Genistein Inhibition of Topoisomerase IIα Expression Participated by Sp1 and Sp3 in HeLa Cell
Abstract
:1. Introduction
2. Results
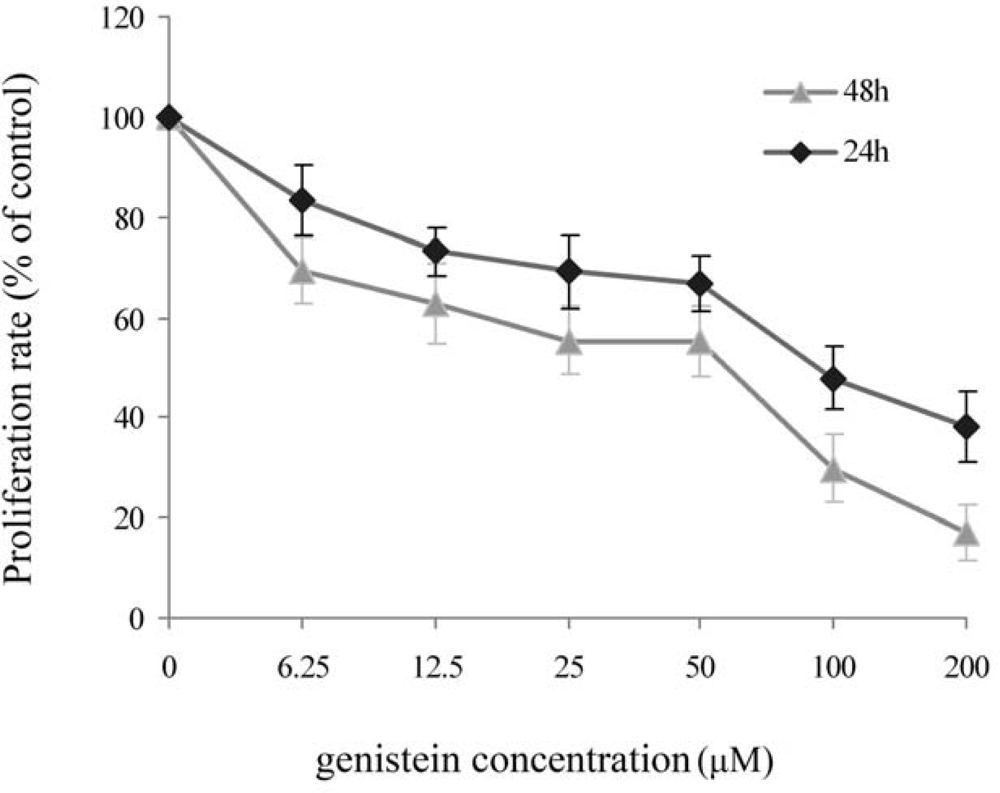
2.1. MTT Assay for Genistein Inhibition of HeLa Cell
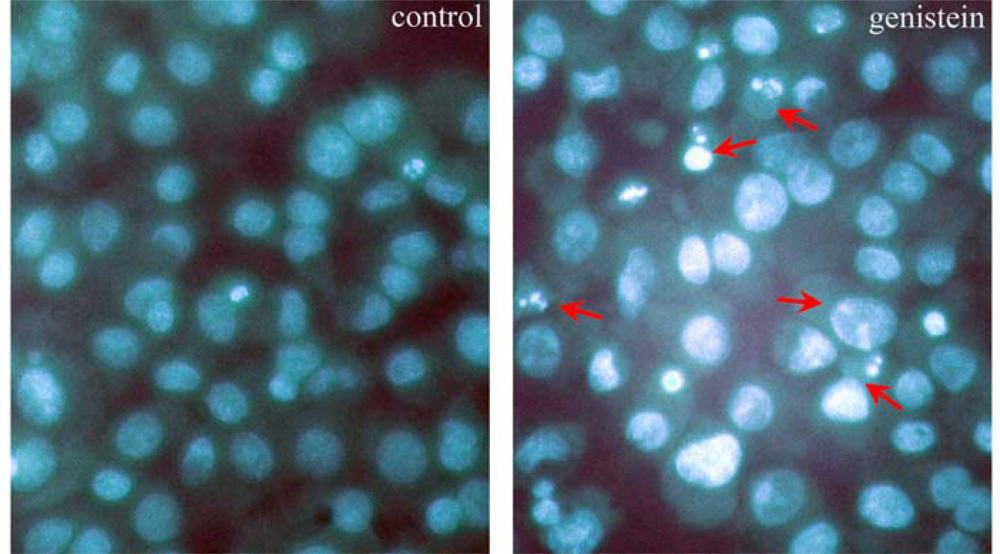
2.2. Observation of the Nuclear Morphological Changes
2.3. Cell Cycle Distribution Revealed by Flow Cytometry
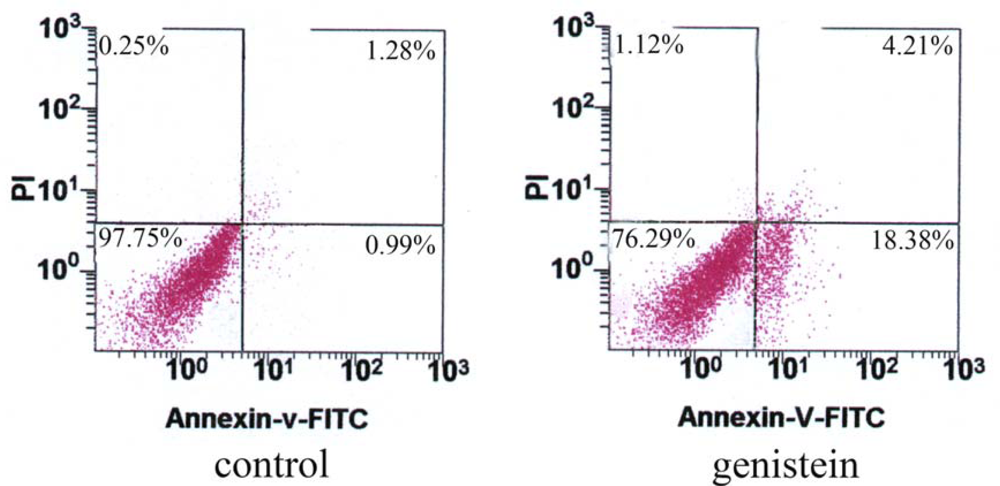
2.4. Apoptosis Revealed by Flow Cytometry Using Annexin V/PI Double-staining
2.5. Results of Topo IIα, Sp1 and Sp3 mRNA Expression with Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
2.6. Western Blot Analysis of Topo II α, Sp1 and Sp3 Protein Expression
2.7. The Occupancy of Sp1 and Sp3 Proteins at GC1 and GC2 of Topo II α Promoter Regions by ChIP
3. Discussion
4. Experimental Section
4.1. Drugs and Reagents
4.2. Cell Culture
4.3. MTT Assay for Genistein Inhibition of HeLa Cell
4.4. Observation of Nuclear Morphological Changes by Hoechst 33258 Staining
4.5. Cell Cycle and Apoptosis Analysis with Flow Cytometry
4.6. Apoptosis Analysis by Annexin V-FITC/PI with Flow Cytometry
4.7. RT-PCR for Topo IIα, Sp1 and Sp3 mRNA Expression
4.8. Western Blot Analysis Topo IIα, Sp1 and Sp3 Protein Expression
4.9. Chromatin Immunoprecipitation (ChIP) Assays Analysis Sp1 and Sp3 Protein Binding to Topo IIα Promoter
| GC1 (proximal) | Sense: 5′-ACTCAGCCGTTCATAGGT-3′, |
| Antisense | 5′-AGCCGCTTCTCCACA-3′; |
| GC2 (distal) | Sense: 5′-GGGGTCTCGCTATGTT-3′ |
| Antisense | 5′-CTGGCTGCTTGGTTG-3′ |
4.10. Statistics
5. Conclusions
References
