Reactivity of Heteropolytungstate and Heteropolymolybdate Metal Transition Salts in the Synthesis of Dimethyl Carbonate from Methanol and CO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.2. Catalytic Activity of the Series of Heteropoly Compounds
2.3. Catalytic Activity of Co1.5PW12O40
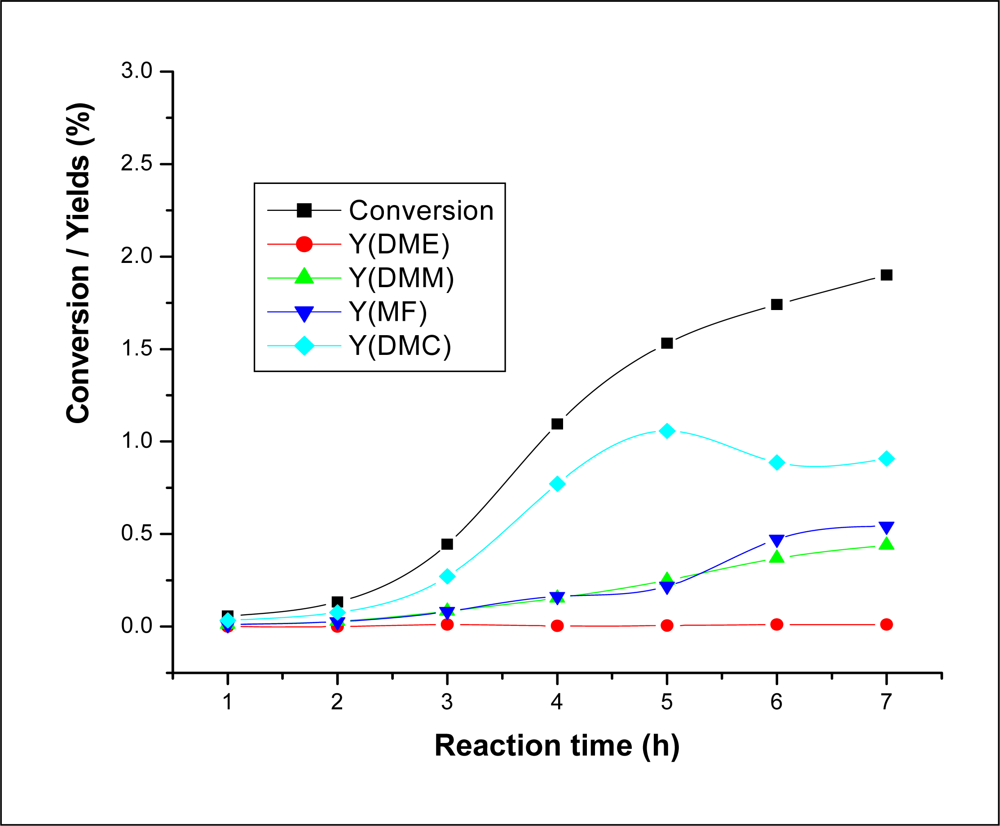
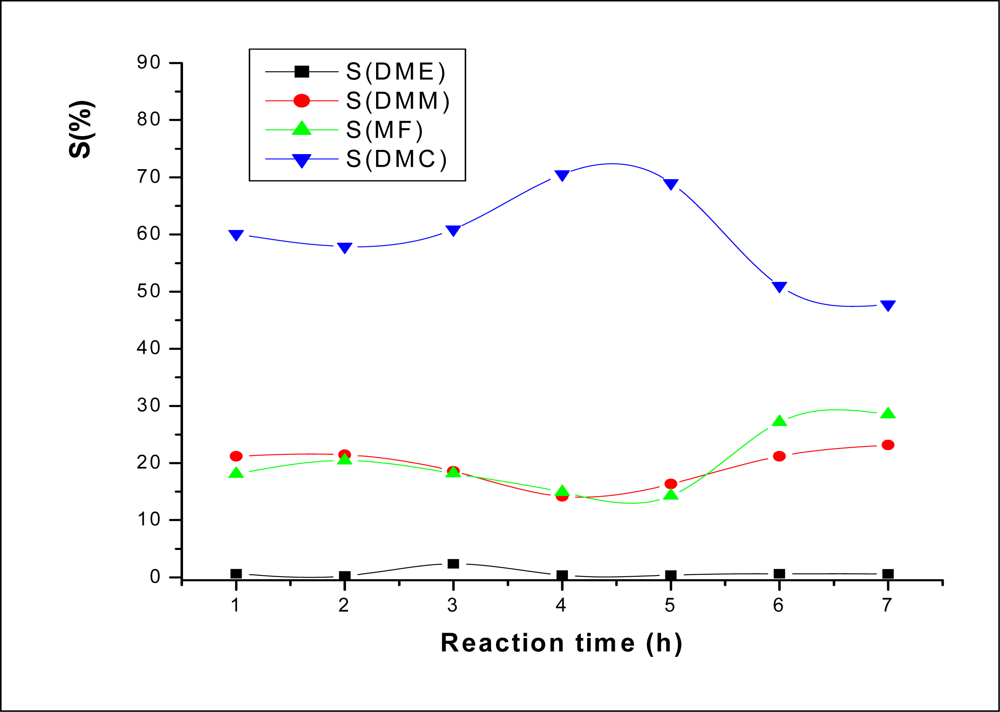
2.3.1. Effect of Reaction Time
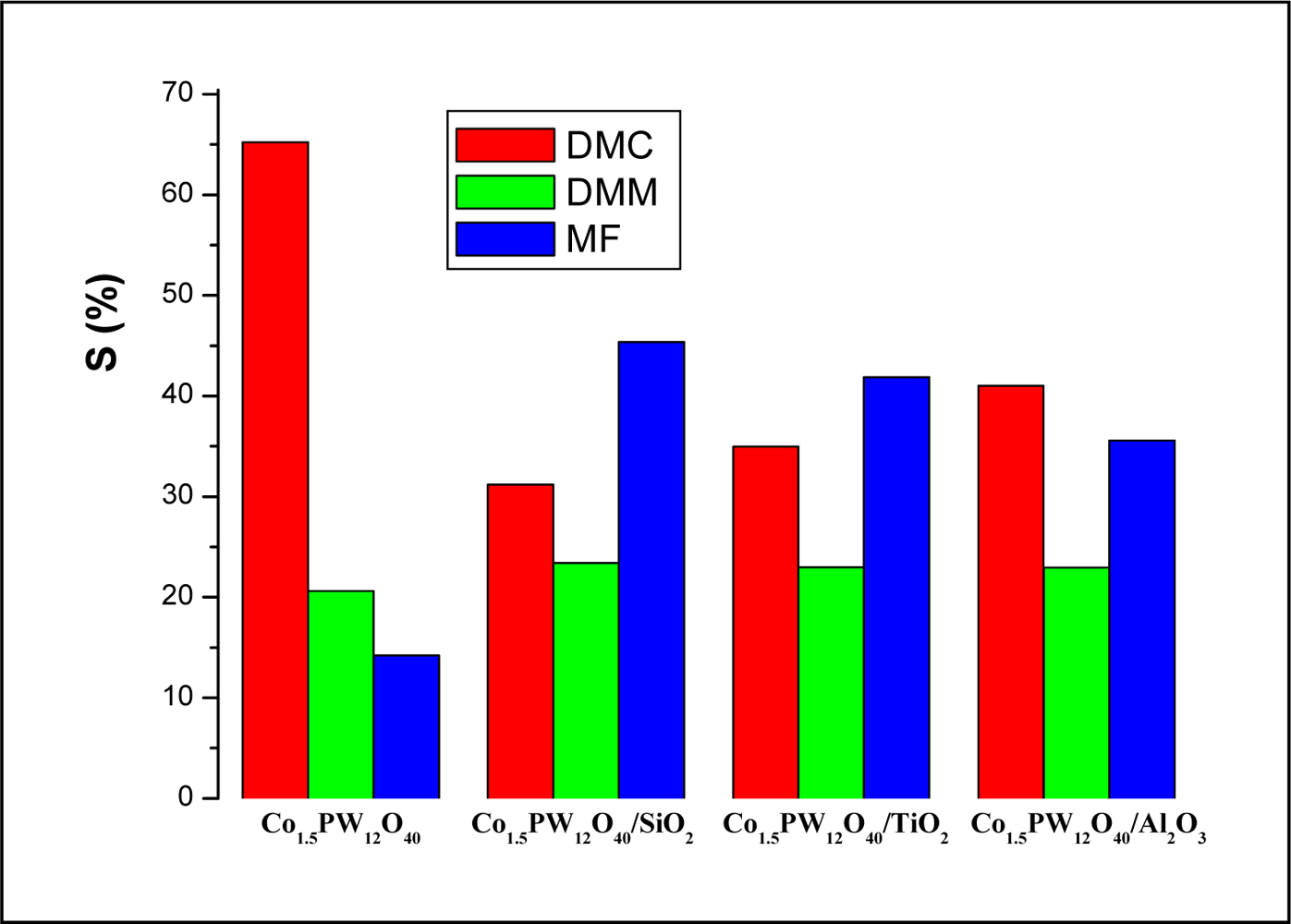
2.3.2. Effect of the Support
3. Experimental Section
3.1. Catalyst Preparation
3.2. Physicochemical Techniques
3.3. Reaction Procedure
4. Conclusion
References
- Centi, G; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar]
- Yin, X; Moss, JR. Recent developments in the activation of carbon dioxide by metal complexes. Coordinat. Chem. Rev 1999, 181, 27–59. [Google Scholar]
- Zhag, K; Kogelschatz, U; Eliasson, B. Conversion of greenhouse gases to synthesis gas and higher hydrocarbons. Energy Fuel 2001, 15, 395–401. [Google Scholar]
- Inui, T; Yamamoto, T. Effective synthesis of ethanol from CO2 on polyfunctional composite catalysts. Catal. Today 1998, 45, 209–221. [Google Scholar]
- Zevenhoven, R; Eloneva, S; Teir, S. Chemical fixation of CO2 in carbonates: Routes to valuable products and long-term storage. Catal. Today 2006, 115, 73–79. [Google Scholar]
- Aouissi, A; Al-Othman, ZA; Al-Amro, A. Gas-phase synthesis of dimethyl carbonate from methanol and carbon dioxide over Co1.5PW12O40 keggin-type heteropolyanion. Int. J. Mol. Sci 2010, 11, 1343–1351. [Google Scholar]
