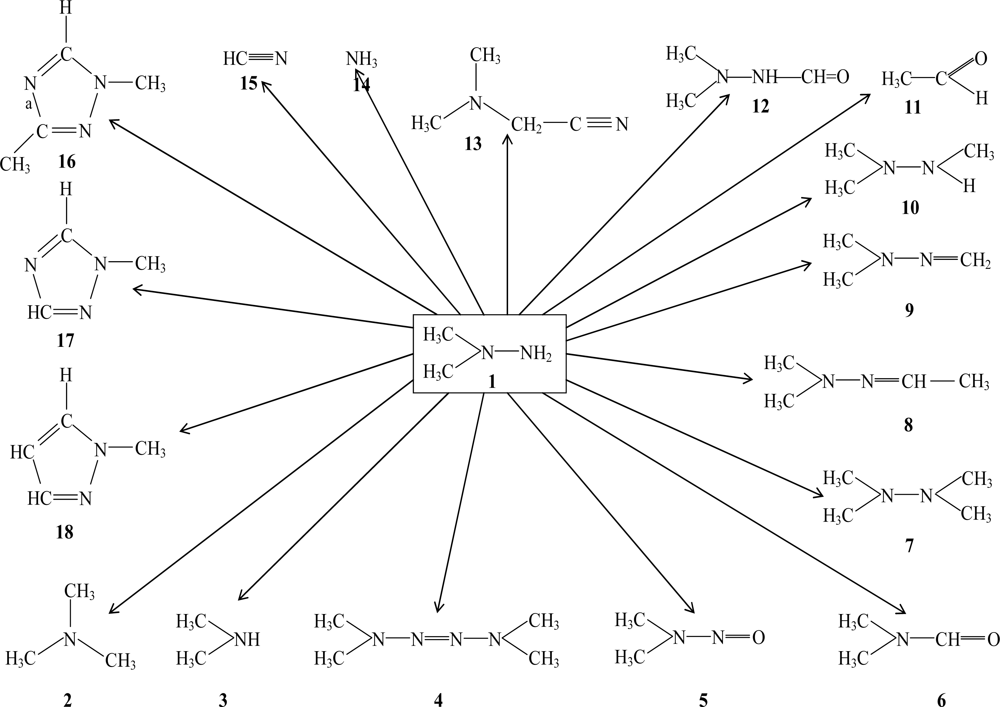
The Interplay between QSAR/QSPR Studiesand Partial Order Ranking and Formal Concept Analyses
Abstract
:1. Introduction
2. Results and Discussion
2.1. Residual Rocket Fuel and its Transformation products
2.2. Environmental Behavior of Rocket Fuel and its Transformation Products
2.3. Ecotoxicology of Rocket Fuel and its Transformation Products
2.4. Human Health Impact by Rocket Fuel and its Transformation Products
3. Methodology
3.1. Quantitative Structure-Activity/Property Relationships (QSAR/QSPR)
3.1.1. Physico-chemical data
3.1.2. Environmental persistence
| BDP3 | Predicted Half-Lives (days) |
|---|---|
| Hours | 0.17 |
| Hours to Days | 1.25 |
| Days | 2.33 |
| Days to Weeks | 8.67 |
| Weeks | 15 |
| Weeks to Months | 37.5 |
| Months | 60 |
| Recalcitrant | 180 |
3.1.3. Environmental toxicity
3.1.4. Absorption, Distribution, Metabolism and Excretion (ADME)
3.1.5. ToxBoxes
3.1.6. Prediction of Activity Spectra for Substances (PASS)
3.2. Partial Order Ranking (POR)
3.2.1. Linear extensions and ranking probabilities
3.2.2. Averaged ranks
3.2.3. Hierarchical POR
3.3. Formal Concept Analysis (FCA)
3.3.1. Line diagrams
4. Conclusions
Acknowledgments
Appendix
| No. | F1000 | F100 | F10 | F5 | F1 | D1000 | D100 | D10 | D5 | D1 | A1000 | A100 | A10 | A5 | A1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | X | X | X | X | X | X | X | X | X | X | X | ||||
| 2 | X | X | X | X | X | ||||||||||
| 3 | X | X | X | X | X | ||||||||||
| 4 | X | ||||||||||||||
| 5 | X | X | X | ||||||||||||
| 6 | |||||||||||||||
| 7 | X | X | X | X | X | X | X | X | X | X | X | X | |||
| 8 | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| 9 | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| 10 | X | X | X | X | X | X | X | X | X | X | X | X | |||
| 11 | X | X | X | X | |||||||||||
| 12 | X | X | X | X | X | X | X | X | X | ||||||
| 13 | X | X | X | X | X | ||||||||||
| 14 | X | X | X | X | |||||||||||
| 15 | |||||||||||||||
| 16 | |||||||||||||||
| 17 | |||||||||||||||
| 18 | X |
| No. | C5 | C6 | C7 | C8 | C9 | M5 | M6 | M7 | M8 | M9 | T5 | T6 | T7 | T8 | T9 | E5 | E6 | E7 | E8 | E9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| 2 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| 3 | X | |||||||||||||||||||
| 4 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| 5 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| 6 | X | X | X | X | X | X | X | X | X | X | ||||||||||
| 7 | X | X | X | X | X | X | X | X | X | |||||||||||
| 8 | X | X | X | X | X | |||||||||||||||
| 9 | X | X | ||||||||||||||||||
| 10 | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| 11 | X | X | ||||||||||||||||||
| 12 | X | X | X | X | X | X | ||||||||||||||
| 13 | ||||||||||||||||||||
| 15 | ||||||||||||||||||||
| 16 | ||||||||||||||||||||
| 17 | ||||||||||||||||||||
| 18 |
References and Notes
- Voigt, K; Brüggemann, R; Pudenz, S. Chemical databases evaluated by order theoretical tools. Anal. Bioanal. Chem 2004, 380, 467–474. [Google Scholar]
- Voigt, K; Brüggemann, R; Pudenz, S. A multi-criteria evaluation of environmental databases using the Hasse Diagram Technique (ProRank) software. Environ. Modell. Softw 2006b, 21, 1587–1597. [Google Scholar]
- Voigt, K; Brüggemann, R; Pudenz, S. Information quality of environmental and chemical databases exemplified by high production volume chemicals and pharmaceuticals. Online Inf. Rev 2006, 30, 8–23. [Google Scholar]
- Carlsen, L; Kenesova, OA; Batyrbekova, SE. A preliminary assessment of the potential environmental and human health impact of unsymmetrical dimethylhydrazine as a result of space activities. Chemosphere 2007, 67, 1108–1116. [Google Scholar]
- Carlsen, L; Kenessov, BN; Batyrbekova, SYe. A QSAR/QSTR study on the environmental health impact by the rocket fuel heptyl and its transformation products. Environ Health Insights. 2008, 1, pp. 11–20.