- Lee, YW; Lee, WH. Protective effects of genistein on proinflammatory pathways in human brain microvascular endothelial cells. J. Nutr. Biochem 2008, 19, 819–825. [Google Scholar]
- Okamura, S; Sawada, Y; Satoh, T; Sakamoto, H; Saito, Y; Sumino, H; Takizawa, T; Kogure, T; Chaichantipyuth, C; Higuchi, Y; Ishikawa, T; Sakamaki, T. Pueraria mirifica phytoestrogens improve dyslipidemia in postmenopausal women probably by activating estrogen receptor subtypes. Tohoku J. Exp. Med 2008, 216, 341–351. [Google Scholar]
- Davis, TA; Mungunsukh, O; Zins, S; Day, RM; Landauer, MR. Genistein induces radioprotection by hematopoietic stem cell quiescence. Int. J. Radiat. Biol 2008, 84, 713–726. [Google Scholar]
- Mandraju, RK; Kondapi, AK. Regulation of topoisomerase II alpha and beta in HIV-1 infected and uninfected neuroblastoma and astrocytoma cells: involvement of distinct nordihydroguaretic acid sensitive inflammatory pathways. Arch. Biochem. Biophys 2007, 461, 40–49. [Google Scholar]
- Mandraju, RK; Kannapiran, P; Kondapi, AK. Distinct roles of Topoisomerase II isoforms: DNA damage accelerating alpha, double strand break repair promoting beta. Arch. Biochem. Biophys 2008, 470, 27–34. [Google Scholar]
- Katz, J; Blake, E; Medrano, TA; Sun, Y; Shiverick, KT. Isoflavones and gamma irradiation inhibit cell growth in human salivary gland cells. Cancer Lett 2008, 270, 87–94. [Google Scholar]
- Schmidt, F; Knobbe, CB; Frank, B; Wolburg, H; Weller, M. The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines. Oncol. Rep 2008, 19, 1061–1066. [Google Scholar]
