Carbonic Anhydrase I Is Recognized by an SOD1 Antibody upon Biotinylation of Human Spinal Cord Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Protein Biotinylation
2.3. Western Analysis
2.4. Ion Exchange Chromatography
2.5. Purification of the 32 kDa Protein
2.6. Mass Spectrometry
3. Results
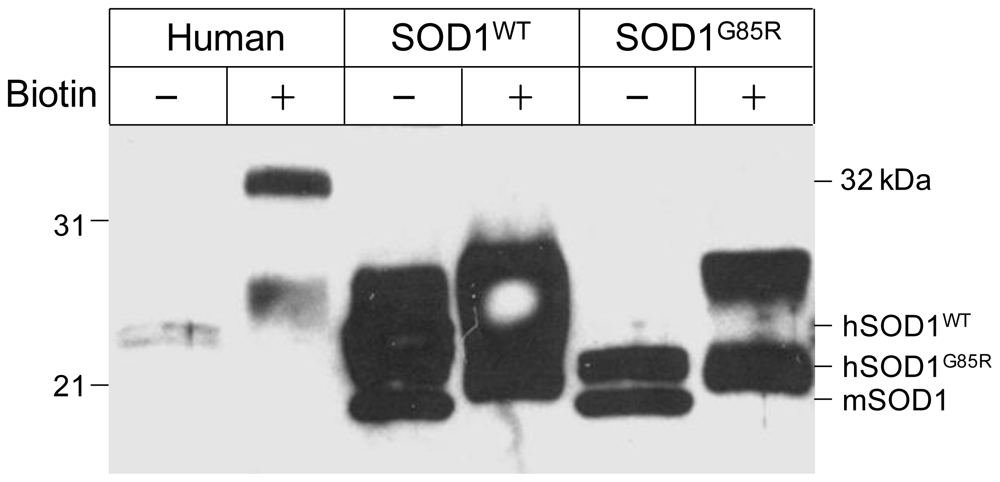
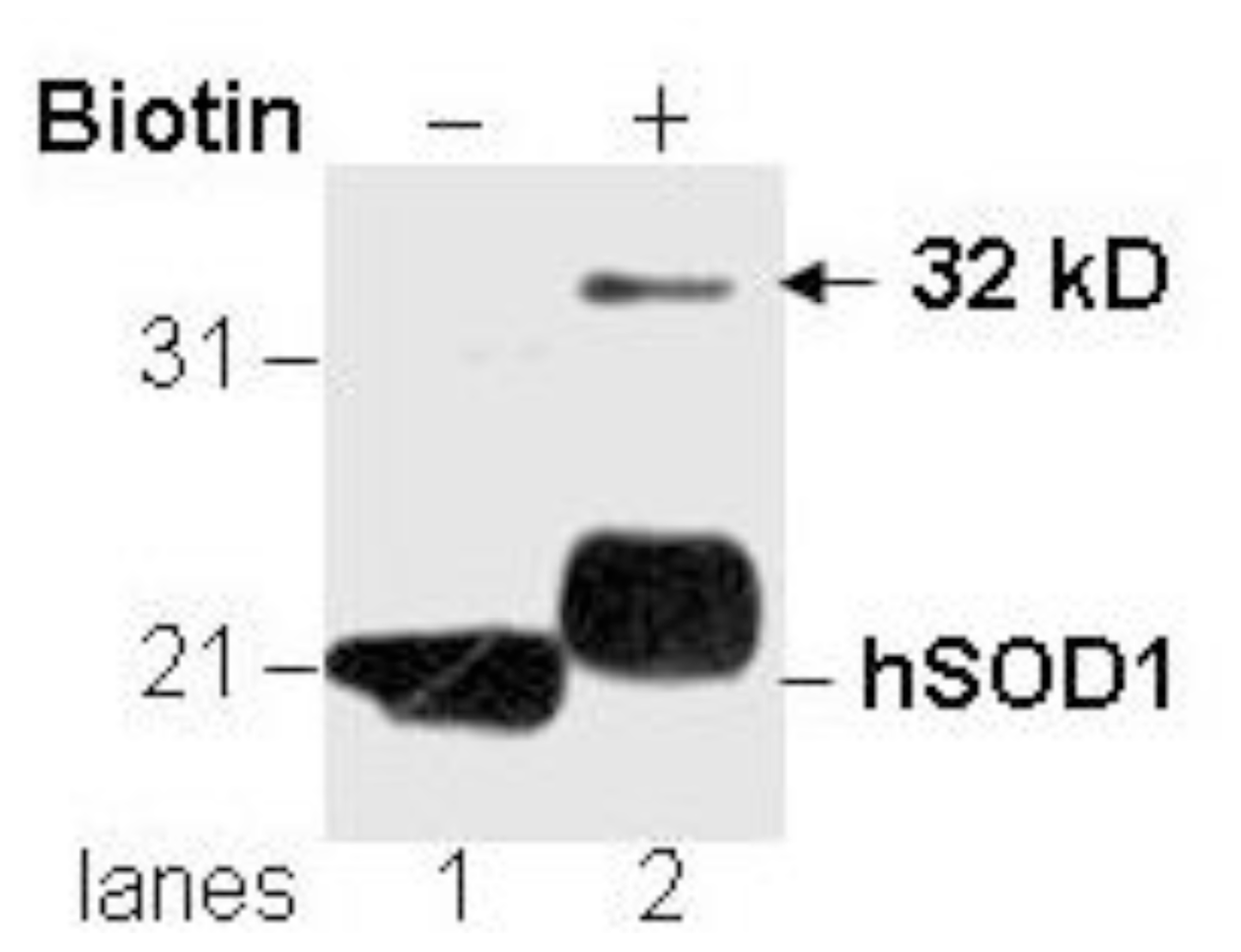
3.1. Novel Immunoreactivity Recognized by Anti-SOD1 Antibody after Biotinylation
3.2. Purification and Identification of the Protein That Gives Rise to the 32 kDa-IR
3.3. Human CA I Is Necessary and Sufficient to Give Rise to the 32 kDa-IR Species upon Biotinyation
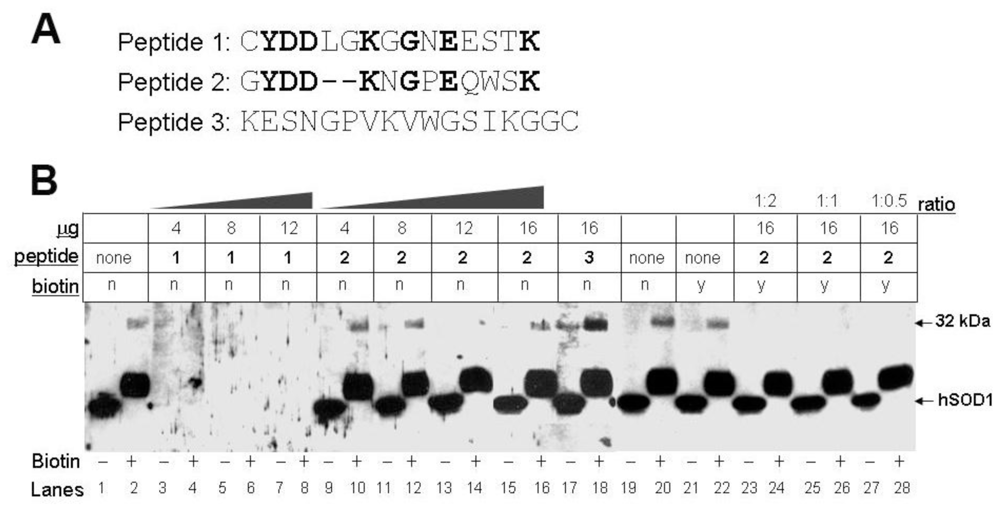
3.4. Biotinylation of an Epitope-Like Sequence in Human CA I Resulted in the Novel Immunoreactivity
4. Conclusions
Supplementary data
Acknowledgements
References
- DeSantis, G; Jones, JB. Chemical modification of enzymes for enhanced functionality. Curr. Opin. Biotechnol 1999, 10, 324–330. [Google Scholar]
- Leitner, A; Lindner, W. Applications of chemical tagging approaches in combination with 2DE and mass spectrometry. Methods Mol. Biol 2009, 519, 83–101. [Google Scholar]
- Carrico, IS. Chemoselective modification of proteins: hitting the target. Chem. Soc. Rev 2008, 37, 1423–1431. [Google Scholar]
- Eaton, P. Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic. Biol. Med 2006, 40, 1889–1899. [Google Scholar]
- Olivares-Corichi, IM; Ceballos, G; Ortega-Camarillo, C; Guzman-Grenfell, AM; Hicks, JJ. Reactive oxygen species (ROS) induce chemical and structural changes on human insulin in vitro, including alterations in its immunoreactivity. Front. Biosci 2005, 10, 838–843. [Google Scholar]
