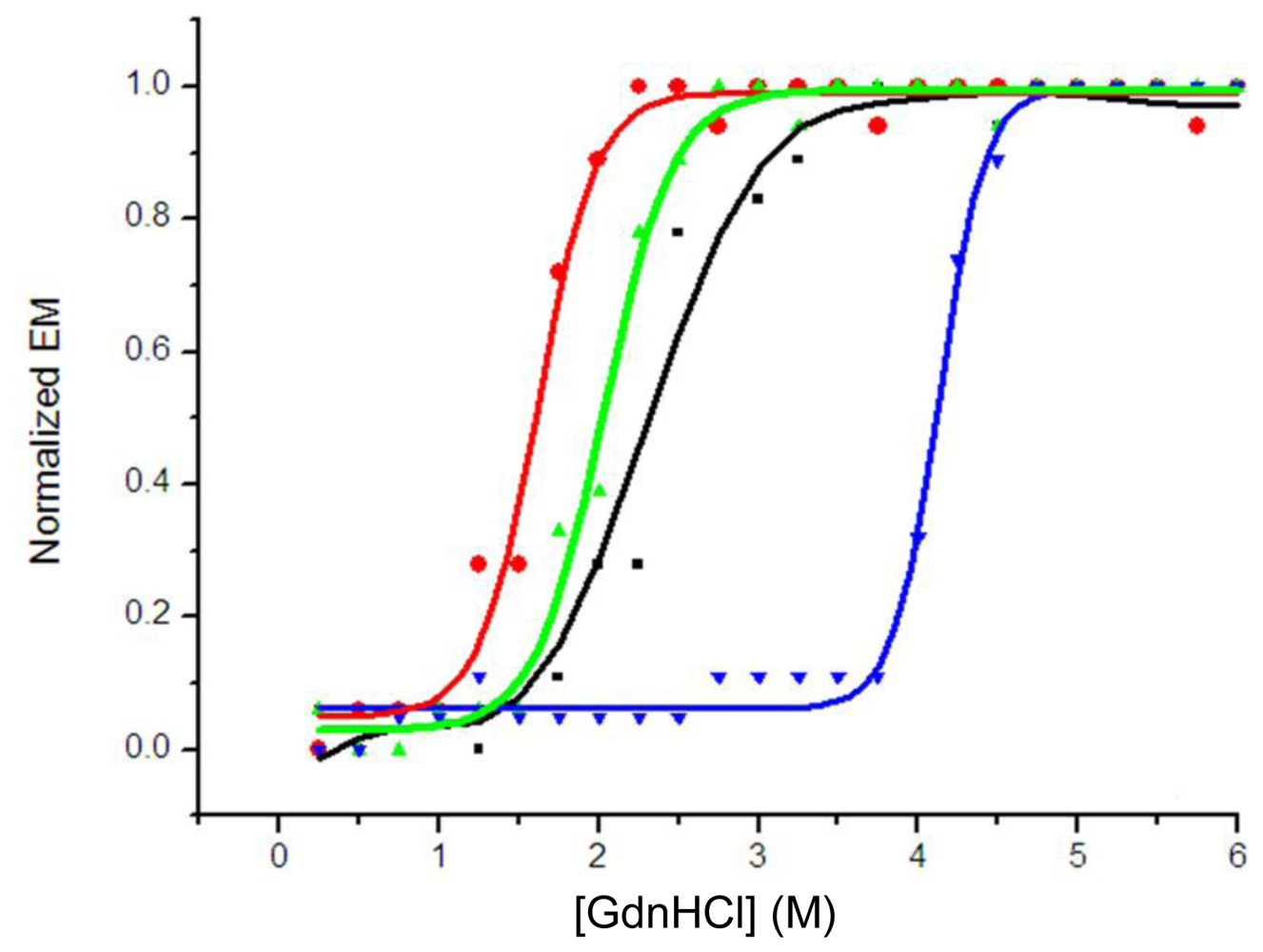

| Site Pair | Locations | Distance (Å) |
|---|---|---|
| H44-L100 | FR H2, FR L4 | 5.36 |
| H46-L98 | FR H2, FR L4 | 6.23 |
| H101-L44 | FR H4, FR L2 | 6.58 |
| H103-L42 | FR H4, FR L2 | 6.98 |
| H103-L43 | FR H4, FR L2 | 5.73 |
| Primer | Sequence |
|---|---|
| LMB | CGACCCGCCACCGCCGCTG |
| pHEN | CTATGCGGCCCCATTCA |
| L100Back | ATGTGCGGCCGCCCGTTTGATTTCCACCTTGGTCCCACAGCC |
| H44For | GCTCCAGGGAAGTGTCTGGAGTGGGTC |
| H44Back | GACCCACTCCAGACACTTCCCTGGAGC |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, J.-X.; Yang, L.; Gu, Z.-N.; Chen, H.-Q.; Tian, F.-W.; Chen, Y.-Q.; Zhang, H.; Chen, W. Stabilization of the Single-Chain Fragment Variable by an Interdomain Disulfide Bond and Its Effect on Antibody Affinity. Int. J. Mol. Sci. 2011, 12, 1-11. https://doi.org/10.3390/ijms12010001
Zhao J-X, Yang L, Gu Z-N, Chen H-Q, Tian F-W, Chen Y-Q, Zhang H, Chen W. Stabilization of the Single-Chain Fragment Variable by an Interdomain Disulfide Bond and Its Effect on Antibody Affinity. International Journal of Molecular Sciences. 2011; 12(1):1-11. https://doi.org/10.3390/ijms12010001
Chicago/Turabian StyleZhao, Jian-Xin, Lian Yang, Zhen-Nan Gu, Hai-Qin Chen, Feng-Wei Tian, Yong-Quan Chen, Hao Zhang, and Wei Chen. 2011. "Stabilization of the Single-Chain Fragment Variable by an Interdomain Disulfide Bond and Its Effect on Antibody Affinity" International Journal of Molecular Sciences 12, no. 1: 1-11. https://doi.org/10.3390/ijms12010001
APA StyleZhao, J.-X., Yang, L., Gu, Z.-N., Chen, H.-Q., Tian, F.-W., Chen, Y.-Q., Zhang, H., & Chen, W. (2011). Stabilization of the Single-Chain Fragment Variable by an Interdomain Disulfide Bond and Its Effect on Antibody Affinity. International Journal of Molecular Sciences, 12(1), 1-11. https://doi.org/10.3390/ijms12010001