- Chodon, D; Ramamurty, N; Sakthisekaran, D. Preliminary studies on induction of apoptosis by genistein on HepG2 cell line. Toxicol. in Vitro 2007, 21, 887–891. [Google Scholar]
- Privat, M; Aubel, C; Arnould, S; Communal, Y; Ferrara, M; Bignon, YJ. Breast cancer cell response to genistein is conditioned by BRCA1 mutations. Biochem. Biophys. Res. Commun 2009, 379, 785–789. [Google Scholar]
- Bandele, OJ; Osheroff, N. Bioflavonoids as poisons of human topoisomerase II alpha and II beta. Biochemistry 2007, 46, 6097–6108. [Google Scholar]
- Michael McClain, R; Wolz, E; Davidovich, A; Bausch, J. Genetic toxicity studies with genistein. Food Chem. Toxicol 2006, 44, 42–55. [Google Scholar]
- Kaufmann, SH. Cell death induced by topoisomerase-targeted drugs: more questions than answers. Biochim. Biophys. Acta 1998, 1400, 195–211. [Google Scholar]
- Markovits, J; Linassier, C; Fosse, P; Couprie, J; Pierre, J; Jacquemin-Sablon, A; Saucier, JM; Le Pecq, JB; Larsen, AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res 1989, 49, 5111–5117. [Google Scholar]
- Notarnicola, M; Messa, C; Orlando, A; D'Attoma, B; Tutino, V; Rivizzigno, R; Caruso, MG. Effect of genistein on cholesterol metabolism-related genes in a colon cancer cell line. Genes Nutr 2008, 3, 35–40. [Google Scholar]
- Akiyama, T; Ishida, J; Nakagawa, S; Ogawara, H; Watanabe, S; Itoh, N; Shibuya, M; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem 1987, 262, 5592–5595. [Google Scholar]