- Sugo, T; Watanabe, K; Naraki, T; Matsuda, M. Chemical modification of gamma-carboxyglutamic acid residues in prothrombin elicits a conformation similar to that of abnormal (des-gamma-carboxy)prothrombin. J. Biochem 1990, 108, 382–387. [Google Scholar]
- Beleza-Meireles, A; Al-Chalabi, A. Genetic studies of amyotrophic lateral sclerosis: Controversies and perspectives. Amyotroph. Lat. Scler 2009, 10, 1–14. [Google Scholar]
- Strong, MJ. The basic aspects of therapeutics in amyotrophic lateral sclerosis. Pharmacol. Therap 2003, 98, 379–414. [Google Scholar]
- Gruzman, A; Wood, WL; Alpert, E; Prasad, MD; Miller, RG; Rothstein, JD; Bowser, R; Hamilton, R; Wood, TD; Cleveland, DW; Lingappa, VR; Liu, J. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12524–12529. [Google Scholar]
- Pardo, CA; Xu, Z; Borchelt, DR; Price, DL; Sisodia, SS; Cleveland, DW. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc. Natl. Acad. Sci. USA 1995, 92, 954–958. [Google Scholar]
- Huang, X; Miller, W. A time-efficient, linear-space local similarity algorithm. Adv. Appl. Math 1991, 12, 337–357. [Google Scholar]
- Hernandez, S; Casanovas, A; Piedrafita, L; Tarabal, O; Esquerda, JE. Neurotoxic species of misfolded SOD1G93A recognized by antibodies against the P2X4 subunit of the ATP receptor accumulate in damaged neurons of transgenic animal models of amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol 2010, 69, 176–187. [Google Scholar]
- Riley, DA; Ellis, S; Bain, JLW. Ultrastructural cytochemical localization of carbonic anhydrase activity in rat peripheral sensory and motor nerves, dorsal root ganglia and dorsal column nuclei. Neuroscience 1984, 13, 189–206. [Google Scholar]
- Obara, M; Szeliga, M; Albrecht, J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem. Int 2008, 52, 905–919. [Google Scholar]
