ECM-Based Materials in Cardiovascular Applications: Inherent Healing Potential and Augmentation of Native Regenerative Processes
Abstract
:1. Introduction
2. Origin of ECM Material: Source, Preparation, Biochemical Properties, Storage and Commercial Availability
3. Factors and Molecular Mechanisms of the ECM Bioactivity during Healing
3.1. The Early Response and Healing of Vascular Graft Material
3.2. Antimicrobial Resistance of ECM Materials
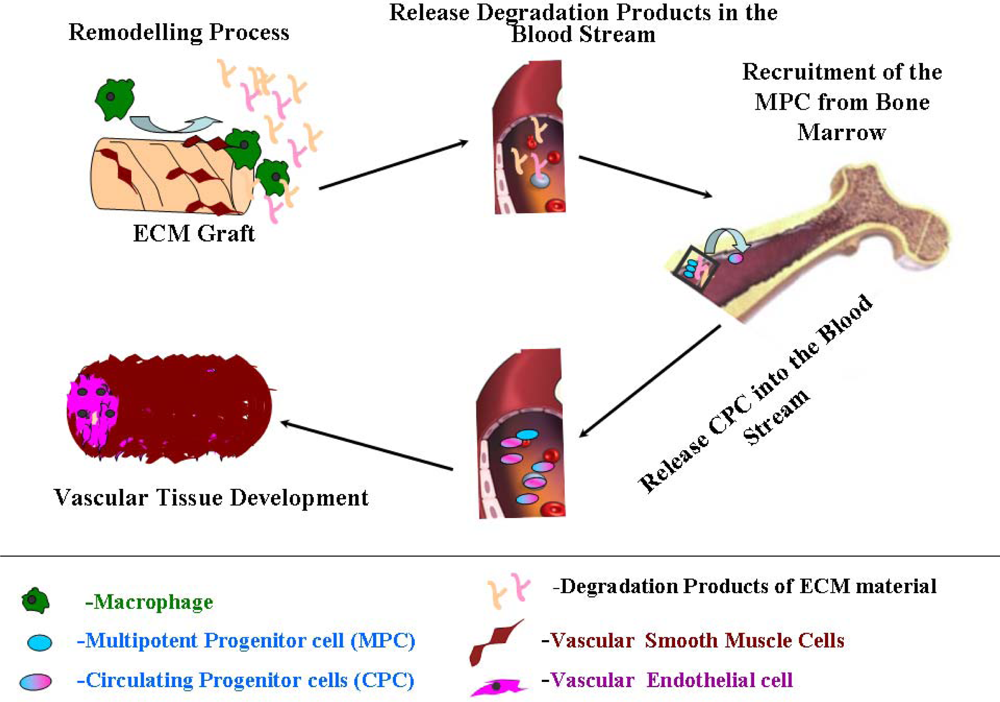
4. Vascular Tissue Development
4.1. Therapeutic Properties of the Remodeling Products of ECM Material
4.2. Bioenergetics of Vascular Healing
5. Vascular tissue Functionality and Homeostasis Maintenance