- Zou, H; Zhan, S; Cao, K. Apoptotic activity of genistein on human lung adenocarcinoma SPC-A-1 cells and preliminary exploration of its mechanisms using microarray. Biomed. Pharmacother 2008, 62, 583–589. [Google Scholar]
- Yamaguchi, M; Weitzmann, MN. The estrogen 17beta-estradiol and phytoestrogen genistein mediate differential effects on osteoblastic NF-kappaB activity. Int. J. Mol. Med 2009, 23, 297–301. [Google Scholar]
- Ismail, IA; Kang, KS; Lee, HA; Kim, JW; Sohn, YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation. Eur. J. Pharmacol 2007, 575, 12–20. [Google Scholar]
- Caruso, MG; Messa, C; Orlando, A; D'Attoma, B; Notarnicola, M. Early induction of LDL receptor gene expression by genistein in DLD-1 colon cancer cell line. Fitoterapia 2008, 79, 524–528. [Google Scholar]
- Banerjee, S; Li, Y; Wang, Z; Sarkar, FH. Multi-targeted therapy of cancer by genistein. Cancer Lett 2008, 269, 226–242. [Google Scholar]
- Rucinska, A; Roszczyk, M; Gabryelak, T. Cytotoxicity of the isoflavone genistein in NIH 3T3 cells. Cell Biol. Int 2008, 32, 1019–1023. [Google Scholar]
- Hochhauser, D; Stanway, CA; Harris, AL; Hickson, ID. Cloning and characterization of the 5′-flanking region of the human topoisomerase II alpha gene. J. Biol. Chem 1992, 267, 18961–18965. [Google Scholar]
- Suske, G. The Sp-family of transcription factors. Gene 1999, 238, 291–300. [Google Scholar]
- Magan, N; Szremska, AP; Isaacs, RJ; Stowell, KM. Modulation of DNA topoisomerase II alpha promoter activity by members of the Sp (specificity protein) and NF-Y (nuclear factor Y) families of transcription factors. Biochem. J 2003, 374, 723–729. [Google Scholar]