- Aouissi, A; Allaoui, LA; Aldhayan, D. Catalytic reactivity of 12-molybdophosphoric acid and its Copper and Zinc salts in CO2 methanol reforming. Asian J. Chem 2006, 18, 3009–3016. [Google Scholar]
- Aouissi, A; Allaoui, LA. Effect of the Brønsted acidity on the behavior of CO2 methanol reaction. J. Mol. Catal. A: Chem 2006, 259, 281. [Google Scholar]
- Guo, XC; Qin, ZF; Wang, GF. Wang, J.G. Critical temperatures and pressures of reacting mixture in synthesis of dimethyl carbonate with methanol and carbon dioxide. Chin. Chem. Lett 2008, 19, 249–252. [Google Scholar]
- Jiang, C; Guo, Y; Wang, C; Hua, C; Wuc, Y; Wang, E. Synthesis of dimethyl carbonate from methanol and carbon dioxide in the presence of polyoxometalates under mild conditions. Appl. Catal. A: Gen 2003, 256, 203–212. [Google Scholar]
- Bian, J; Xiao, M; Wang, S; Lu, Y; Meng, Y. Direct synthesis of DMC from CH3OH and CO2 over V-doped Cu–Ni/AC catalysts. Catal. Commun 2009, 10, 1142–1145. [Google Scholar]
- Bian, J; Xiao, M; Wang, S; Lu, Y; Meng, Y. Novel application of thermally expanded graphite as the support of catalysts for direct synthesis of DMC from CH3OH and CO2. J. Colloid Interf. Sci 2009, 334, 50–57. [Google Scholar]
- Wu, XL; Xiao, M; Meng, YZ; Lu, YX. Direct synthesis of dimethyl carbonate (DMC) using Cu-Ni/VSO as catalyst. J. Mol. Catal. A: Chem 2006, 249, 93–97. [Google Scholar]
- Jiang, C; Guo, Y; Wang, C; Hu, C; Wu, Y; Wang, E. Synthesis of dimethyl carbonate from methanol and carbon dioxide in the presence of polyoxometalates under mild conditions. Appl. Catal. A: Gen 2003, 256, 203–212. [Google Scholar]