5.1. Biomechanical Properties of Graft Materials and Their Importance in Sufficient Reconstruction of Vascular Tree
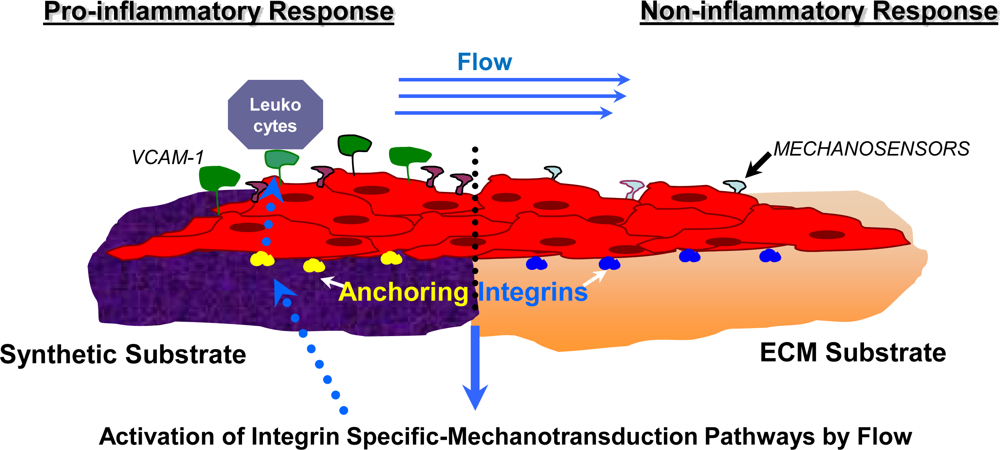
5.2. Mechanotransduction Pathways in the Healing Process of Vascular Graft
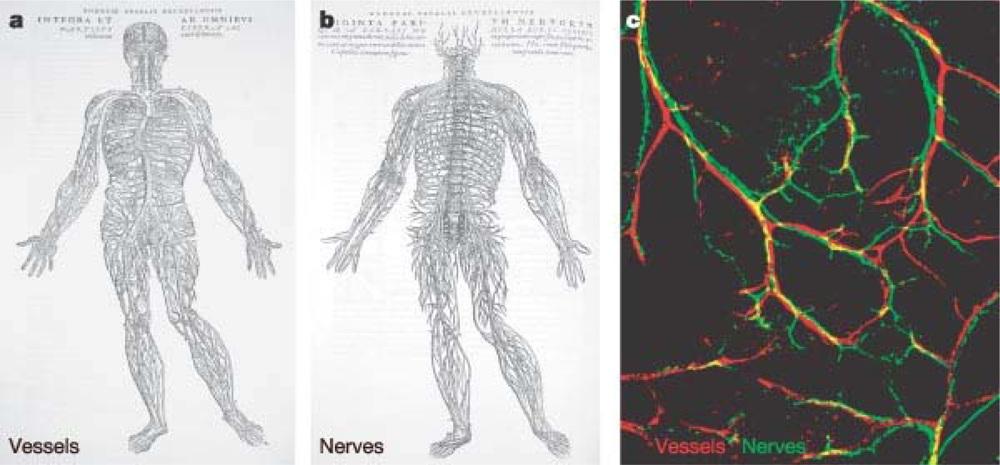
5.3. Restoration of Innervation and Blood Vessel Homeostasis
6. Future Perspectives for Cardiovascular Implants Based on ECM
7. Conclusions
Acknowledgments
References and notes
- Vascular Surgery: Principals and Practice; Hobson, RW; Wilson, SE; Veith, FJ (Eds.) McGraw-Hill: New York, NY, USA, 2003.
- Vascular Graft Update, ASTM STP 898; Kambic, HE; Kantrowitz, A; Sung, P (Eds.) American Society for Testing and Materials International: Philadelphia, PA, USA, 1986.