- Carlsen, L; Kenessov, BN; Batyrbekova, SYe. A QSAR/QSTR study on the human health impact of the rocket fuel 1,1-dimethyl hydrazine and its transformation products. Multicriteria hazard ranking based on partial order methodologies. Environ. Toxicol. Pharmacol 2009, 27, 415–423. [Google Scholar]
- Ivanciuc, T; Ivanciuc, O; Klein, DJ. Posetic Quantitative Superstructure/Activity Relationships (QSSARs) for Chlorobenzenes. J. Chem. Inf. Model 2005, 45, 870–879. [Google Scholar]
- Klein, DJ; Ivanciuc, T; Ryzhov, A; Ivanciuc, O. Combinatorics of Reaction-Network Posets. Comb. Chem. High Throughput Scr 2008, 11, 723–733. [Google Scholar]
- Verdonck, FAM; Boeije, G; Vandenberghe, V; Comber, M; de Wolf, W; Feijtel, T; Holt, M; Koch, K; Lecloux, A; Siebel-Sauer, A; Vanrolleghem, PA. A rule-based screening environmental risk assessment tool derived from EUSES. Chemosphere 2005, 58, 1169–1176. [Google Scholar]
- Sørensen, PB; Brüggemann, R; Carlsen, L; Mogensen, BB; Kreuger, J; Pudenz, S. Analysis of monitoring data of pesticide residues in surface waters using partial order ranking theory. Environ. Toxicol. Chem 2003, 22, 661–670. [Google Scholar]
- Carlsen, L. Giving molecules an identity. On the interplay between QSARs and Partial Order Ranking. Molecules. 2004, 9, pp. 1010–1018.
- Carlsen, L. A QSAR Approach to physico-chemical data for organophosphates with special focus on known and potential nerve agents. Internet Electron J Mol Des. 2005, 4, pp. 355–366.
- Carlsen, L. Partial order ranking of organophosphates with special emphasis on nerve agents, MATCH-Commun. Math. Comput. Chem 2005, 54, 519–534. [Google Scholar]
- Carlsen, L. Interpolation schemes in QSAR. In Partial Order in Environmental Sciences and Chemistry; Brüggemann, R, Carlsen, L, Eds.; Springer: Berlin, 2006; pp. 163–180. [Google Scholar]
- Carlsen, L. A combined QSAR and partial order ranking approach to risk assessment. SAR QSAR Environ. Res 2006, 17, 133–146. [Google Scholar]
- Carlsen, L. Hierarchical partial order ranking. Environ. Pollut 2008, 155, 247–253. [Google Scholar]
- Burmeister, P. Formal concept analysis with ConImp: Introduction to the basic features.
- Formal Concept Analysis: Foundations and Applications; Ganter, B; Stumme, G; Wille, R (Eds.) Springer: Berlin, Germany, 2005.
- Johnson, MH; Maggiora, GM. Concepts and Applications of Molecular Similarity; Wiley: New York, USA, 1990. [Google Scholar]
- Crum-Brown, A; Frazer, T. On the connection between chemical constitution and physiological action. Codeia, Morphia and Nicotia Trans Royal Soc. Edinburgh 1868/1869, 25, 257–274. [Google Scholar]
- Meyer, H. Zur Theorie der Alkoholnarkose, welche Eigenschaft die Anästhetica bedingt ihre narkotische Wirkung. Arch Exp Pathol Pharmakol 1899, 42, 109–118. [Google Scholar]
- Overton, CE. Studien über die Narkose. In Zugleich ein Beitrag zur Allgemeine Pharmakologie; Gustav Fischer Verlag: Jena, Germany, 1901. [Google Scholar]
- Ferguson, J. The use of chemical potentials as indicators for toxicity. Proc. Royal Soc. B 1939, 127, 387. [Google Scholar]
- Hansch, C; Leo, A. Exploring QSAR Fundamentals and Applications in Chemistry and Biology; ACS: Washington DC, USA, 1995. [Google Scholar]
- Bevan, DR. QSAR and Drug Design.
- 3D-QSAR in drug design. In Theory Methods and Applications; Kubinyi, H (Ed.) Kluwer/Escom: Dordrecht, The Netherlands, 1998.
- Neural Networks in QSAR and Drug Design; Devilliers, J (Ed.) Academic Press: London, UK, 1996.
- Kurup, A; Garg, R; Carini, DJ; Hansch, C. Comparative QSAR: Angiotensin II antagonists. Chem. Rev 2001, 101, 2727–2750. [Google Scholar]
- Jaworska, J; Comber, M; Auer, C; Leeuwen, CJ. Summary of a workshop on regulatory acceptance of (Q)SARs for human health and environmental endpoints. Env. Health Perspect 2003, 111, 1358–1360. [Google Scholar]
- Eriksson, L; Jaworska, J; Worth, AP; Cronin, MTD; McDowell, RM; Gramatica, P. Methods for reliability and uncertainty assessment, and for applicability evaluations of classification- and regression-based QSARs. Env. Health Perspect 2003, 111, 1361–1375. [Google Scholar]
- Cronin, MTD; Walker, JD; Jaworska, JS; Comber, MHI; Watts, CD; Worth, AP. Use of QSARs in international decisions-making frameworks to predict ecological effects and environmental fate of chemical substances. Env. Health Perspect 2003, 111, 1376–1390. [Google Scholar]
- Cronin, MTD; Walker, JD; Jaworska, JS; Comber, MHI; Watts, CD; Worth, AP. Use of QSARs in international decisions-making frameworks to predict health effects of chemical substances. Env. Health Perspect 2003, 111, 1391–1401. [Google Scholar]
- Worth, A. QSC QSAR and Combinatorial. Science 2008, 27, 1–132. [Google Scholar]
- EPA Estimation Program Interface (EPI) Suite.
- PASS1. Prediction of Activity Spectra for Substances.
