Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-dressing Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology of CS/SF Blend Nanofibers
2.2. Crosslinking of Fibers
2.3. Mechanical Properties
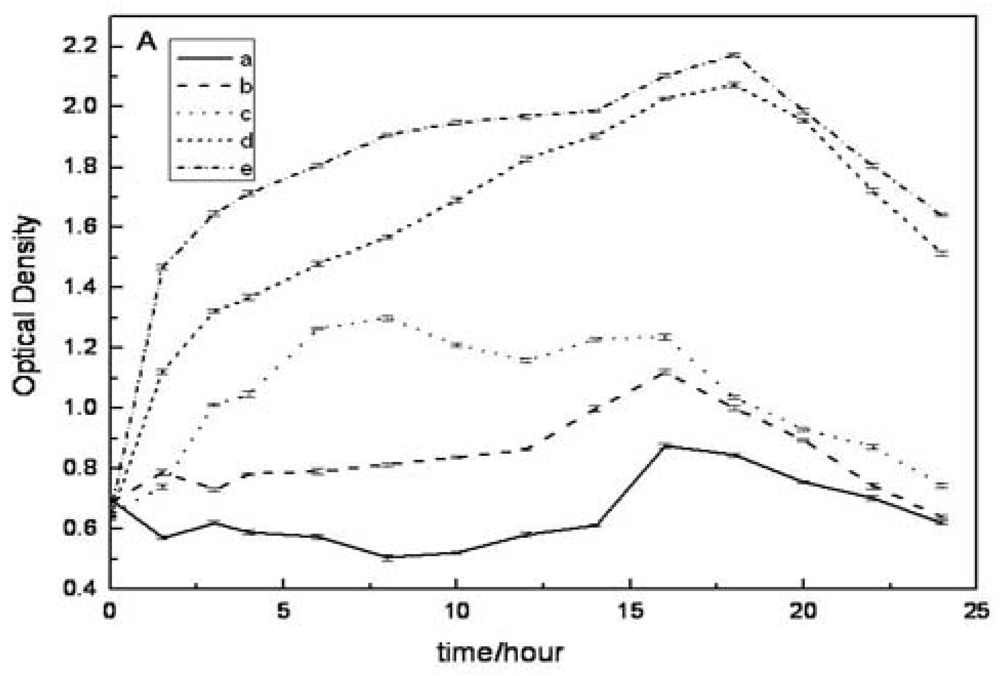
2.4. Evaluation of Antibacterial Activity in Vitro
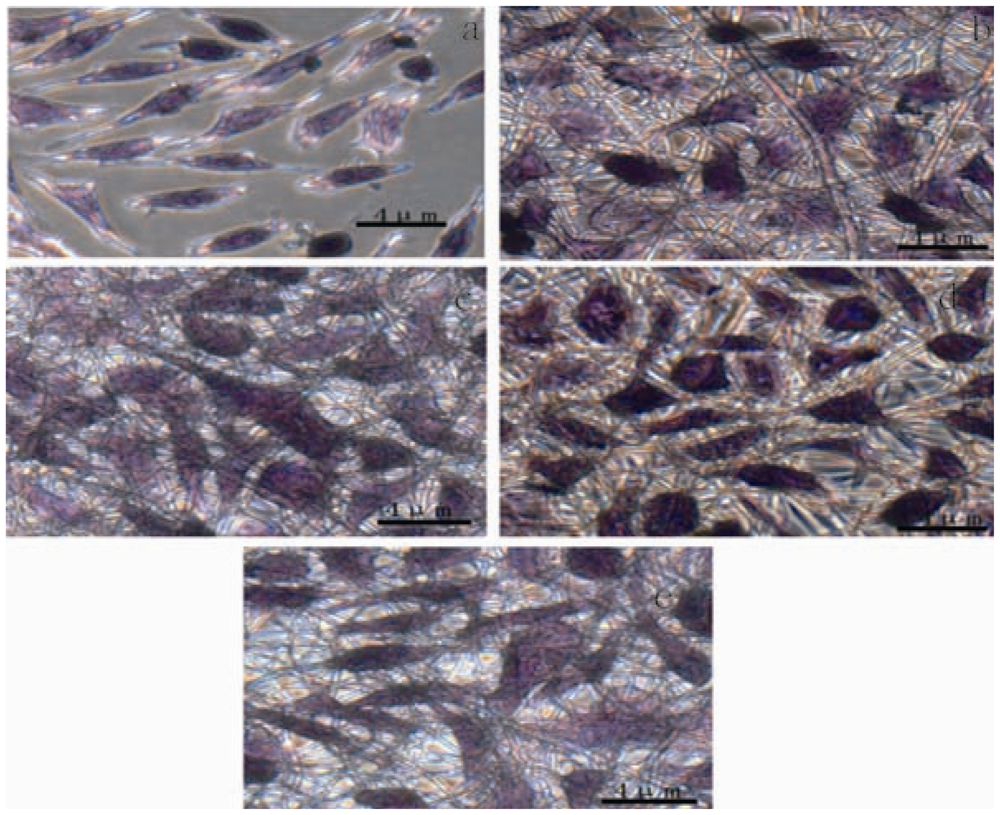
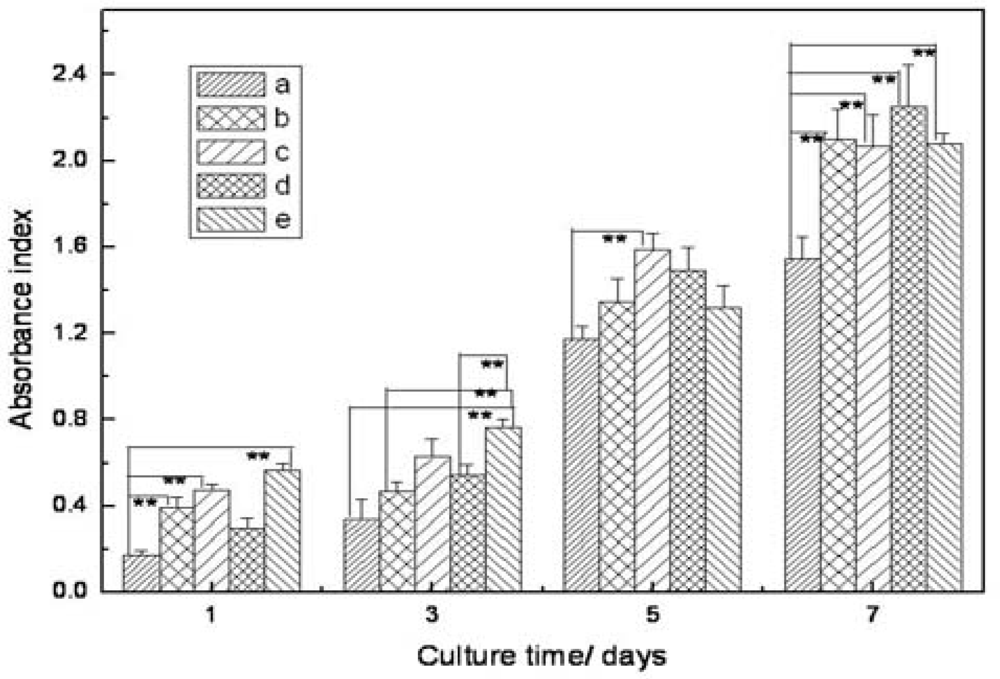
2.5. Cell Morphology and Proliferation
3. Experimental Section
3.1. Materials
3.2. Preparation of Regenerated SF
3.3. Electrospinning
3.4. Glutaraldehyde Vapor Crosslinking of Nanofibers
3.5. Scanning Electron Microscopy Analysis
3.6. Mechanical Properties
3.7. Antibacterial Assessment
3.8. Hematoxylin and Eosin (H&E) Staining and MTT Analysis
3.9. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Li, Y; Chen, XG; Liu, N. Physicochemical characterization and antibacterial property of chitosan acetates. Carbohydr. Polymer 2007, 67, 227–232. [Google Scholar]
- Ignatova, M; Starbova, K; Markova, N; Manolova, N; Rashkova, I. Electrospun nano-fibre membranes with antibacterial properties from quaternised chitosan and poly(vinyl alcohol). Carbohydr. Res 2006, 341, 2098–2107. [Google Scholar]
- Devlieghere, F; Vermeulen, A; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol 2004, 21, 703–714. [Google Scholar]
- Hartgerink, JD; Beniash, E; Stupp, SI. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. USA 2002, 99, 5133–5138. [Google Scholar]