- Kizlink, J; Pastucha, I. Preparation of dimethyl carbonate from methanol and carbon dioxide in the presence of Sn(IV) and Ti(IV) alkoxides and metal acetates. Collect. Czech. Chem. Commun 1995, 60, 687. [Google Scholar]
- Jung, KT; Bell, AT. Effects of catalyst phase structure on the elementary processes involved in the synthesis of dimethyl carbonate from methanol and carbon dioxide over zirconia. Topics Catal 2002, 20, 97–105. [Google Scholar]
- Pacheco, MA; Marshall, CL. Review on Dimethyl carbonate manufacture and its characteristics as a fuel additive. Energ. Fuel 1997, 11, 2–29. [Google Scholar]
- Ono, Y. Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block. Appl. Catal. A: Gen 1997, 155, 133–166. [Google Scholar]
- Niemelä, M; Nokkosmäki, M. Activation of carbon dioxide on Fe-catalysts. Catal. Today 2005, 100, 269–274. [Google Scholar]
- Kim, BJ; Cho, KS; Park, SJ. Copper oxide-decorated porous carbons for carbon dioxide adsorption behaviors. J. Colloid Interf. Sci 2010, 342, 575–578. [Google Scholar]
- Walther, D; Ruben, M; Rau, S. Carbon dioxide and metal centres: from reactions inspired by nature to reactions in compressed carbon dioxide as solvent. Coord. Chem. Rev 1999, 182, 67–100. [Google Scholar]
- Yin, X; Moss, JR. Recent developments in the activation of carbon dioxide by metal complexes. Coord. Chem. Rev 1999, 181, 27–59. [Google Scholar]
- Wang, D; Zhang, X; Gao, Y; Xiao, F; Wei, W; Sun, Y. Zn/Fe mixed oxide: Heterogeneous catalyst for the synthesis of dimethyl carbonate from methyl carbamate and methanol. Catal. Commun 2010, 11, 430–433. [Google Scholar]
