Serum Concentrations of TNF α and Its Soluble Receptors in Patients with Adrenal Tumors Treated by Surgery
Abstract
:1. Introduction
2. Materials and Methods
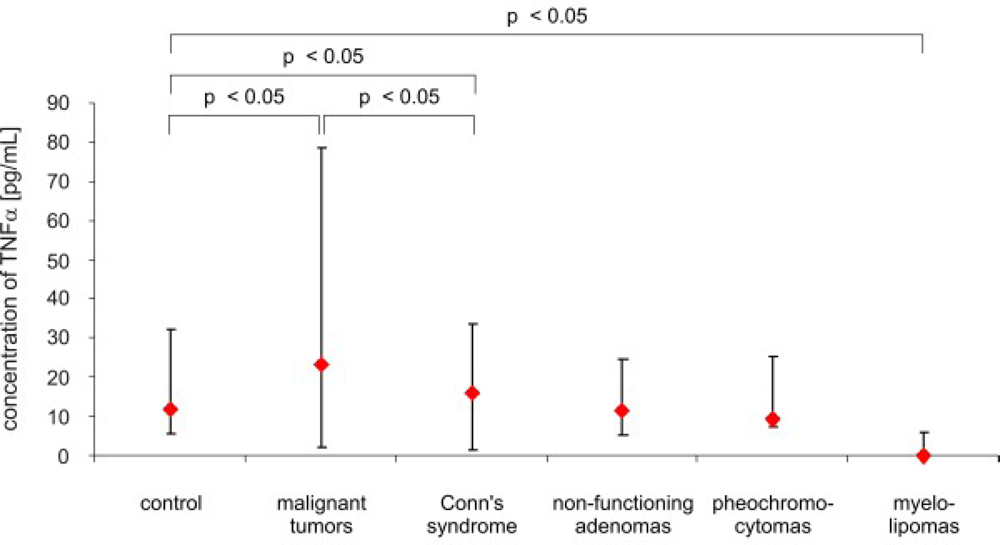
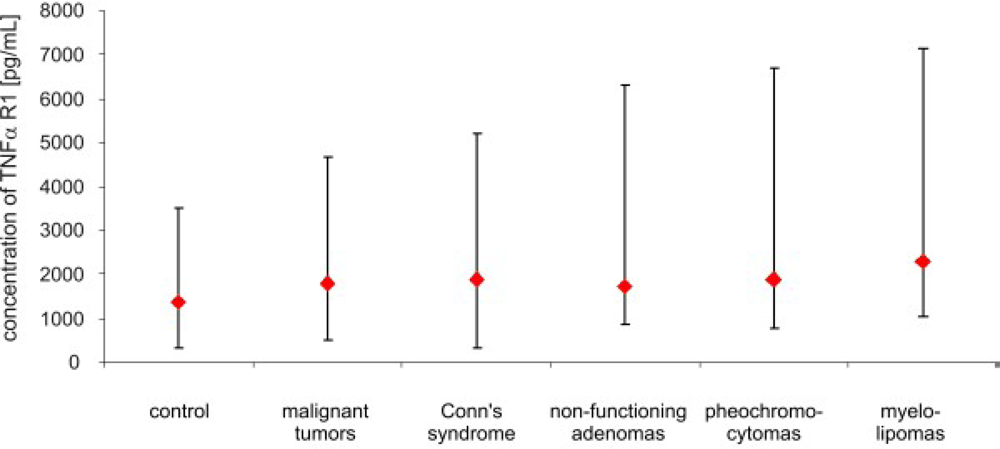
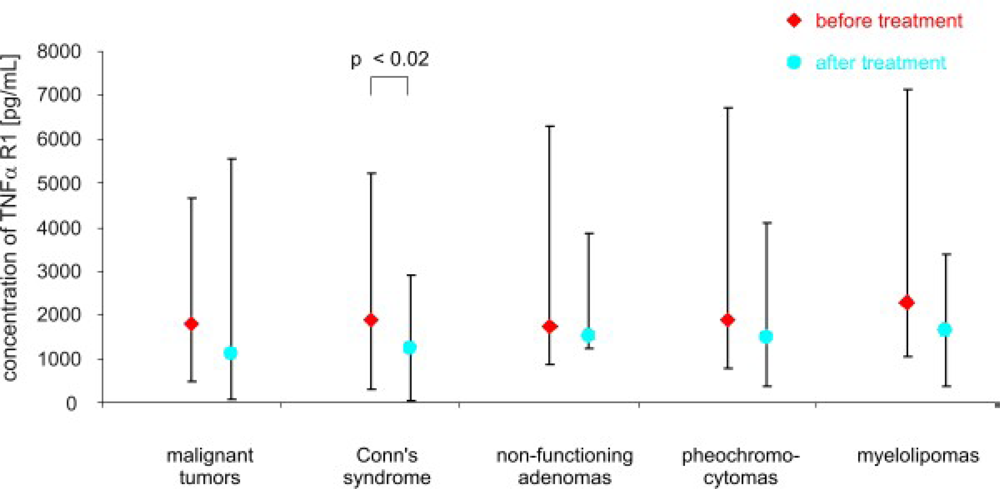
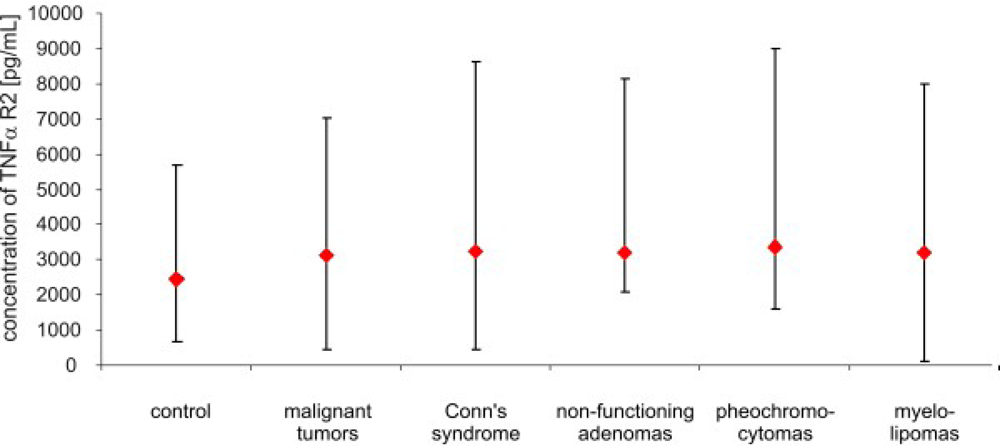
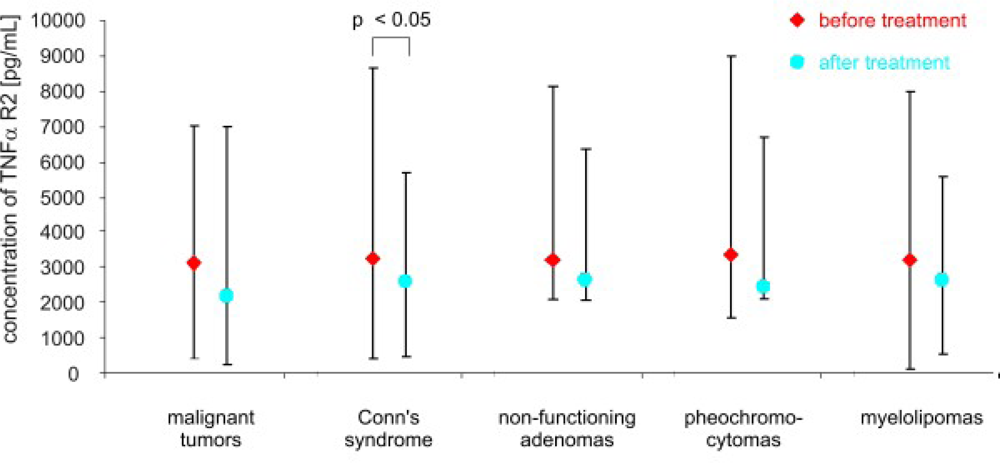
3. Results
4. Discussion
5. Conclusions
Acknowledgments
References
- Jurczyńska, J; Zieleniewski, W; Stępień, H; Komorowski, J. Angiogenic and anti-angiogenic factors in adrenal tumours. Endokrynol. Pol 2006, 57, 633–640. [Google Scholar]
- Jurczyńska, J; Stępień, T; Ławnicka, H; Stępień, H; Krupiński, R; Kołomecki, K; Kuzdak, K; Komorowski, J. Peripheral blood concentrations of vascular endothelial growth factor and its soluble receptor (R1 and R2) in patients with adrenal cortex tumors treated by surgery. Endokrynol. Pol 2009, 60, 9–13. [Google Scholar]
- Hiemar, U; Heim, ME. Tumor necrosis factor for the treatment of malignancies. Oncology 1994, 51, 142–153. [Google Scholar]
- Dembic, Z; Loetscher, H; Gubler, U; Pan, Y; C Lahm, HW; Gentz, R; Brockhaus, M; Lesslauer, W. Two human TNF receptors have similar extracellular, but distinct intracellular, domain sequences. Cytokine 1990, 2, 231–237. [Google Scholar]
- Smith, CA; Davis, T; Anderson, D; Solam, L; Beckmann, MP; Jerzy, R; Dower, SK; Cosman, D; Goodwin, RG. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 1990, 248, 1019–1023. [Google Scholar]
- Aderka, D; Engelmann, H; Maor, Y; Brakebusch, C; Wallach, D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J. Exp. Med 1992, 175, 323–329. [Google Scholar]
- Chouaib, S; Branellec, D; Buurman, WA. More insights into the complex physiology of TNF. Immunol. Today 1991, 12, 141–142. [Google Scholar]
- Corti, A; Marcucci, F. Tumour necrosis factor: Strategies for improving the therapeutic index. J. Drug. Target 1998, 5, 403–413. [Google Scholar]
- Kwon, B; Youn, BS; Kwon, BS. Functions of newly identified members of the tumor necrosis factor receptor/ligand superfamilies in lymphocytes. Curr. Opin. Immunol 1999, 11, 340–345. [Google Scholar]