- Hulteen, JC; Chen, HX; Chambliss, CK; Martin, CR. Template synthesis of carbon nanotubule and nanofiber arrays. Nanostruct. Mater 1997, 9, 133–136. [Google Scholar]
- Ma, PX; Zhang, RY. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res 1999, 46, 60–72. [Google Scholar]
- Reneker, DH; Yarin, AL; Fong, H; Koombhongse, S. Bending instabilityof electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys 2000, 87, 4531–4547. [Google Scholar]
- Srinivasan, G; Reneker, DH. Structure and morphology of small-diameter electrospun aramid fibers. Polymer Int 1995, 3, 195–201. [Google Scholar]
- Reneker, DH; Chun, I. Nanometre diameter fibres of polymer, produced byelectrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar]
- Yarin, AL; Koombhongse, S; Reneker, DH. Bending instability in electrospinning of nanofibers. J. Appl. Phys 2001, 89, 3018–3026. [Google Scholar]
- Jayaraman, K; Kotaki, M; Zhang, YZ; Mo, XM; Ramakrishna, S. Recent advances in polymer nanofibers. J. Nanosci. Nanotechnol 2004, 4, 52–65. [Google Scholar]
- Li, D; Xia, Y. Electrospinning of nanofibers reinventing the wheel. Adv. Mater 2004, 16, 1151. [Google Scholar]
- Ohkawa, K; Cha, D; Kim, H; Nishida, A; Yamamoto, H. Electrospinning of chitosan. Macromol. Rapid Commun 2004, 25, 1600–1605. [Google Scholar]
- Desai, K; Kit, K; Li, J; Zivanovic, S. Morphological and surface properties of electrospun chitosan nanofibers. Biomacromolecules 2008, 9, 1000–1006. [Google Scholar]
- Xu, J; Zhang, J; Gao, W; Liang, H; Wang, H; Li, J. Preparation of chitosan/PLA blend micro/nanofibers by electrospinning. Mater. Lett 2009, 63, 658–660. [Google Scholar]