- Bezuidenhout, D; Zilla, P. Vascular grafts. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, GE, Bowlin, GL, Eds.; Informa Health Care: New York, NY, USA, 2008. [Google Scholar]
- Davis, L; Dower, T; Zilla, P. The lack of healing in conventional vascular grafts. In Tissue Engineering of Vascular Prosthetic Grafts; Zilla, P, Greisler, HP, Eds.; Landes Company: Austin, TX, USA, 1999; pp. 3–44. [Google Scholar]
- Greisler, HP. Characteristics and healing of vascular grafts. In Vascular Surgery: Theory and Practice; Callow, AD, Ernst, CB, Eds.; Appleton & Lange: Stamford (Conn), New York, NY, USA, 1995; pp. 1181–1212. [Google Scholar]
- Anderson, M. Procedures in the retrieval and evaluation of vascular grafts. In Vascular Graft Update: Safety and Performance; Kambic, HE, Kantrowitz, A, Sung, P, Eds.; American Society for Testing and Materials: Philadelphia, Pennsylvania, USA, 1986; pp. 156–165. [Google Scholar]
- Clowes, W; Gown, AM; Hanson, SR; Reidy, MA. Mechanisms of arterial graft failure. 1. Role of cellular proliferation in early healing of PTFE prostheses. Amer. J. Pathol 1985, 118, 43–54. [Google Scholar]
- Clowes, AW; Kinkman, TR; Reidy, MA. Mechanisms of arterial graft healing. A rapid transmural capillary ingrowth provides a source of intimal endothelium and smooth muscle in porous PTFE prostheses. Amer. J. Pathol 1986, 123, 220–230. [Google Scholar]
- Burkel, WE. The development of cellular linings in artificial vascular prostheses. In Biocompatible Polymers, Metals and Composites; Szycher, M, Ed.; Technomic Publ Co, Inc: Lancaster, PA, USA, 1983; pp. 165–178. [Google Scholar]
- Nanotechnology and Tissue Engineering: The scaffold; Laurencin, CT; Nair, L (Eds.) CRC Press: New York, NY, USA, 2008.
- Ratner, BD; Hoffman, AS; Schoen, FJ; Lemons, JE. Biomaterials Science: An Introduction to Materials and Medicine, 2nd ed; Elsevier: Oxford, UK, 2004. [Google Scholar]
- Biomaterials Engineering and Devices: Fundamentals and Vascular and Carrier Applications; Wise, DL (Ed.) Humana Press: Totowa, NJ, USA, 2000.
- Functional Materials and Biomaterials; Liu, XD (Ed.) Springer: Berlin, Germany, 2007.
- Szycher, M. Blood compatibility and vascular prostheses. In High Performance Biomaterials: A Complete Guide to Medical and Pharmceutical Applications; Szycher, M, Ed.; CRC Press, LLC: Boca Raton, FL, USA, 1991. [Google Scholar]
- Inayat-Hussain, S; Rajab, NF. In vitro testing of biomaterial toxicity and biocompatibility. In Cellular Response to Biomaterials; Di Silvio, L, Ed.; Woodhead Publishing Ltd: Cambridge, UK, 2008. [Google Scholar]
- Fortunato, JE; Glagov, S; Bassiouny, HS. Biomechanical factors as regulators of biological responses to vascular grafts. Semin. Vasc. Surg 1999, 12, 27–37. [Google Scholar]
- Badylak, SF; Freytes, DO; Gilbert, TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater 2009, 5, 1–13. [Google Scholar]
- Gilbert, TW; Sellaro, TL; Badylak, SF. Decellularisation of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar]
- Sandusky, GE; Lantz, GC; Badylak, SF. Healing comparison of small intestine submucosa and ePTFE grafts in the canine carotid artery. J. Surg. Res 1995, 58, 415–420. [Google Scholar]
- Badylak, SF; Coffey, AC; Lantz, GC; Tacker, WA; Geddes, LA. Comparison of the resistance to infection of intestinal submucosa arterial autografts versus polytetrafluoroethylene arterial prostheses in a dog model. J. Vasc. Surg 1994, 19, 465–472. [Google Scholar]
- Prevel, CD; Eppley, BL; McCarty, M; Jackson, JR; Voytik, SL; Hiles, MC; Badylak, SF. Experimental evaluation of small intestinal submucosa as a microvascular graft material. Microsurgery 1994, 15, 586–591. [Google Scholar]
- Lantz, GC; Badylak, SF; Hiles, MC; Coffey, AC; Geddes, LA; Kokini, K; Sandusky, GE; Morff, RJ. Small intestinal submucosa as a vascular graft: A review. J. Invest. Surg 1993, 6, 297–310. [Google Scholar]
- Hiles, MC; Badylak, SF; Geddes, LA; Kokini, K; Morff, RJ. Porosity of porcine small-intestinal submucosa for use as a vascular graft. J. Biomed. Mater. Res 1993, 27, 139–144. [Google Scholar]