- Sedgwick, JD; Riminton, DS; Cyster, JG; Körner, H. Tumor necrosis factor: A masterregulator of leukocyte movement. Immunol. Today 2000, 21, 110–113. [Google Scholar]
- MacEwan, DJ. A TNF ligands and receptors-a matter of life and death. Br. J. Pharmacol 2002, 135, 855–875. [Google Scholar]
- MacEwan, DJ. B TNF receptor subtype signaling: Differences and cellular consequences. Cell Signal 2002, 14, 477–492. [Google Scholar]
- Wellmer, A; Gerber, J; Ragheb, J; Zysk, G; Kunst, T; Smirnov, A; Brück, W; Nau, R. Effect of deficiency of tumor necrosis factor α or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect. Immun 2001, 69, 6881–6886. [Google Scholar]
- Pasparakis, M; Alexopoulou, L; Episkopou, V; Kollias, GP. A Immune and inflammatory responses in TNF α -deficient mice: A critical requirement for TNF α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med 1996, 184, 1397–411. [Google Scholar]
- Mocellin, S; Nitti, D. TNF and cancer: The two sides of the coin. Front. Biosci 2008, 13, 2774–2783. [Google Scholar]
- Leibovich, SJ; Polverini, PJ; Shepard, HM; Wiseman, DM; Shively, V; Nuseir, N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nature 1987, 329, 630–632. [Google Scholar]
- Schweigerer, L; Malerstein, B; Gospodarowicz, D. Tumor necrosis factor inhibits the proliferation of cultured capillar endothelial cells. Biochem. Biophys. Res. Commun 1987, 143, 997–1004. [Google Scholar]
- Call, GB; Husein, OF; McIlmoil, Ch J; Adams, A; Heckman, RA; Judd, AM. Bovine adrenal cells secrete interleukin-6 and tumor necrosis factor in vitro. Gen. Comp. Endocrinol 2000, 118, 249–261. [Google Scholar]
- González-Hernández, JA; Bornstein, SR; Ehrhart-Bornstein, M; Späth-Schwalbe, E; Jirikowski, G; Scherbaum, WA. Interleukin-6 messenger ribonucleic acid expression in human adrenal gland in vivo: New clue to a paracrine or autocrine regulation of adrenal function. J. Clin. Endocrinol. Metab 1994, 79, 1492–1497. [Google Scholar]