- Park, WH; Jeong, L; Yoo, DI; Hudson, S. Effect of chitosan on morphology and conformation of silk fibroin nanofibers. Polymer 2004, 45, 7151–7157. [Google Scholar]
- Sakabe, H; Ito, H; Miyamoto, T; Noishiki, Y; Ha, WS. In vivo blood compatibility of regenerated silk fibroin. Sen-i Gakkaishi 1989, 45, 487–490. [Google Scholar]
- Santin, M; Motta, A; Freddi, G; Cannas, M. In vitro evaluation of the inflammatory potential of the silk fibroin. J. Biomed. Mater. Res 1999, 46, 382–389. [Google Scholar]
- Purna, SK; Babu, M. Collagen based dressings–a review. Burns 2000, 26, 54–62. [Google Scholar]
- Huang, ZM; Zhang, YZ; Ramakrishna, S; Lim, CT. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361. [Google Scholar]
- Chen, ZG; Wei, B; Mo, XM. Mechanical properties of electrospun collagen–chitosan complex single fibers and membrane. Mater. Sci. Eng. C 2009, 29, 2428–2435. [Google Scholar]
- Wang, X; Du, Y; Liu, H. Preparation, characterization and antibacterial activity of chitosan–Zn complex. Carbohydr. Polymers 2004, 56, 21–26. [Google Scholar]
- Chen, ZG; Wang, PW; Wei, B; Mo, XM; Cui, FZ. Electrospun collagen–chitosan nanofiber: A biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater 2010, 6, 372–382. [Google Scholar]
- Zhou, Y; Yang, D; Chen, X; Xu, Q; Lu, F; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar]
- Stephan, T; Dubas, PK. Coating of polyelectrolyte multilayer thin films on nanofibrous scaffolds to improve cell adhesion. J. Appl. Polymer Sci 2009, 114, 1574–1579. [Google Scholar]