- Montilla, F; Clara, E; Avile’s, T; Casimiro, T; Ricardo, AA; Nunes da Ponte, M. Transition-metal-mediated activation of arylisocyanates in supercritical carbon dioxide. J. Organomet. Chem 2001, 626, 227–232. [Google Scholar]
- Rocchiccioli-Deltcheff, C; Fournier, M. Catalysis by polyoxometalates. Part 3,–influence of Vanadium (V) on the thermal stability of 12-metallophosphoric acids from in situ infrared studies. J. Chem. Soc. Faraday Trans 1991, 87, 3913–3920. [Google Scholar]
- Fournier, M; Feumi-Jantou, C; Rabia, C; Herve, G; Launay, S. Polyoxometalates catalyst materials: X-Ray thermal stability study of phosphorus-containing heteropolyacids H3+xPM12−xVxO40.13–14H2O (M=Mo, W; x=0–1). J. Mater. Chem 1992, 2, 971–978. [Google Scholar]
- Gao, R; Chen, H; Le, Y; Dai, W-L; Fan, K. Highly active and selective Cs2.5H0.5PW12O40/SBA- 15 composite material in the oxidation of cyclopentane-1,2-diol to glutaric acid by aqueous H2O2. Appl. Catal. A: Gen 2009, 352, 61–65. [Google Scholar]
- Fuchs, VM; Pizzio, LR; Blanco, MN. Synthesis and characterization of aluminum or copper tungstophosphate and tungstosilicate immobilized in a polymeric blend. Eur. Polym. J 2008, 44, 801–807. [Google Scholar]
- Louis, C; Tatibouët, JM; Che, M. Catalytic properties of silica-supported molybdenum catalysts in methanol oxidation: The influence of molybdenum dispersion. J. Catal 1988, 109, 354–366. [Google Scholar]