- Lantz, GC; Badylak, SF; Coffey, AC; Geddes, LA; Sandusky, GE. Small intestinal submucosa as a superior vena cava graft in the dog. J. Surg. Res 1992, 53, 175–181. [Google Scholar]
- Badylak, SF; Lantz, GC; Coffey, A; Geddes, LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res 1989, 47, 74–80. [Google Scholar]
- Prasertsung, I; Kanokpanont, S; Bunaprasert, T; Thanakit, V; Damrongsakkul, S. Development of acellular dermis from porcine skin using periodic pressurized technique. J Biomed. Mater. Res. B Appl. Biomater 2008, 85, 210–219. [Google Scholar]
- Mirsch, MW, II; Schroeder, RFB; Illingworth, B; Borner, WH; Montoya, SI. Use of microorganisms for decellularizing bioprosthetic tissue. US Patent 6121041 2000. [Google Scholar]
- Rashid, ST; Salacinski, HJ; Hamilton, G; Seifalian, AM. The use of animal models in developing the discipline of cardiovascular tissue engineering: A review. Biomaterials 2004, 25, 1627–1637. [Google Scholar]
- Schmidt, CE; Baier, JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar]
- Lü, WD; Zhang, M; Wu, ZS; Hu, TH. Decellularized and photooxidatively crosslinked bovine jugular veins as potential tissue engineering scaffolds. Interact. CardioVasc. Thorac. Surg 2009, 8, 301–305. [Google Scholar]
- Derham, C; Yow, H; Ingram, J; Fisher, J; Ingham, E; Korrosis, SA; Homer-Vanniasinkam, S. Tissue engineering small-diameter vascular grafts: Preparation of a biocompatible porcine ureteric scaffold. Tissue Eng. Part A 2008, 14, 1871–1882. [Google Scholar]
- Wilshaw, SP; Kearney, J; Fisher, J; Ingham, E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng. Part A 2008, 14, 463–472. [Google Scholar]
- Nyland, J; Larsen, N; Burden, R; Chang, H; Caborn, DN. Biomechanical and tissue handling property comparison of decellularized and cryopreserved tibialis anterior tendons following extreme incubation and rehydration. Knee Surg. Sports Traumatol. Arthrosc 2009, 17, 83–91. [Google Scholar]
- Weadock, K; Olson, RM; Silver, FH. Evaluation of collagen crosslinking techniques. Biomater. Med. Devices Artif. Organs 1983, 11, 293–318. [Google Scholar]
- Sacks, MS; Hamamoto, H; Connolly, JM; Gorman, RC; Gorman, JH, III; Levy, RJ. In vivo biomechanical assessment of triglycidylamine crosslinked pericardium. Biomaterials 2007, 28, 5390–5398. [Google Scholar]
- Garcia, Y; Hemantkumar, N; Collighan, R; Griffin, M; Rodriguez-Cabello, JC; Pandit, A. In vitro characterization of a collagen scaffold enzymatically cross-linked with a tailored elastin-like polymer. Tissue Eng. Part A 2009, 15, 887–899. [Google Scholar]
- Somers, P; De Somer, F; Cornelissen, M; Bouchez, S; Gasthuys, F; Narine, K; Cox, E; van Nooten, G. Genipin blues: An alternative non-toxic crosslinker for heart valves? J. Heart Valve Dis 2008, 17, 682–688. [Google Scholar]
- Freytes, DO; Martin, J; Velankar, SS; Lee, AS; Badylak, SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 2008, 29, 1630–1637. [Google Scholar]
- Brockbank, KG; MacLellan, WR. Optimized preservation of extracellular matrix in cardiac tissues: Implications for long-term graft durability. Ann. Thorac. Surg 2007, 83, 1641–1650. [Google Scholar]
- Freytes, DO; Tullius, RS; Valentinm, JE; Stewart-Akers, AM; Badylak, SF. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J Biomed. Mater. Res. A 2008, 87, 862–872. [Google Scholar]
- Piterina, AV; Davis, LM; Meaney, CL; Walsh, MT; Badylak, SF; McGloughlin, TM. Characterisation of structural features a full-thickness acellular matrices deriving from animal organs. Proceedings of Bioengineering in Ireland 15, Limerick, Ireland, Jan 30–31; 2009. [Google Scholar]
- Piterina, AV; Callanan, A; Davis, LM; Meaney, CL; Walsh, MT; McGloughlin, TM. ECM matrices as an advanced scaffold for vascular tissue engineering. Bio—Med Mater Eng 2009. [Google Scholar]
- Piterina, AV; Davis, LM; Meaney, CL; Cloonan, AJ; Walsh, MT; McGloughlin, TM. Cell-seeded decellularised extracellular matrices as an advanced approach for tissue engineering. Proceedings of International Conference on Tissue Engineering ICTE, Leiria, Portugal, July 9–11, 2009.