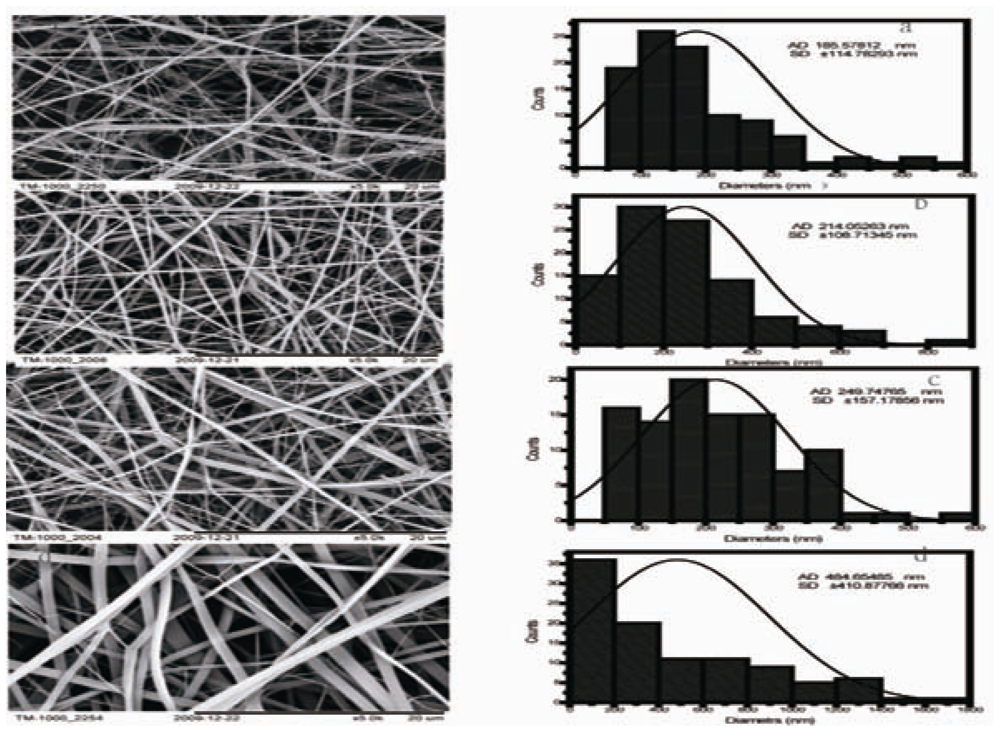
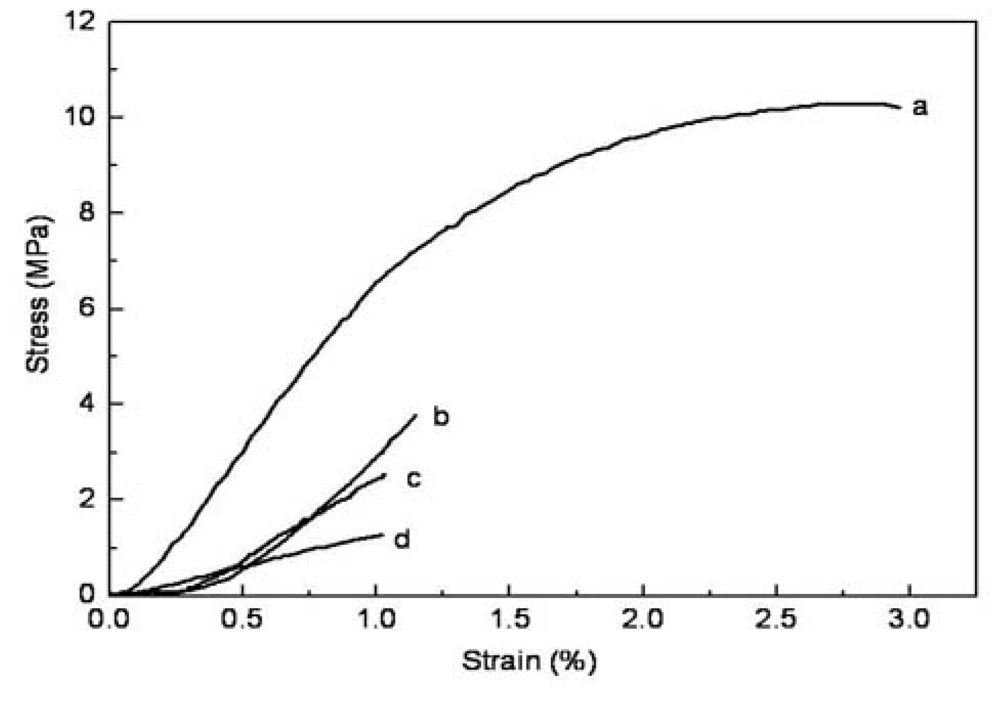
| Crosslinked CS/SF (wt/wt) | 0:10 | 2:8 | 5:5 | 8:2 |
|---|---|---|---|---|
| Tensile stress (MPa) | 10.3 ± 0.24 | 1.2 ± 0.13 | 1.1 ± 0.22 | 1.0 ± 0.21 |
| Ultimate strain (%) | 2.8 ± 0.22 | 3.8 ± 0.21 | 2.5 ± 0.25 | 1.3 ± 0.20 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cai, Z.-x.; Mo, X.-m.; Zhang, K.-h.; Fan, L.-p.; Yin, A.-l.; He, C.-l.; Wang, H.-s. Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-dressing Applications. Int. J. Mol. Sci. 2010, 11, 3529-3539. https://doi.org/10.3390/ijms11093529
Cai Z-x, Mo X-m, Zhang K-h, Fan L-p, Yin A-l, He C-l, Wang H-s. Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-dressing Applications. International Journal of Molecular Sciences. 2010; 11(9):3529-3539. https://doi.org/10.3390/ijms11093529
Chicago/Turabian StyleCai, Zeng-xiao, Xiu-mei Mo, Kui-hua Zhang, Lin-peng Fan, An-lin Yin, Chuang-long He, and Hong-sheng Wang. 2010. "Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-dressing Applications" International Journal of Molecular Sciences 11, no. 9: 3529-3539. https://doi.org/10.3390/ijms11093529
APA StyleCai, Z.-x., Mo, X.-m., Zhang, K.-h., Fan, L.-p., Yin, A.-l., He, C.-l., & Wang, H.-s. (2010). Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-dressing Applications. International Journal of Molecular Sciences, 11(9), 3529-3539. https://doi.org/10.3390/ijms11093529