- Pharma Algorithms 1. ADME/Tox WEB.
- Nauryzbaev, MK; Batyrbekova, SE; Tassibekov, KhS; Kenesov, BN; Vorozheikin, AP; Proskuryakov, YuV. Ecological problems of central Asia resulting from space rocket debris. In History and Society in Central and Inner Asia, Toronto Studies in Central and Inner Asia; No 7, Asian Institute: University of Toronto, Toronto, 2005; pp. 327–349. [Google Scholar]
- System analysis of environmental objects in the territories of Kazakhstan, which suffered negative influence through Baikonur space port activity, , Final technical Report of ISTC K451.2, Center of Physical-Chemical Methods of Analysis, al-Farabi Kazakh National University in Almaty, Kazakhstan, 2006.
- European Commission. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.
- European Commision. Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market Part I EUR 20418 EN/1. 2003. European Communities. 2003.
- Adushkin, VV; Kozlov, CI; Petrov, AV. Ecological Problems and Risks of Rocket-Space Techniques Impacts on Environment; Ankil: Moscow, Russia, 2000. [Google Scholar]
- Carlsen, L. Formal concept analyses of the ecotoxicological and health effects of residual rocket fuel and its transformation products. 2009, in press. [Google Scholar]
- Ivanciuc, O; Ivanciuc; Klein, DJ. Modeling the Bioconcentration Factors and BioaccumulationFactors of Polychlorinated Biphenyls with Posetic Quantitative Super– Structure/Activity Relationships (QSSAR). Mol. Diversity 2006, 10, 133–145. [Google Scholar]
- Partial Order in Environmental Sciences and Chemistry; Brüggemann, R; Carlsen, L (Eds.) Springer: Berlin, Germany, 2006.
- Walker, JD; Carlsen, L. QSARs for Identifying and Prioritizing Substances with Persistence and Bioconcentration Potential. SAR QSAR Environ. Res 2002, 13, 713–726. [Google Scholar]
- .
- .
- Sazonovas, A. Descriptions, incl. validation of the sigle ADME Boxes and ToxBoxes modules. Personal communication from A. 2009. [Google Scholar]
- PASS2. List of predicted activities.
- PASS3. Quality of prediction.
- PASS4. Basic elements of PASS.
- Poroikov, VV; Filimonov, DA; Ihlenfeldt, W-D; Gloriozova, TA; Lagunin, AA; Borodina, YuV; Stepanchikova, AV; Nicklaus, MC. PASS Biological activity spectrum predictions in the enhanced open NCI database browser. J. Chem. Inform. Comput. Sci 2003, 43, 228–236. [Google Scholar]
- PASS5. Limitations.