- Mayeux, V; Pons, F; Baldy-Moulinier, M; Valmier, J. Early postnatal muscle contractile activity regulates the carbonic anhydrase phenotype of proprioceptive neurons in young and mature mice: evidence for a critical period in development. Neuroscience 1996, 71, 787–795. [Google Scholar]
- Heath, R; Schwartz, MS; Brown, IRF; Carter, ND. Carbonic anhydrase III in neuromuscular disorders. J. Neurol. Sci 1983, 59, 383–88. [Google Scholar]



© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, J.; Akhavan, A.; Lu, M.; Gruzman, A.; Lingappa, V.R.; An, J.; Bowser, R. Carbonic Anhydrase I Is Recognized by an SOD1 Antibody upon Biotinylation of Human Spinal Cord Extracts. Int. J. Mol. Sci. 2010, 11, 4051-4062. https://doi.org/10.3390/ijms11104051
Liu J, Akhavan A, Lu M, Gruzman A, Lingappa VR, An J, Bowser R. Carbonic Anhydrase I Is Recognized by an SOD1 Antibody upon Biotinylation of Human Spinal Cord Extracts. International Journal of Molecular Sciences. 2010; 11(10):4051-4062. https://doi.org/10.3390/ijms11104051
Chicago/Turabian StyleLiu, Jian, Armin Akhavan, Mengde Lu, Arie Gruzman, Vishwanath R. Lingappa, Jiyan An, and Robert Bowser. 2010. "Carbonic Anhydrase I Is Recognized by an SOD1 Antibody upon Biotinylation of Human Spinal Cord Extracts" International Journal of Molecular Sciences 11, no. 10: 4051-4062. https://doi.org/10.3390/ijms11104051
APA StyleLiu, J., Akhavan, A., Lu, M., Gruzman, A., Lingappa, V. R., An, J., & Bowser, R. (2010). Carbonic Anhydrase I Is Recognized by an SOD1 Antibody upon Biotinylation of Human Spinal Cord Extracts. International Journal of Molecular Sciences, 11(10), 4051-4062. https://doi.org/10.3390/ijms11104051