- González-Hernández, JA; Ehrhart-Bornstein, M; Späth-Schwalbe, E; Scherbaum, WA; Bornstein, SR. Human adrenal cells express tumor necrosis factor- α messenger ribonucleic acid: Evidence for paracrine control of adrenal function. J. Clin. Endocrinol. Metab 1996, 81, 807–813. [Google Scholar]
- Päth, G; Bornstein, SR; Späth-Schwalbe, E; Scherbaum, WA. Direct effects of interleukin-6 on human adrenal cells. Endocr. Res 1996, 22, 867–873. [Google Scholar]
- Wilder, RL. Neuroendocrine-immune system interactions and autoimmunity. Annu. Rev. Immunol 1995, 13, 307–338. [Google Scholar]
- Cimino, G; Amadori, S; Cava, MC; De Sanctis, V; Petti, MC; Di Gregorio, AO; Sgadari, C; Vegna, L; Cimino, G; Mandelli, F. Serum interleukin-2 (IL-2), soluble IL-2 receptors and tumor necrosis factor-α levels are significantly increased in acute myeloid leukemia patients. Leukemia 1991, 5, 32–35. [Google Scholar]
- Yurkovetsky, ZR; Kirkwood, JM; Edington, HD; Marrangoni, AM; Velikokhatnaya, L; Winans, MT; Gorelik, E; Lokshin, AE. Multiplex analysis of serum cytokines in melanoma patients treated interferon- α 2b. Clin. Cancer Res 2007, 13, 2422–2428. [Google Scholar]
- Dosquet, C; Coudert, MC; Lepagne, E; Cabane, J; Richard, F. Are angiogenic factors, cytokines and soluble adhesion molecules prognostic factors in patients with renal cell carcinoma? Clin. Cancer Res 1997, 3, 2451–2458. [Google Scholar]
- Talar-Wojnarowska, R; Gąsiorowska, A; Smolarz, B; Romanowicz-Makowska, H; Kulig, A; Malecka-Panas, E. Tumor necrosis factor α and interferon γ genes polymorphisms and serum levels in pancreatic adenocarcinoma. Neoplasma 2009, 56, 56–62. [Google Scholar]
- Vila-del Sol, V; Punzón, C; Fresno, M. IFN-gamma-induced TNF-alpha expression is regulated by interferon regulatory factors 1 and 8 in mouse macrophages. J. Immunol 2008, 181, 4461–4470. [Google Scholar]
- Creyghton, WM; de Waard-Siebinga, I; Danen, EH; Luyten, GP; van Muijen, GN; Jager, MJ. Cytokine-mediated modulation of integrin, ICAM-1 and CD44 expression on human uveal melanoma cells in vitro. Melanoma Res 1995, 5, 235–242. [Google Scholar]
