Model Free Approach for Non-Isothermal Decomposition of Un-Irradiated and g-Irradiated Silver Acetate: New Route for Synthesis of Ag2O Nanoparticles
Abstract
:1. Introduction
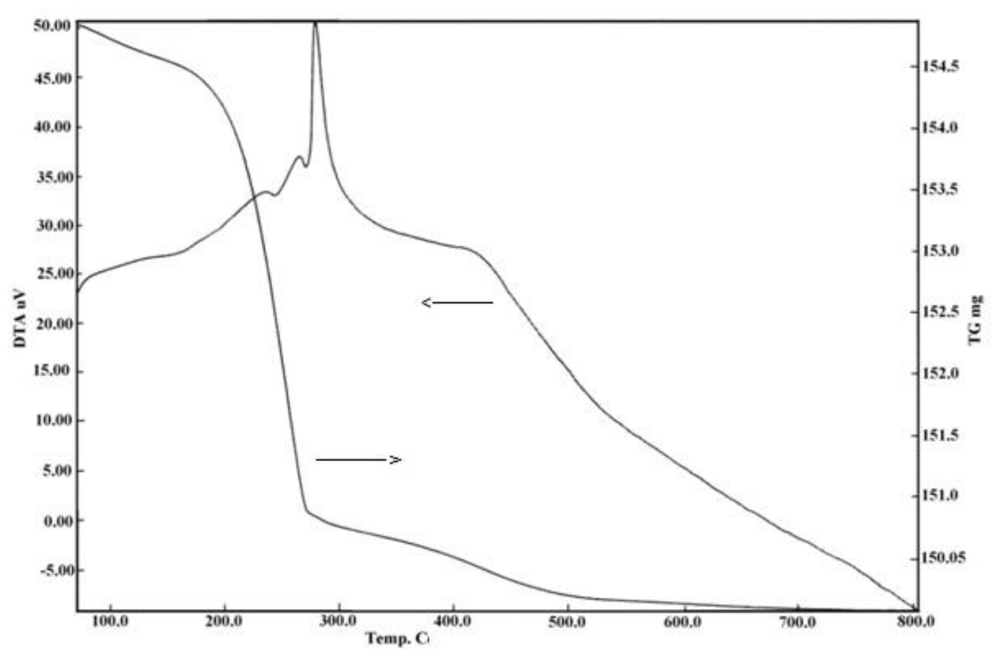
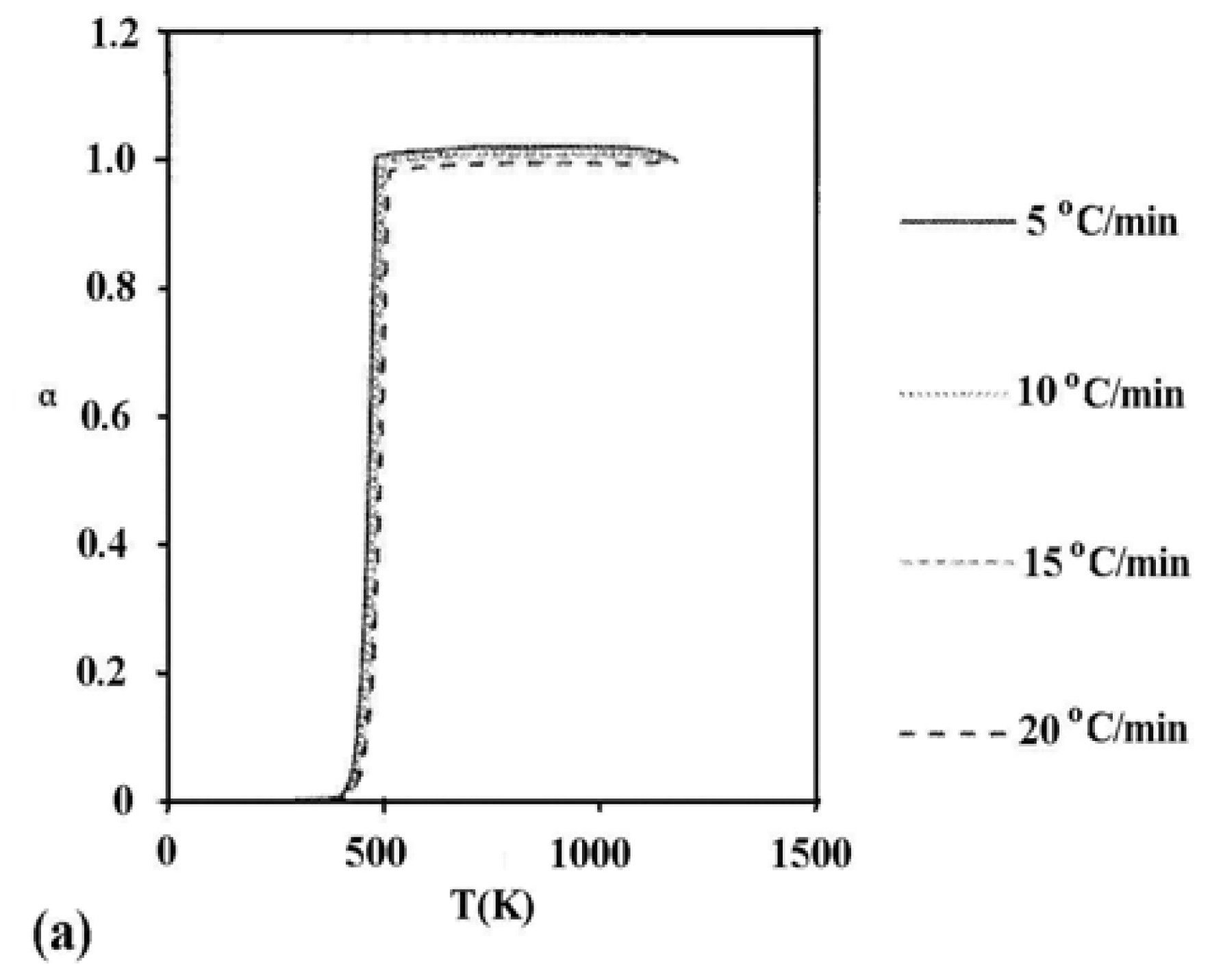
2. Results and Discussion
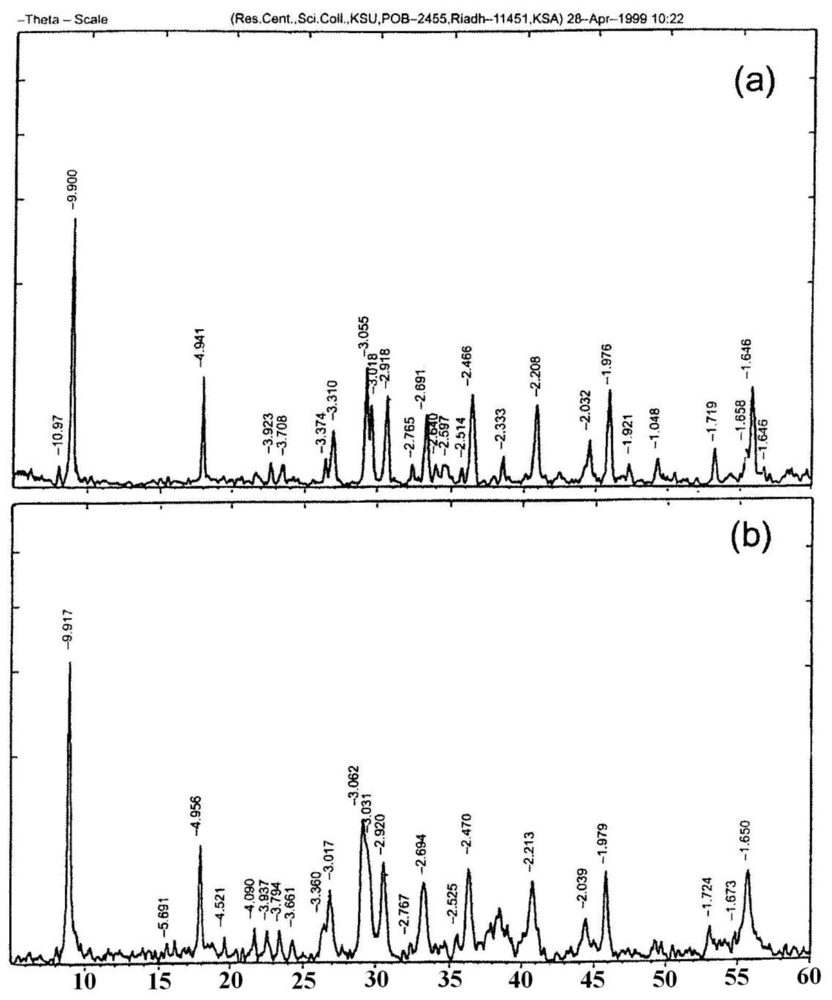
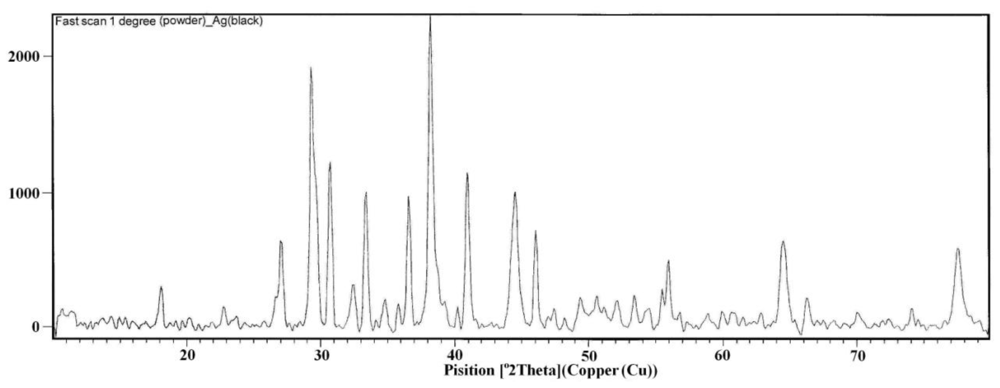
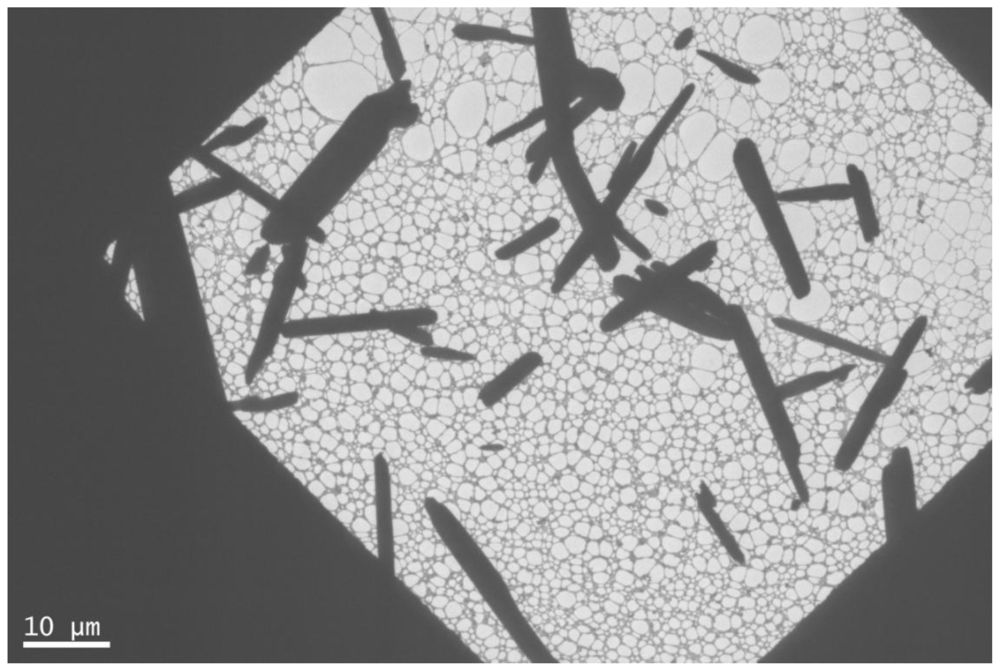
2.1. IR, XRD, SEM and TEM Measurements
2.2. Role of Irradiation
3. Experimental Section
3.1. Synthesis of Ag2O Nanoparticles
4. Conclusions
Acknowledgements
References
- Mahfouz, RM; Siddiqui, MR; Al-Ahmari, SA; Alkyali, WZ. Kinetics analysis of thermal decomposition of un-irradiated and γ-irradiated tris(acetylacetonate)-Ruthenium(III)[Ru(AcAc)3]. Prog. React. Kinet. Mechan 2007, 32, 1–27. [Google Scholar]