- Brown, B; Lindberg, K; Reing, J; Stolz, DB; Badylak, SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng 2006, 12, 519–526. [Google Scholar]
- Hodde, J; Record, R; Tullius, R; Badylak, S. Fibronectin peptides mediate HMEC adhesion to porcine-derived extracellular matrix. Biomaterials 2002, 23, 1841–1848. [Google Scholar]
- Hodde, JP; Record, RD; Liang, HA; Badylak, SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium 2001, 8, 11–24. [Google Scholar]
- Helton, WS; Fisichella, PM; Berger, R; Horgan, S; Espat, NJ; Abcarian, H. Short-term outcomes with small intestinal submucosa for ventral abdominal hernia. Arch. Surg 2005, 140, 549–562. [Google Scholar]
- Badylak, SF; Vorp, DA; Spievack, AR; Simmons-Byrd, A; Hanke, J; Freytes, DO; Thapa, A; Gilbert, TW; Nieponice, A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J. Surg. Res 2005, 128, 87–97. [Google Scholar]
- Rosalia, M; Mark, PC; Anthony, JC; Martin, K; Kirstan, KM; Richard, CR. Small intestinal submucosa bladder neck slings for incontinence associated with neuropathic bladder. J. Urology 2005, 174, 1680–1682. [Google Scholar]
- Malcarney, HL; Bonar, F; Murrell, GAC. Early Inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant. Am. J. Sport Med 2005, 33, 907–911. [Google Scholar]
- El-Assmy, A; Hafez, AT; El-Sherbiny, MT; El-Hamid, MA; Mohsen, T; Nour, EM; Bazeed, M. Use of single layer small intestinal submucosa for long segment ureteral replacement: A pilot study. J. Urology 2004, 171, 1939–1942. [Google Scholar]
- De Ugarte, DA; Choi, E; Weitzbuch, H; Wulur, I; Caulkins, C; Wu, B; Fonkalsrud, EW; Atkinson, JB; Dunn, JCY. Mucosal regeneration of a duodenal defect using small intestine submucosa. Am. Surgeon 2004, 70, 49–51. [Google Scholar]
- Jones, JS; Rackley, RR; Berglund, R; Abdelmalak, JB; Deorco, G; Vasavada, SP. Porcine small intestinal submucosa as a percutaneous mid-urethral sling: 2-year results. BJU Int 2005, 96, 103–106. [Google Scholar]
- Ziats, NP; Miller, KM; Anderson, JM. In vitro and in vivo interactions of cells with biomaterials. Biomaterials 1988, 9, 5–13. [Google Scholar]
- Tang, L; Ugarova, TP; Plow, EF; Eaton, JW. Molecular determinants of acute inflammatory responses to biomaterials. J. Clin. Invest 1996, 97, 1329–1334. [Google Scholar]
- Anderson, JM; Rodriguez, A; Chang, DT. Foreign body reaction to biomaterials. Semin. Immunol 2008, 20, 86–100. [Google Scholar]