- Guardiola-Serrano, F; Haendeler, J; Lukosz, M; Sturm, K; Melchner, H; Altschmied, J. Gene trapping identifies a putative tumor suppressor and a new inducer of cell migration. Biochem. Biophys. Res. Commun 2008, 376, 748–752. [Google Scholar]
- Austgulen, R; Liabakk, NB; Brockhaus, M; Espevik, T. Soluble TNF receptors in amniotic fluid and in urine from pregnant women. J. Reprod. Immunol 1992, 22, 105–16. [Google Scholar]
- Austgulen, R; Liabakk, NB; Espevik, T. Secretion of soluble receptors for tumor necrosis factor. An immunologic buffer mechanism during normal pregnancy? Tidsskr. Nor. Laegeforen 1992, 112, 3545–3547. [Google Scholar]
- Kalinkovich, A; Engelmann, H; Harpaz, N; Burstein, R; Barak, V; Kalickman, I; Wallach, D; Bentwich, Z. Elevated serum levels of soluble tumour necrosis factor receptors (sTNF-R) in patients with HIV infection. Clin. Exp. Immunol 1992, 89, 351–355. [Google Scholar]
- Andus, T; Gross, V; Holstege, A; Ott, M; Weber, M; David, M; Gallati, H; Gerok, W; Schölmerich, J. High concentrations of soluble tumor necrosis factor receptors in ascites. Hepatology 1992, 16, 749–755. [Google Scholar]

© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Komorowski, J.; Jurczynska, J.; Stepien, T.; Kolomecki, K.; Kuzdak, K.; Stepien, H. Serum Concentrations of TNF α and Its Soluble Receptors in Patients with Adrenal Tumors Treated by Surgery. Int. J. Mol. Sci. 2010, 11, 2281-2290. https://doi.org/10.3390/ijms11062281
Komorowski J, Jurczynska J, Stepien T, Kolomecki K, Kuzdak K, Stepien H. Serum Concentrations of TNF α and Its Soluble Receptors in Patients with Adrenal Tumors Treated by Surgery. International Journal of Molecular Sciences. 2010; 11(6):2281-2290. https://doi.org/10.3390/ijms11062281
Chicago/Turabian StyleKomorowski, Jan, Jolanta Jurczynska, Tomasz Stepien, Krzysztof Kolomecki, Krzysztof Kuzdak, and Henryk Stepien. 2010. "Serum Concentrations of TNF α and Its Soluble Receptors in Patients with Adrenal Tumors Treated by Surgery" International Journal of Molecular Sciences 11, no. 6: 2281-2290. https://doi.org/10.3390/ijms11062281