- Davey, BA; Priestley, HA. Introduction to lattices and Order; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Brüggemann, R; Halfon, E; Welzl, G; Voigt, K; Steinberg, CEW. Applying the concept of partially ordered sets on the ranking of near-shore sediments by a battery of tests. J. Chem. Inf. Comput. Sci 2001, 41, 918–925. [Google Scholar]
- Halfon, E; Reggiani, MG. On the ranking of chemicals for environmental hazard. Environ. Sci. Technol 1986, 20, 1173–1179. [Google Scholar]
- Hasse, H. Über die Klassenzahl abelscher Zahlkörper; Akademie Verlag: Berlin, Germany, 1952. [Google Scholar]
- Brüggemann, R; Halfon, E; Bücherl, C. Theoretical base of the program “Hasse”, GSF-Bericht 20/95, Neuherberg, Germany, 1995. The software may be obtained by contacting Dr. R. Brüggemann, Institute of Freshwater Ecology and Inland Fisheries, Berlin ([email protected]).
- European Chemical Bureau. DART. Decision Analysis by Ranking Techniques.
- Brüggemann, R; Restrepo, G; Voigt, K. Towards a New and Advanced Partial Order Program: PyHasse. In Multicriteria Ordering and Ranking: Partial Orders, Ambiguities and Applied Issues; Systems Research Institute Polish Academy of Sciences: Warsaw; pp. 11–33, The software may be obtained by contacting Dr. R. Brüggemann, Institute of Freshwater Ecology and Inland Fisheries, Berlin ([email protected]).
- Fishburn, PC. On the family of linear extensions of a partial order. J. Combinat. Theory 1974, 17, 240–243. [Google Scholar]
- Graham, RL. Linear Extensions of Partial Orders and the FKG Inequality. In Ordered Sets; Rival, I, Ed.; Reidel Publishing Company: Dordrecht, The Netherlands, 1982; pp. 213–236. [Google Scholar]
- Winkler, PM. Average height in a partially ordered set. Discrete Math 1982, 39, 337–341. [Google Scholar]
- Winkler, PM. Correlation among partial orders. Siam. J. Alg. Disc. Meth 1983, 4, 1–7. [Google Scholar]
- Sørensen, PB; Lerche, DB; Carlsen, L; Brüggemann, R. Statistically approach for estimating the total set of linear orders. A possible way for analysing larger partial order sets. In Order Theoretical Tools in Environmental Science and Decision Systems; Brüggemann, R, Pudenz, S, Lühr, H-P, Eds.; Berichte des IGB, Leibniz-Institut of Freshwater Ecology and Inland Fisheries: Berlin, Heft 14, Sonderheft IV, 2001; pp. 87–97. [Google Scholar]
- Lerche, D; Brüggemann, R; Sørensen, P; Carlsen, L; Nielsen, OJ. A comparison of partial order technique with three methods of multi-criteria analysis for ranking of chemical substances. J. Chem. Inf. Comput. Sci 2002, 42, 1086–1098. [Google Scholar]