- Al-Khamis, KM; Al-Othman, ZA; Mahfouz, RM. Kinetics studies of the non-isothermal decomposition of un-irrdiated and γ-irradiated gallium acetylacetonate. Prog. React. Kinet. Mechan 2010, 35, 131–151. [Google Scholar]
- Balan, L; Malval, JP; Schneider, R; Burget, D. Silver nanoparticles: new synthesis characterization and photophysical properties. Mater. Chem. Phys 2007, 104, 417–421. [Google Scholar]
- Mahfouz, RM; Bumajdad, A; Al-Sagheer, FA. γ-Irradiation effects on the kinetic and mechanism of the thermal decomposition of silver acetate. Radiat. Effects Defects Solids 2009, 164, 170–177. [Google Scholar]
- Logvinenko, V; Polunina, O; Mikhailov, K; Bokhonov, BJ. Study of thermal decomposition of silver acetate. Therm. Anal. Cal 2007, 90, 813–816. [Google Scholar]
- Mahfouz, RM; Monshi, MAS; Abd El-Salam, NM. Kinetics of the thermal decomposition of γ-irradiated gadolinium acetate. Thermochim. Acta 2002, 38, 95–101. [Google Scholar]
- Galwey, AK; Herley, PJ; Mohamed, AM. Thermal decomposition of γ-irradiated silver malonate. J. Chem. Soc. Faraday Trans 1988, 184, 729–738. [Google Scholar]
- Spinks, JWT; Woods, RJ. An Introduction to Radiation Chemistry; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Doyle, CD. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci 1961, 5, 285–292. [Google Scholar]
- Vyazovkin, S; Wight, C. Model-free and model-fitting approaches to kinetic analysis of isothermal and non-isothermal data. Themochim. Acta 1999, 340–341, 53–68. [Google Scholar]
- Cai, J; Yao, F; Yi, W; He, F. New temperature integral approximation for non-isothermal kinetics. AlChE J 2006, 52, 1554–1557. [Google Scholar]



© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Siddiqui, M.R.H.; Alshehri, S.; Warad, I.K.; El-Salam, N.M.A.; Mahfouz, R.M. Model Free Approach for Non-Isothermal Decomposition of Un-Irradiated and g-Irradiated Silver Acetate: New Route for Synthesis of Ag2O Nanoparticles. Int. J. Mol. Sci. 2010, 11, 3600-3609. https://doi.org/10.3390/ijms11093600
Siddiqui MRH, Alshehri S, Warad IK, El-Salam NMA, Mahfouz RM. Model Free Approach for Non-Isothermal Decomposition of Un-Irradiated and g-Irradiated Silver Acetate: New Route for Synthesis of Ag2O Nanoparticles. International Journal of Molecular Sciences. 2010; 11(9):3600-3609. https://doi.org/10.3390/ijms11093600
Chicago/Turabian StyleSiddiqui, Mohammed Rafiq H., Saad Alshehri, Ismail Kh. Warad, Naser M. Abd El-Salam, and Refaat M. Mahfouz. 2010. "Model Free Approach for Non-Isothermal Decomposition of Un-Irradiated and g-Irradiated Silver Acetate: New Route for Synthesis of Ag2O Nanoparticles" International Journal of Molecular Sciences 11, no. 9: 3600-3609. https://doi.org/10.3390/ijms11093600