- Damyanova, S; Cubeiro, ML; Fierro, JLG. Acid-redox properties of titania-supported 12-molybdophosphates for methanol oxidation. J. Mol. Catal. A: Chem 1999, 142, 85–100. [Google Scholar]
- Venezia, AM; Parola, VL; Pawelec, B; Fierro, JLG. Hydrogenation of aromatics over Au–Pd/SiO2–Al2O3 catalysts; support acidity effect. Appl. Catal. A: Gen 2004, 264, 43–51. [Google Scholar]
- Borque, MP; Lopez-Agudo, A; Olguin, E. Catalytic activities of Co(Ni) Mo/TiO2–Al2O3 catalysts in gasoil and thiophene HDS and pyridine HDN: Effect of the TiO2–Al2O3 composition. Appl. Catal. A: Gen 1999, 180, 53–61. [Google Scholar]
- Rocchiccioli-Deltcheff, C; Fournier, M; Franck, R; Thouvenot, R. Vibrational investigations of polyoxometalates. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem 1983, 22, 207–216. [Google Scholar]
- Rocchiccioli-Deltcheff, C; Aouissi, A; Launay, S; Fournier, M. Silica-supported 12-molybdophosphoric acid catalysts: Influence of the thermal treatments and of the Mo contents on their behavior, from IR, Raman, X-ray diffraction studies, and catalytic reactivity in the methanol oxidation. J. Mol. Catal. A: Chem 1996, 114, 331–342. [Google Scholar]
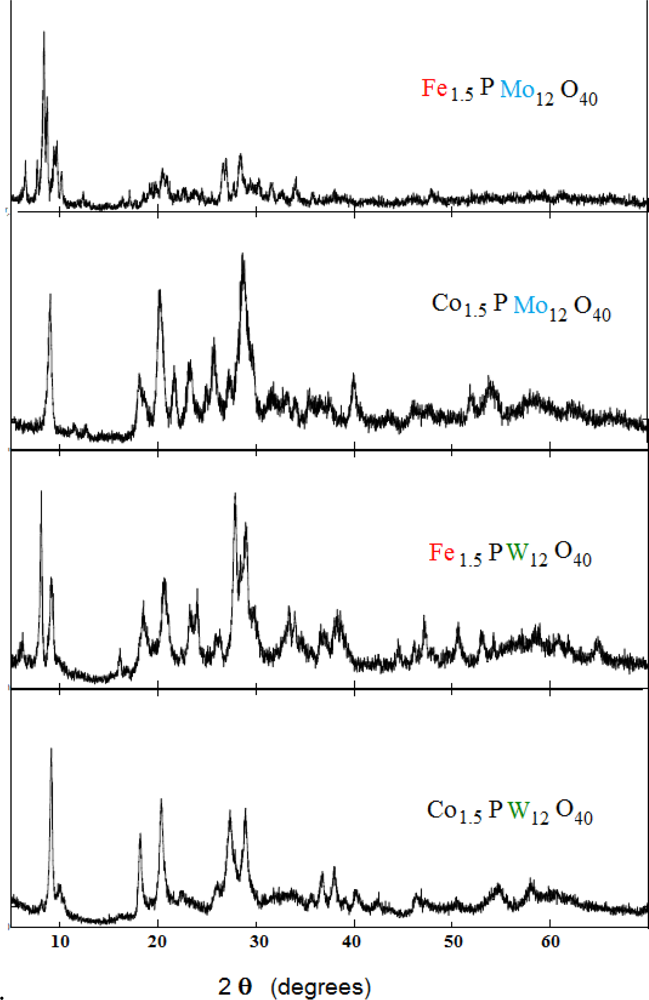
| Catalyst | Frequency (cm−1) | |||
|---|---|---|---|---|
| νas (P-Oa) | νas (M-Od) | νas (M-Ob-M) | νas(M-Oc-M) | |
| Fe1.5PMo12O40 | 1064.71 | 960.55 | 867.97 | 783.10 |
| Co1.5PMo12O40 | 1062.78 | 960.55 | 871.82 | 785.03 |
| Fe1.5PW12O40 | 1080.14 | 981.77 | 894.97 | 806.25 |
| Co1.5PW12O40 | 1080.14 | 979.84 | 894.97 | 790.00 |
| Catalyst Xc (%) | S (%) | ||||
|---|---|---|---|---|---|