- Lok, CN; Lang, AJ; Mirski, SE; Cole, SP. Characterization of the human topoisomerase IIbeta (TOP2B) promoter activity: essential roles of the nuclear factor-Y (NF-Y)- and specificity protein-1 (Sp1)-binding sites. Biochem. J 2002, 368, 741–751. [Google Scholar]
- Xu, R; Zhang, P; Huang, J; Ge, S; Lu, J; Qian, G. Sp1 and Sp3 regulate basal transcription of the survivin gene. Biochem. Biophys. Res. Commun 2007, 356, 286–292. [Google Scholar]
- Williams, AO; Isaacs, RJ; Stowell, KM. Down-regulation of human topoisomerase IIalpha expression correlates with relative amounts of specificity factors Sp1 and Sp3 bound at proximal and distal promoter regions. BMC Mol. Biol 2007, 8, 36. [Google Scholar]
- Hua, P; Tsai, WJ; Kuo, SM. Estrogen response element-independent regulation of gene expression by genistein in intestinal cells. Biochim. Biophys. Acta 2003, 1627, 63–70. [Google Scholar]
- Maeno, T; Tanaka, T; Sando, Y; Suga, T; Maeno, Y; Nakagawa, J; Hosono, T; Sato, M; Akiyama, H; Kishi, S; Nagai, R; Kurabayashi, M. Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioloalveolar carcinoma cells. Am. J. Respir. Cell Mol. Biol 2002, 26, 246–253. [Google Scholar]
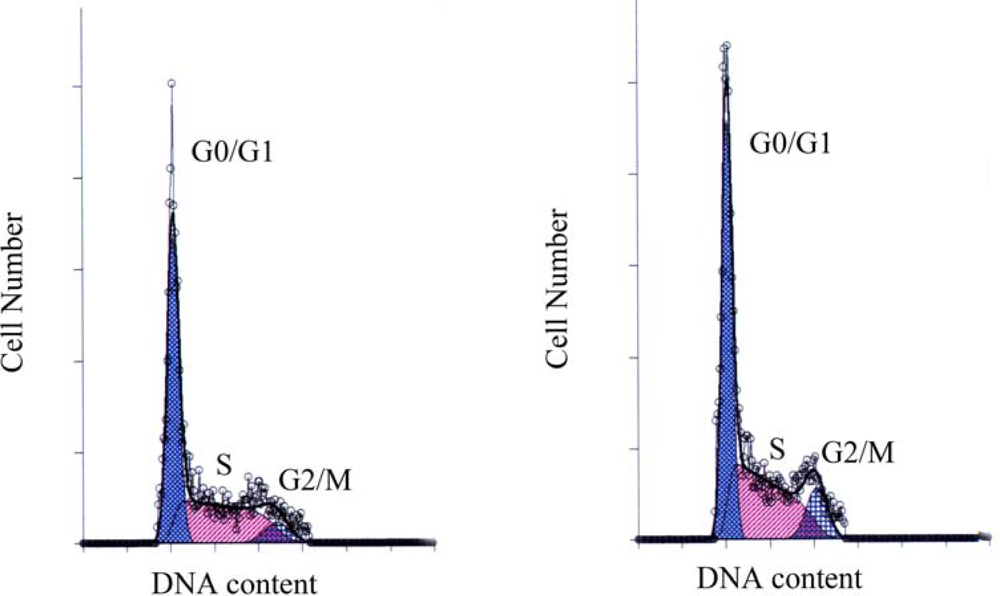
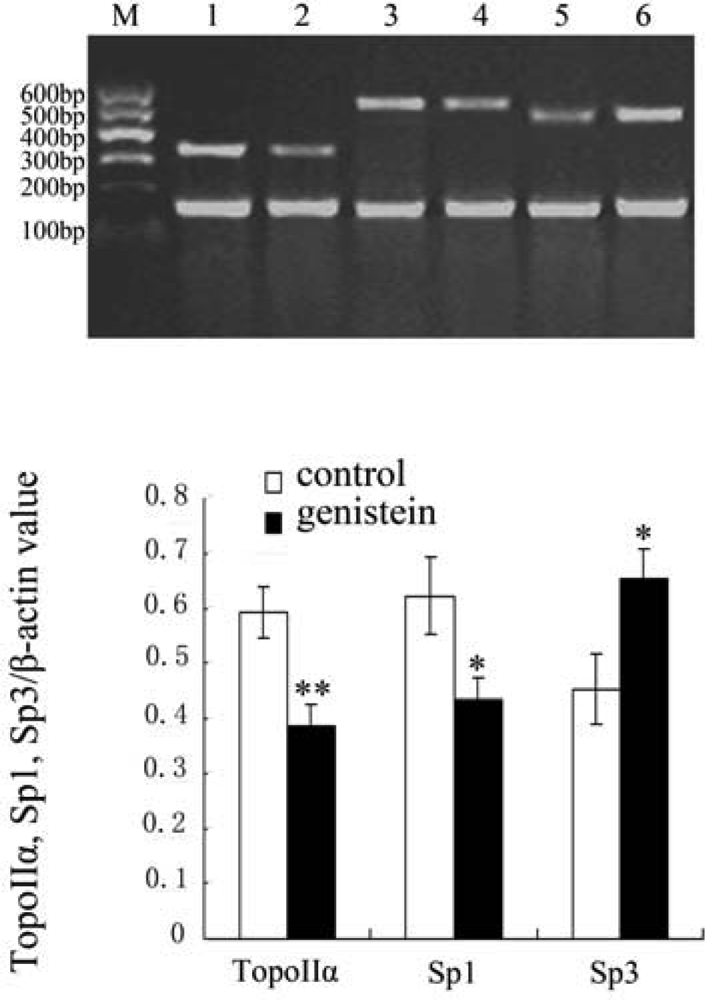
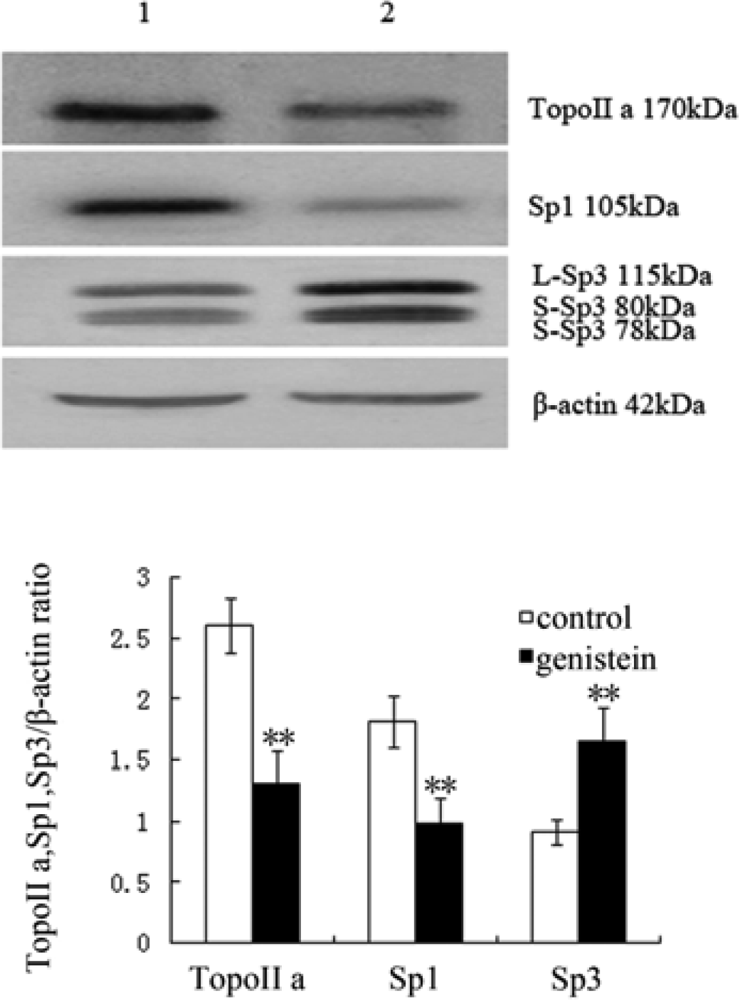

| Group | G0/G1 | S | G2/M |
|---|---|---|---|
| Control | 50.64 ± 2.35 | 42.31 ± 2.66 | 7.06 ± 0.33 |
| Genistein | 45.77 ± 4.03 | 42.40 ± 3.60 | 11.83 ± 0.61* |
| Name | Primer sequence | Length | Annealing temperature |
|---|---|---|---|