- Janatova, J. Activation and control of complement, inflammation, and infection associated with the use of biomedical polymers. ASAIO J 2000, 46, 53–62. [Google Scholar]
- Xia, Z; Triffitt, JT. A review on macrophage responses to biomaterials. Biomed. Mater 2006, 1, 1–9. [Google Scholar]
- Anderson, JM; Miller, KM. Biomaterial biocompatibility and the macrophage. Biomaterials 1984, 5, 5–10. [Google Scholar]
- Valentin, JE; Stewart-Akers, AM; Gilbert, TW; Badylak, SF. Macrophage participation in the degradation and remodeling of ECM scaffolds. Tissue Eng Part A 2009, 15. [Google Scholar]
- Mensik, A; Brouwer, A; van den Burg, EH; Geurts, S; Jongen, WMF; Lakemond, CMM; Meijerman, I; van der Wijk, T. Modulation of intercellular communication between smooth muscle cells by growth factors and cytokines. Eur. J. Pharmacol 1996, 310, 73–81. [Google Scholar]
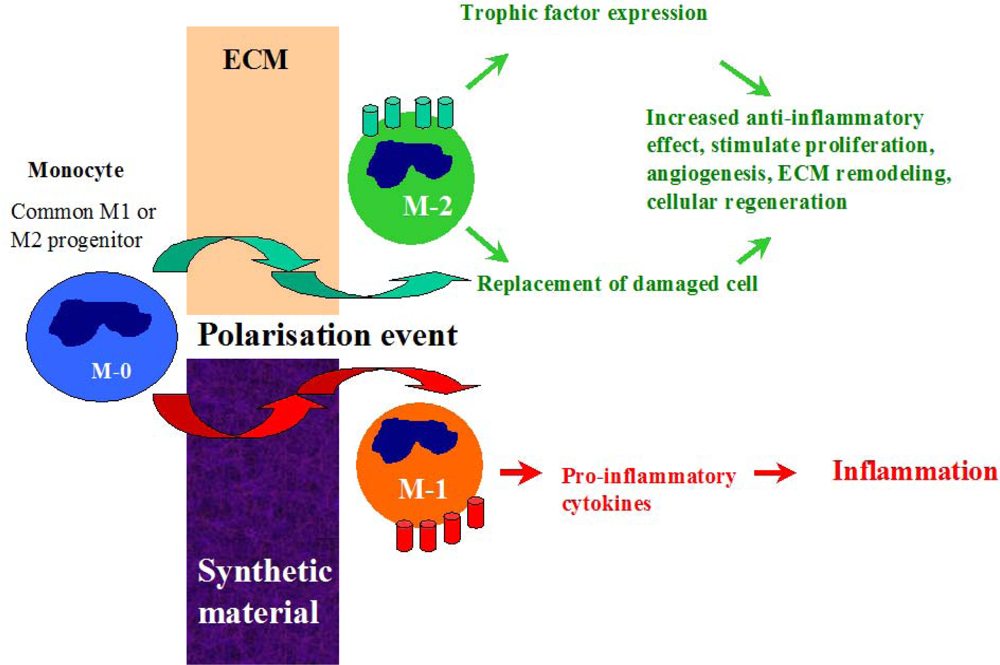
- Mills, D; Kincaid, K; Alt, JM; Heilman, MJ; Hill, AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol 2000, 164, 6166–6173. [Google Scholar]
- Badylak, SF; Gilbert, TW. Immune response to biologic scaffold materials. Semin. Immunol 2008, 20, 109–116. [Google Scholar]
- Rehman, J; Li, J; Orschell, CM; March, KL. Peripheral blood ‘endothelial progenitor cells’ are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003, 107, 1164–1169. [Google Scholar]
- Palmer, EM; Beilfuss, BA; Nagai, T; Semnani, RT; Badylak, SF; van Seventer, GA. Human helper T cell activation and differentiation is suppressed by porcine small intestinal submucosa. Tissue Eng 2002, 8, 893–900. [Google Scholar]
- Allman, AJ; McPherson, TB; Merrill, LC; Badylak, SF; Metzger, DW. The Th2-restricted immune response to xenogeneic small intestinal submucosa does not influence systemic protective immunity to viral and bacterial pathogens. Tissue Eng 2002, 8, 53–62. [Google Scholar]
- Brodbeck, WG; Voskerician, G; Ziats, NP; Nakayama, Y; Matsuda, T; Anderson, JM. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J. Biomed. Mater. Res. A 2003, 64, 320–329. [Google Scholar]
- Zetrenne, E; McIntosh, BC; McRae, MH; Gusberg, R; Evans, GR; Narayan, D. Prosthetic vascular graft infection: A multi-center review of surgical management. Yale J. Biol. Med 2007, 80, 113–121. [Google Scholar]
- Chiesa, R; Astore, D; Frigerio, S; Garriboli, L; Piccolo, G; Castellano, R; Scalamogna, M; Odero, A; Pirrelli, S; Biasi, G; Mingazzini, P; Biglioli, P; Polvani, G; Guarino, A; Agrifoglio, G; Tori, A; Spina, G. Vascular prosthetic graft infection: Epidemiology, bacteriology, pathogenesis and treatment. Acta Chir. Belg 2002, 102, 238–247. [Google Scholar]