- Lerche, D; Sørensen, PB; Brüggemann, R. Improved estimation of ranking probabilities in partial orders using random linear extensions by approximation of the mutual ranking probability. J. Chem. Inf. Comput. Sci 2003, 43, 1471–1480. [Google Scholar]
- Brüggemann, R; Lerche, D; Sørensen, PB; Carlsen, L. Estimation of average ranks by a local partial order model. J. Chem. Inf. Comput. Sci 2004, 44, 618–625. [Google Scholar]
- Sørensen, PB; Mogensen, BB; Carlsen, L; Thomsen, M. The influence of partial order ranking from input parameter uncertainty. Definition of a robustness parameter. Chemosphere 2000, 41, 595–601. [Google Scholar]
- Yevtushenko, SA. System of data analysis “Concept Explorer”. Proceedings of the 7th National Conference on Artificial Intelligence KII-2000, Russia; 2000; pp. 127–134. [Google Scholar]
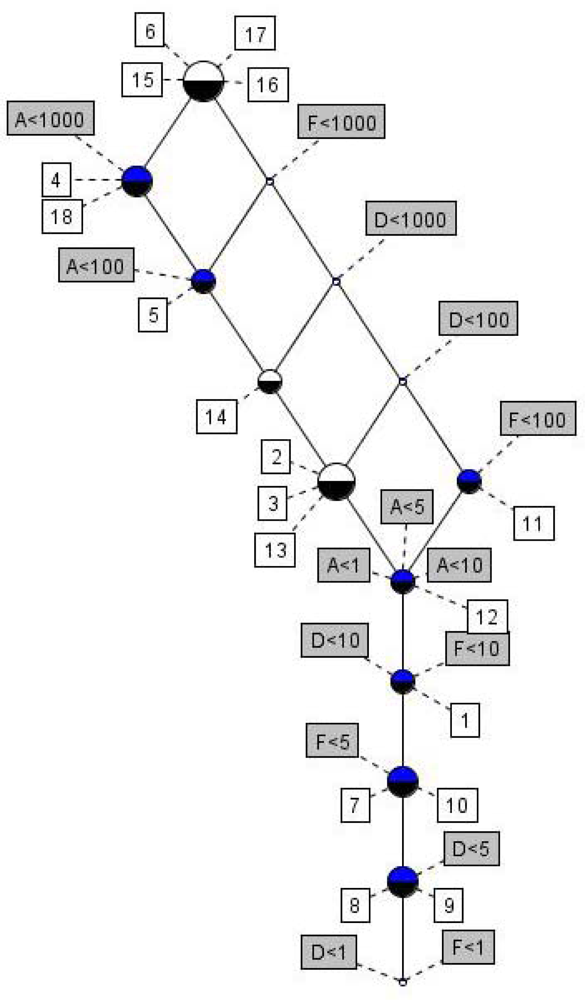
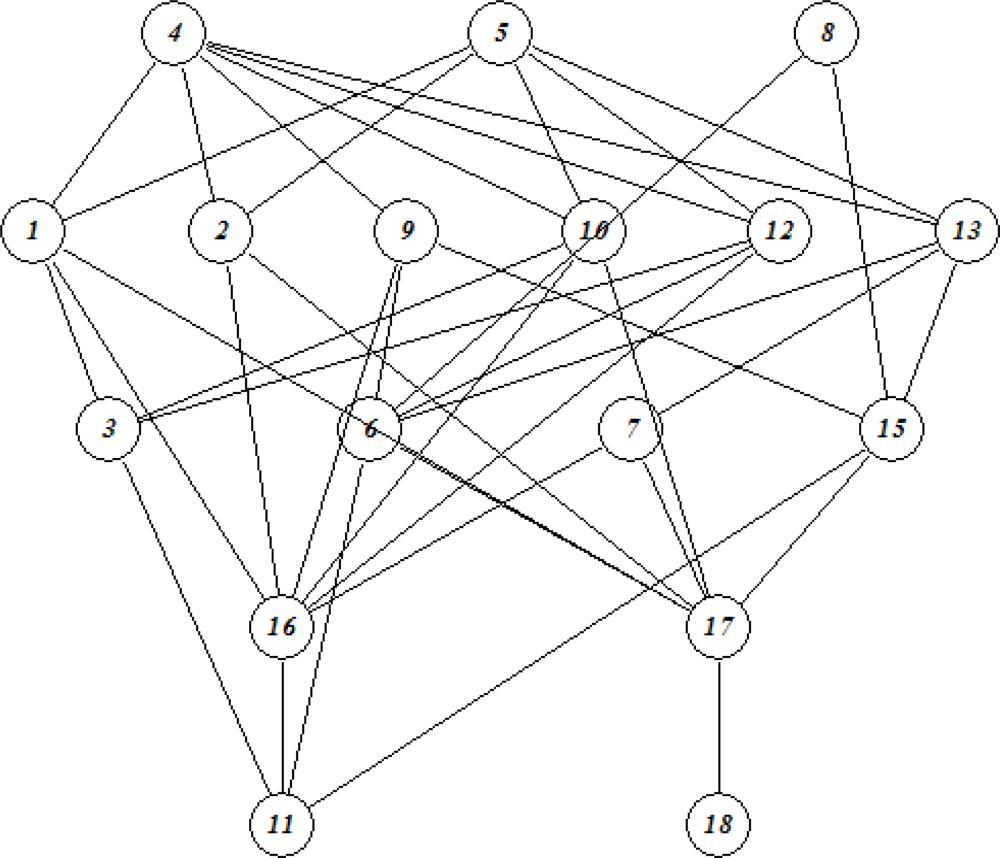
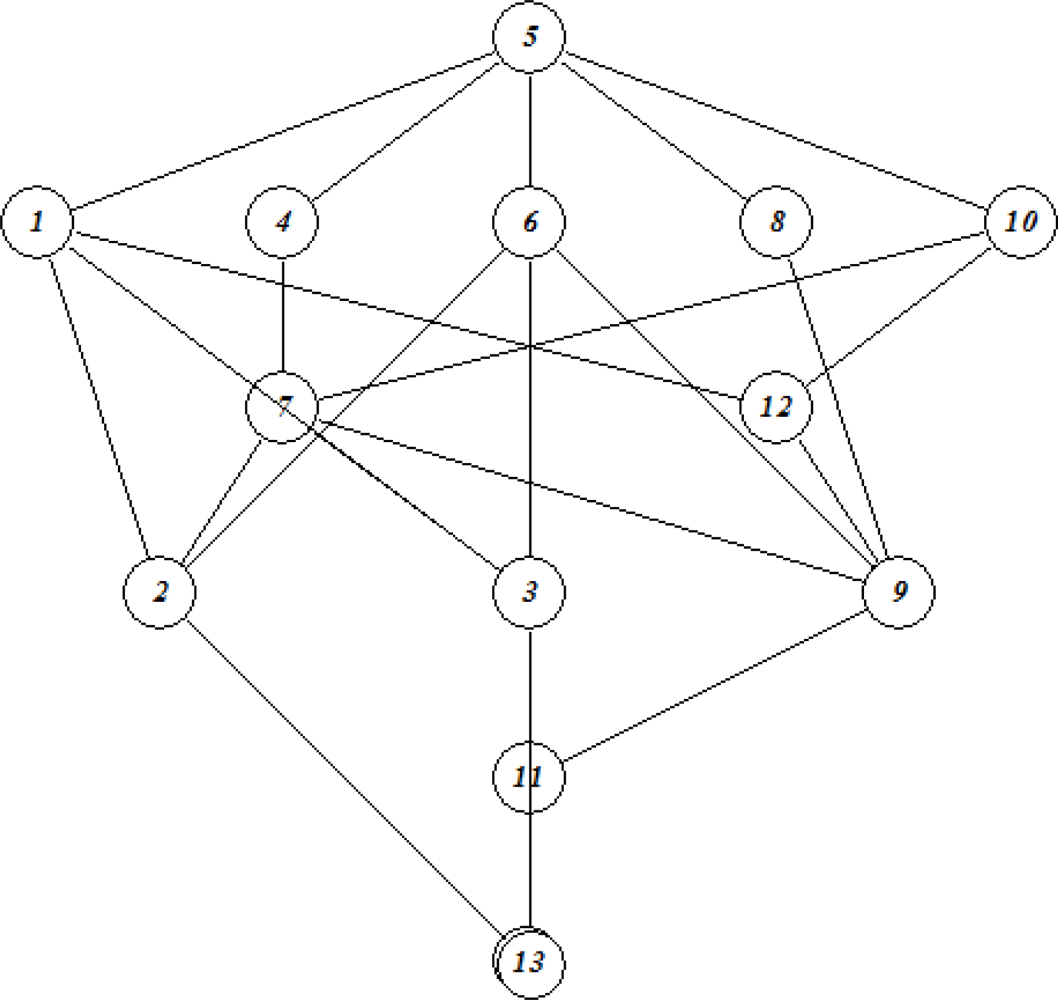
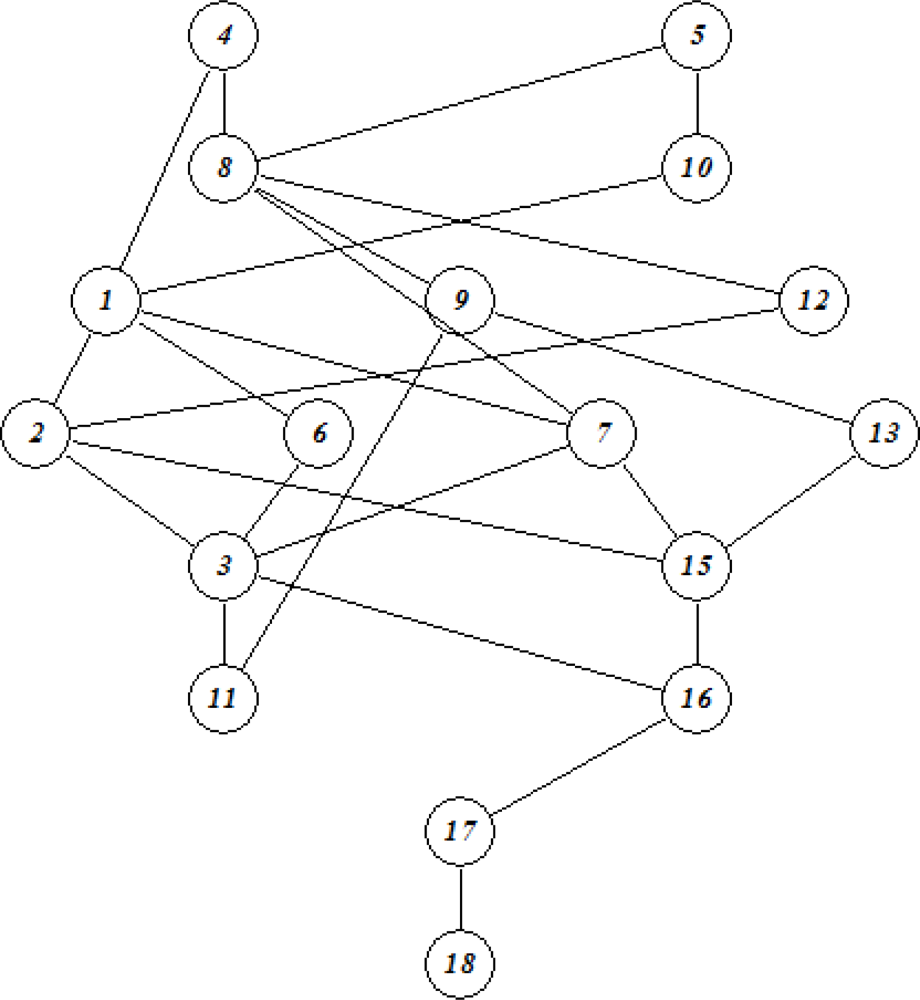
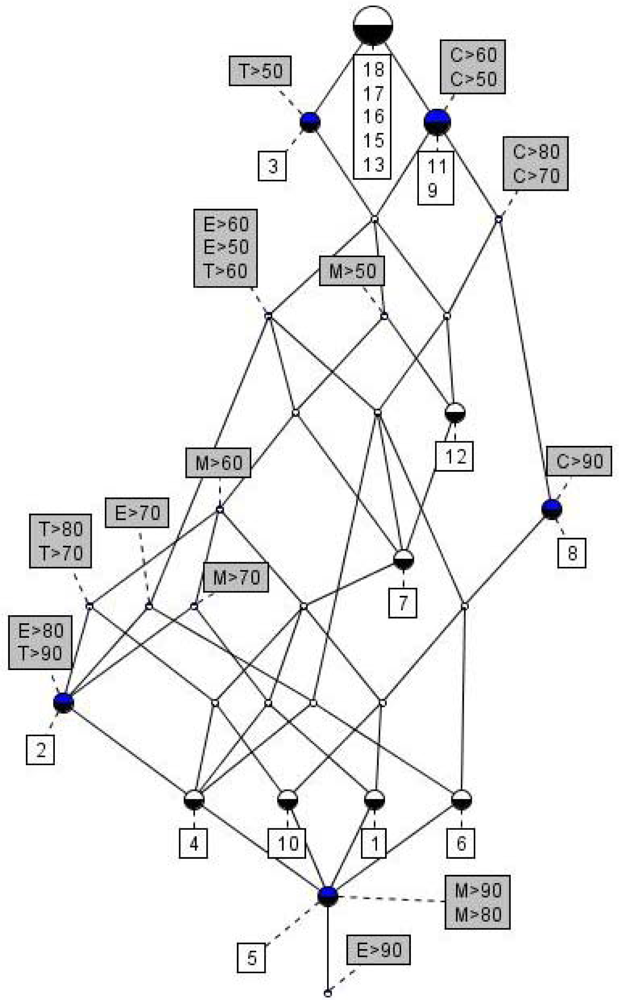
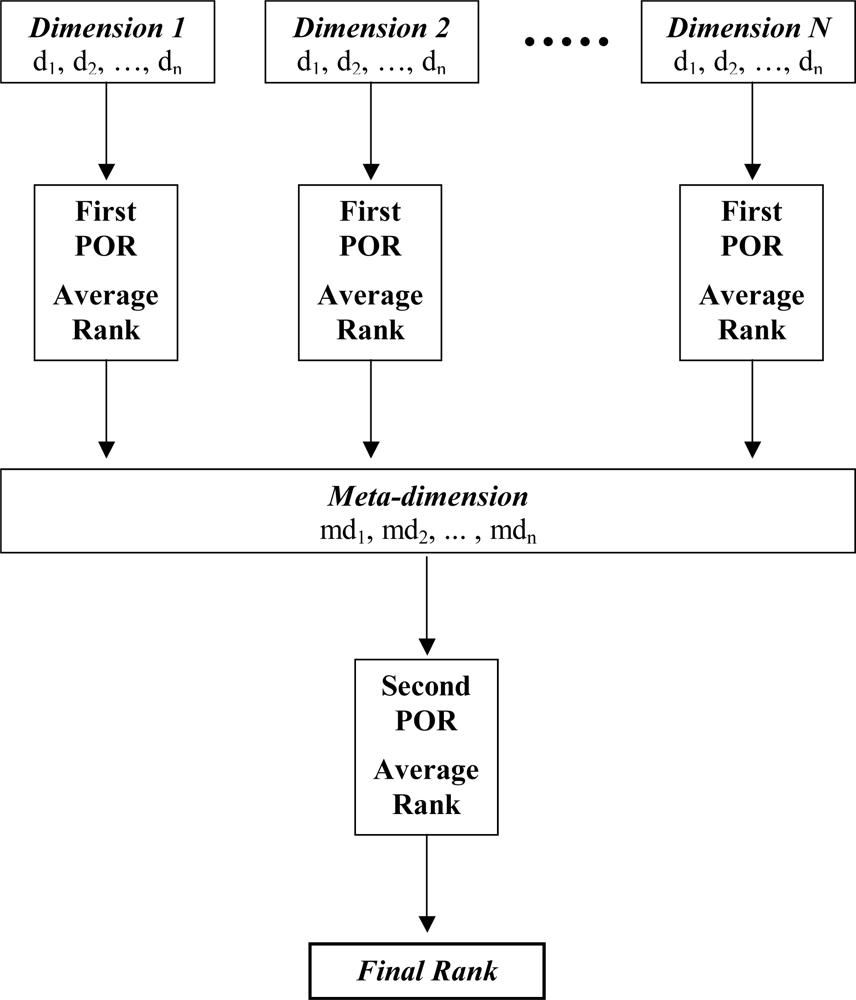
| No | Log SW mg/L | Log KOW | Log KOC | Log HLC atm m3 mole−1 | Log VP mmHg |
|---|---|---|---|---|---|
| 1 | 1×106 (1×106) | −1.19 | 1.29 | 6.95×10−8 | 1.68×102 (1.57×102) |
| 2 | 1×106 (8.9×105) | 0.04 (0.16) | 1.17 | 1.28×10−4 (1.04×10−4) | 1.69×103 (1.61×103) |
| 3 | 1×106 (1.7×106) | −0.17 (−0.38) | 1.12 | 1.81×10−5 (1.77×10−5) | 1.52×103 (1.47×103) |
| 4 | 1×106 | 0.69 | 1.03 | 1.96×108 | 21.3 |
| 5 | 9.6×105 (1×106) | −0.64 (−0.57) | 1.58 | 2.02×10−6 (1.82×10−6) | 4.3 (2.70) |
| 6 | 1×106 (1×106) | −0.93 (−1.01) | 0.38 | 7.38×10−8 (7.39×10−8) | 3.49 (3.87) |
| 7 | 1×106 | −0.52 | 1.53 | 7.39×10−7 | 1.31×102 |
| 8 | 4.5×105 | 0.40 | 1.85 | 5.91×10−5 | 80.3 |
| 9 | 7.78×105 | 0.68 | 1.58 | 4.45×10−5 | 3.30×102 |
| 10 | 1×106 | −0.73 | 1.45 | 1.53×10−7 | 1.45×102 |
| 11 | 4.77×105 (1×106) | −0.17 (−0.34) | 0.18 | 6.78×10−5 (6.67×10−5) | 9.10×102 (9.02×102) |
| 12 | 1×106 | −1.70 | 0.65 | 3.08×10−10 | 0.14 |
| 13 | 1×106 | −0.44 | 1.00 | 1.52×10−8 | 7.12 |
| 14 | 3.02×104 (4.8×105) | 0.23 (−1.38) | 1.16 | 3.45×10−6 | 35.2 (7.51×103) |
| 15 | 3.1×105 (1×106) | −0.69 (−0.25) | 0.43 | 2.42×10−2 (1.33×10−4) | 7.32×102 (7.42×102) |
| 16 | 2.0×105 | 0.33 | 2.37 | 3.60×10−5 | 3.78 |
| 17 | 5.7×105 | −0.21 | 2.16 | 3.26×10−5 | 10.5 |
| 18 | 7.3×104 | 0.61 (0.23) | 1.20 | 7.88×10−5 | 11.5 |