| TopoIIα | Sense: 5′-TGCCTGTTTAGTCGCTTTC-3′ | 349bp | 50.5 °C |
| Antisense 5′-TGAGGTGGTCTTAAGAAT-3′ | |||
| Sp1 | Sense: 5′-TGGTGGGCAGTATGTTGT-3′ | 550bp | 55 °C |
| Antisense 5′-GCTATTGGCATTGGTGAA-3′ | |||
| Sp3 | Sense: 5′-TAAGGTGTATTGCGTCTT-3′ | 516bp | 50 °C |
| Antisense 5′-GCTATTGGCATTGGTGAA-3′ | |||
| β-actin | Sense: 5′-CCA ACTGGGACGACAT-3′ | 135bp | 54 °C |
| Antisense: 5′-TCTGGGTCATCTTCTCG-3′ |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, N.; Yan, Y.; Li, W.; Wang, Y.; Zheng, L.; Han, S.; Yan, Y.; Li, Y. Genistein Inhibition of Topoisomerase IIα Expression Participated by Sp1 and Sp3 in HeLa Cell. Int. J. Mol. Sci. 2009, 10, 3255-3268. https://doi.org/10.3390/ijms10073255
Zhou N, Yan Y, Li W, Wang Y, Zheng L, Han S, Yan Y, Li Y. Genistein Inhibition of Topoisomerase IIα Expression Participated by Sp1 and Sp3 in HeLa Cell. International Journal of Molecular Sciences. 2009; 10(7):3255-3268. https://doi.org/10.3390/ijms10073255
Chicago/Turabian StyleZhou, Najing, Yunli Yan, Wenling Li, Yanling Wang, Lifen Zheng, Shuo Han, Yongxin Yan, and Yunzhi Li. 2009. "Genistein Inhibition of Topoisomerase IIα Expression Participated by Sp1 and Sp3 in HeLa Cell" International Journal of Molecular Sciences 10, no. 7: 3255-3268. https://doi.org/10.3390/ijms10073255
APA StyleZhou, N., Yan, Y., Li, W., Wang, Y., Zheng, L., Han, S., Yan, Y., & Li, Y. (2009). Genistein Inhibition of Topoisomerase IIα Expression Participated by Sp1 and Sp3 in HeLa Cell. International Journal of Molecular Sciences, 10(7), 3255-3268. https://doi.org/10.3390/ijms10073255