| DME | DMM | MF | DMC | ||
| Fe1.5P Mo12O40 | 0.18 | 2.00 | 27.99 | 45.84 | 24.18 |
| Co1.5PMo12O40 | 0.51 | 0.70 | 16.11 | 29.07 | 54.12 |
| Fe1.5PW12O40 | 0.51 | 0.10 | 24.43 | 13.60 | 61.87 |
| Co1.5PW12O40 | 1.53 | 0.38 | 16.35 | 14.27 | 69.00 |
| Catalyst (mol%/1g-HPC) | Conv (%) | Conv (%) | Yield (%) | ||
|---|---|---|---|---|---|
| DMM | MF | DMC | |||
| Co1.5PW12O40 | 3.73 | 3.73 | 0.77 | 0.53 | 2.43 |
| Co1.5PW12O40/SiO2 | 1.12 | 37.33 | 0.26 | 0.51 | 0.35 |
| Co1.5PW12O40/TiO2 | 0.89 | 29.67 | 0.21 | 0.37 | 0.31 |
| Co1.5PW12O40/Al2O3 | 1.18 | 39.33 | 0.27 | 0.42 | 0.48 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aouissi, A.; Al-Deyab, S.S.; Al-Owais, A.; Al-Amro, A. Reactivity of Heteropolytungstate and Heteropolymolybdate Metal Transition Salts in the Synthesis of Dimethyl Carbonate from Methanol and CO2. Int. J. Mol. Sci. 2010, 11, 2770-2779. https://doi.org/10.3390/ijms11072770
Aouissi A, Al-Deyab SS, Al-Owais A, Al-Amro A. Reactivity of Heteropolytungstate and Heteropolymolybdate Metal Transition Salts in the Synthesis of Dimethyl Carbonate from Methanol and CO2. International Journal of Molecular Sciences. 2010; 11(7):2770-2779. https://doi.org/10.3390/ijms11072770
Chicago/Turabian StyleAouissi, Ahmed, Salem S. Al-Deyab, Ahmad Al-Owais, and Amro Al-Amro. 2010. "Reactivity of Heteropolytungstate and Heteropolymolybdate Metal Transition Salts in the Synthesis of Dimethyl Carbonate from Methanol and CO2" International Journal of Molecular Sciences 11, no. 7: 2770-2779. https://doi.org/10.3390/ijms11072770