| No. | BDP3a | Ultimate biodegradation half life within | Fast Anaerobic biodegradation? | Residence half life in riversb | Residence half life in lakesb |
|---|---|---|---|---|---|
| 1 | 3.0664 | Weeks | Yes | 272 d | 8.1 y |
| 2 | 2.8137 | Weeks | No | 5.1 h | 5.0 d |
| 3 | 3.1240 | Weeks | Yes | 22.9 h | 12.8 d |
| 4 | 2.9425 | Weeks | Yes | 3.67 y | 40.1 y |
| 5 | 2.6503 | Weeks to Months | Yes | 11.6 d | 130 d |
| 6 | 2.9834 | Weeks | No | 282 d | 8.4 y |
| 7 | 3.0044 | Weeks | Yes | 31.0 d | 340 d |
| 8 | 3.0088 | Weeks | No | 10.1 h | 7.9 d |
| 9 | 3.0398 | Weeks | Yes | 12.0 h | 8.4 d |
| 10 | 3.0354 | Weeks | Yes | 137 d | 4.1 y |
| 11 | 3.1241 | Weeks | Yes | 6.5 h | 5.3 d |
| 12 | 3.0045 | Weeks | Yes | 204 y | 2220 y |
| 13 | 2.6761 | Weeks to Months | No | 4.0 y | 44.0 y |
| 14 | 3.1615 | Weeks | No | 6.7 d | 6.7 d |
| 15 | 3.1394 | Weeks | Yes | 2.8 h | 3.1 d |
| 16 | 2.9097 | Weeks | No | 17.0 h | 11.2 d |
| 17 | 3.0155 | Weeks | Yes | 17.3 h | 11.1 d |
| 18 | 3.0177 | Weeks | No | 7.7 h | 6.6 d |
| No. | LC50 (mg/L) | EC50 (mg/L) | ||
|---|---|---|---|---|
| Fisha | Fishb | Daphnidsc | Green algae | |
| 1 | 48500 | 5.9 | 6.2 | 0.53d |
| 2 | 4050 | 290 | 16.8 | 16.1b |
| 3 | 4700 | 300 | 17.0 | 15.1b |
| 4 | 2160 | 1470 | 1450 | 830b |
| 5 | 19800 | 1000 | 5200 | 39.8b |
| 6 | 35000 | 30800 | 27000 | 14200b |
| 7 | 18500 | 4.4 | 6.1 | 0.67d |
| 8 | 2850 | 1.7 | 3.5 | 0.53d |
| 9 | 1350 | 1.1 | 2.5 | 0.42d |
| 10 | 23800 | 4.6 | 5.8 | 0.59d |
| 11 | 4600 | 17.8 | 48.8 | 1820b |
| 12 | 200000 | 14.4 | 12.2 | 0.88d |
| 13 | 15100 | 850 | 45.5 | 37.0b |
| 14 | 800 | 580 | 550 | 310b |
| 15 | 8000 | 6775 | 6025 | 3225b |
| 16 | 3700 | 2675 | 2550 | 1450b |
| 17 | 9400 | 7350 | 6775 | 3725b |
| 18 | 1800 | 1225 | 1200 | 690b |
| No | Passive absorption (Human intestinal)a | Absorption rate constant (min−1) | PPB%b | Binding constant log KaHSA | Vdc (L/kg) | P-Glycoprotein inhibitor | P-Glycoprotein substrate |
|---|---|---|---|---|---|---|---|
| 1 | 94 (10/90) | 0.012 | 14.71 | 1.70 | 0.94 | 0.003 | 0.031 |
| 2 | 99 (53/47) | 0.019 | 13.96 | 1.80 | 2.00 | 0.002 | 0.002 |
| 3 | 99 (18/82) | 0.018 | 11.74 | 1.65 | 2.29 | 0.004 | 0.014 |
| 4 | 100 (98/2) | 0.044 | 17.27 | 2.38 | 1.13 | 0.003 | 0.008 |
| 5 | 100 (88/12) | 0.022 | 7.25 | 2.19 | 1.01 | 0.003 | 0.006 |
| 6 | 94 (76/24) | 0.012 | 3.84 | 2.00 | 0.96 | 0.004 | 0.007 |
| 7 | 100 (95/5) | 0.031 | 17.49 | 2.24 | 1.22 | 0.003 | 0.010 |
| 8 | 100 (98/2) | 0.050 | 26.73 | 2.41 | 1.24 | 0.009 | 0.006 |
| 9 | 100 (93/7) | 0.031 | 19.85 | 2.22 | 1.14 | 0.005 | 0.005 |
| 10 | 93 (34/66) | 0.011 | 20.17 | 1.81 | 1.22 | 0.004 | 0.039 |
| 11 | 100 (74/26) | 0.022 | 5.67 | 2.10 | 1.03 | 0.004 | 0.006 |
| 12 | 89 (76/24) | 0.009 | 7.33 | 2.05 | 0.94 | 0.004 | 0.011 |
| 13 | 99 (91/9) | 0.019 | 22.82 | 2.14 | 1.02 | 0.009 | 0.006 |
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 15 | 99 (50/50) | 0.021 | 3.87 | 2.02 | 0.99 | 0.005 | 0.005 |
| 16 | 100 (94/6) | 0.027 | 12.51 | 2.47 | 1.04 | 0.005 | 0.009 |
| 17 | 99 (85/15) | 0.017 | 8.90 | 2.33 | 1.01 | 0.003 | 0.008 |
| 18 | 100 (94/6) | 0.032 | 12.64 | 2.49 | 1.14 | 0.005 | 0.009 |
| No | Probability for adverse health effectsa | |||||
|---|---|---|---|---|---|---|
| Blood | Cardiovascular | Gastrointestinal | Kidney | Liver | Lungs | |
| 1 | 0.57 | 0.40 | 0.65 T | 0.28 | 0.48 T | 0.34 T |
| 2 | 0.44 | 0.34 | 0.80 | 0.20 | 0.18 | 0.27 |
| 3 | 0.20 | 0.31 | 0.26 | 0.11 | 0.20 T | 0.20 T |
| 4 | 0.79 | 0.07 | 0.92 | 0.57 | 0.85 | 0.74 |
| 5 | 0.76 | 0.06 | 0.97 | 0.75 T | 0.93 T | 0.71 T |
| 6 | 0.27 | 0.12 | 0.65 | 0.14 | 0.05 | 0.40 |
| 7 | 0.52 | 0.33 | 0.83 | 0.19 | 0.10 | 0.17 T |
| 8 | 0.63 | 0.06 | 0.84 | 0.31 | 0.05 | 0.75 |
| 9 | 0.32 | 0.08 | 0.90 | 0.42 | 0.07 | 0.72 |
| 10 | 0.53 | 0.64 | 0.66 | 0.14 | 0.29 T | 0.29 T |
| 11 | 0.19 | 0.08 | 0.25 | 0.09 | 0.04 | 0.04 T |
| 12 | 0.48 | 0.14 | 0.71 | 0.15 | 0.28 | 0.42 |
| 13 | 0.47 | 0.21 | 0.89 | 0.18 | 0.12 | 0.47 |
| 14 | n/a | N/a | n/a | n/a | n/a | n/a |
| 15 | 0.10 | 0.08 | 0.81 | 0.09 | 0.05 | 0.27 |
| 16 | 0.14 | 0.02 | 0.46 | 0.03 | 0.06 | 0.04 |
| 17 | 0.12 | 0.02 | 0.46 | 0.07 | 0.02 | 0.05 |
| 18 | 0.08 | 0.02 | 0.36 | 0.04 | 0.02 | 0.05 |
| No | Carcinogenic | Mutagenic | Teratogenic | Embryotoxic |
|---|---|---|---|---|
| 1 | 0.955 (0.002) | 0.762 (0.006) | 0.689 (0.031) | 0.672 (0.016) |
| 2 | 0.619 (0.001) | NE | NE | 0.527 (0.043) |
| 3 | NE | NE | 0.563 (0.062) | NE |
| 4 | 0.894 (0.003) | 0.792 (0.005) | 0.946 (0.006) | 0.816(0.007) |
| 5 | 0.980 (0.001) | 0.969 (0.002) | 0.952 (0.005) | 0.866 (0.005) |
| 6 | 0.951 (0.002) | NE | 0.614 (0.048) | 0.795 (0.009) |
| 7 | 0.827 (0.006) | 0.539 (0.010) | 0.698 (0.030) | 0.604 (0.026) |
| 8 | 0.980 (0.002) | NE | NE | NE |
| 9 | 0.683 (0.012) | NE | NE | NE |
| 10 | 0.923 (0.006) | 0.619 (0.007) | 0.811 (0.012) | 0.681 (0.015) |
| 11 | 0.628 (0.011) | NE | NE | NE |
| 12 | 0.897 (0.003) | 0.524 (0.011) | 0.530 (0.072) | NE |
| 13 | NE | NE | NE | NE |
| 14 | n/a | n/a | n/a | n/a |
| 15 | NE | NE | NE | NE |
| 16 | NE | NE | NE | NE |
| 17 | NE | NE | NE | NE |
| 18 | NE | NE | NE | NE |
| No | Rkav According to ToxBoxesa | Rkav According to PASSb |
|---|---|---|
| 1 | 6.0 | 2.8 |
| 2 | 6.8 | 9.7 |
| 3 | 13.5 | 9.7 |
| 4 | 1.1 | 2.8 |
| 5 | 1.2 | 1.0 |
| 6 | 11.5 | 3.0 |
| 7 | 8.0 | 5.1 |
| 8 | 2.6 | 3.6 |
| 9 | 4.0 | 10.1 |
| 10 | 6.0 | 2.6 |
| 11 | 16.9 | 11.3 |
| 12 | 5.4 | 6.0 |
| 13 | 4.9 | 17.0 |
| 14 | na | na |
| 15 | 10.8 | 17.0 |
| 16 | 15.0 | 17.0 |
| 17 | 15.6 | 17.0 |
| 18 | 16.8 | 17.0 |
| No | Rkav |
|---|---|
| 1 | 5.1 |
| 2 | 9.0 |
| 3 | 12.0 |
| 4 | 1.1 |
| 5 | 1.1 |
| 6 | 8.2 |
| 7 | 8.3 |
| 8 | 3.6 |
| 9 | 6.5 |
| 10 | 2.8 |
| 11 | 16.6 |
| 12 | 6.0 |
| 13 | 9.0 |
| 14 | n/a |
| 15 | 13.2 |
| 16 | 14.8 |
| 17 | 15.9 |
| 18 | 16.9 |
| No of compounds | If | Then |
|---|---|---|
| 7 | M > 50 | C > 60T > 50 |
| 5 | M > 60 | C > 60T > 60E > 60 |
| 1 | M > 90 | C > 90T > 90E > 80 |
| 7 | T > 60 | C > 60E > 60 |
| 4 | T > 80 | C > 60M > 60E > 60 |
| 3 | T > 90 | C > 60E > 80 |
| 4 | E > 70 | C > 60T > 60 |
| 3 | E > 80 | C > 60M > 70T > 90 |
| No of compounds | Pct | If | Then |
|---|---|---|---|
| 7 / 8 | 88 | C > 60T > 50 | T > 60E > 60 |
| 7 / 8 | 88 | C > 60T > 50 | M> 50 |
| 7 / 8 | 88 | C > 80 | T > 50 |
| 6 / 7 | 86 | C > 60T > 60E > 60 | M > 50 |
| 6 / 7 | 86 | C > 60T > 60E > 60 | C > 80 |
| 6 / 7 | 86 | C > 60 M > 50T > 50 | C > 80 |
| 5 / 6 | 83 | C > 60M > 50T > 60E > 60 | M > 60 |
| 5 / 6 | 83 | C > 60M > 50T > 60E > 60 | C > 80 |
| 4 / 5 | 80 | C > 60M > 60T > 60E > 60 | T > 80 |
| 4 / 5 | 80 | C > 60M > 50T > 60E > 60 | M > 70 |
| 4 / 5 | 80 | C > 60M > 50T > 60E > 60 | C > 80 |
| 4 / 5 | 80 | C > 90 | T > 60 E > 60 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carlsen, L. The Interplay between QSAR/QSPR Studiesand Partial Order Ranking and Formal Concept Analyses. Int. J. Mol. Sci. 2009, 10, 1628-1657. https://doi.org/10.3390/ijms10041628
Carlsen L. The Interplay between QSAR/QSPR Studiesand Partial Order Ranking and Formal Concept Analyses. International Journal of Molecular Sciences. 2009; 10(4):1628-1657. https://doi.org/10.3390/ijms10041628
Chicago/Turabian StyleCarlsen, Lars. 2009. "The Interplay between QSAR/QSPR Studiesand Partial Order Ranking and Formal Concept Analyses" International Journal of Molecular Sciences 10, no. 4: 1628-1657. https://doi.org/10.3390/ijms10041628