- Mertens, RA; O’Hara, PJ; Hertzer, NR; Krajewski, LP; Beven, EG. Surgical management of infrainguinal arterial prosthetic graft infections: Review of a 35-year experience. J. Vasc. Surg 1995, 21, 782–791. [Google Scholar]
- Bunt, TJ. Vascular graft infections: An update. Cardiovasc. Surg 2001, 9, 225–233. [Google Scholar]
- Mermel, LA; Farr, BM; Sheretz, RJ; Raad, II; O’Grady, N; Harris, JS; Craven, DE. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis 2001, 32, 1249–1272. [Google Scholar]
- Perera, GB; Fujitani, RM; Kubaska, SM. Aortic graft infection: Update on management and treatment options. Vasc. Endovasc. Surg 2006, 40, 1–10. [Google Scholar]
- Sharp, WJ; Hoballah, JJ; Mohan, CR; Kresowik, TF; Martinasevic, M; Chalmers, RTA; Corson, JD. The management of the infected aortic prosthesis: A current decade of experience. J. Vasc. Surg 1994, 19, 844–850. [Google Scholar]
- Swain, TW, III; Calligaro, KD; Dougherty, MD. Management of infected aortic prosthetic grafts. Vasc. Endovasc. Surg 2004, 38, 75–82. [Google Scholar]
- Padberg, FT, Jr; Calligaro, KD; Sidawy, AN. Complications of arteriovenous hemodialysis access: Recognition and management. J. Vasc. Surg 2008, 48, S55–S80. [Google Scholar]
- Holland, FW; Darling, RC, III; Chang, BB; Shah, DM; Leather, RP. Clostridial aortic graft infection. Ann. Vasc. Surg 1994, 8, 387–389. [Google Scholar]
- Upchurch, GR, Jr; Clair, DG; Whittemore, AD; Mannick, JA. Clostridium septicum bacteremia associated with aortic graft infection. J. Vasc. Surg 1995, 22, 493–495. [Google Scholar]
- Lephart, P; Ferrieri, P; van Burik, JA. Reservoir of Candida albicans infection in a vascular bypass graft demonstrates a stable karyotype over six months. Med. Mycol 2004, 42, 255–260. [Google Scholar]
- van Dijk, J; Herkströter, F; Busscher, H; Weerkamp, A; Jansen, H; Arends, J. Surface-free energy and bacterial adhesion. An in vivo study in beagle dogs. J. Clin. Periodontol 1987, 14, 300–304. [Google Scholar]
- Wadström, T. Molecular aspects of bacterial adhesion, colonization, and development of infections associated with biomaterials. J. Invest. Surg 1989, 2, 353–360. [Google Scholar]
- Fleer, A; Verhoef, J. An evaluation of the role of surface hydrophobicity and extracellular slime in the pathogenesis of foreign-body-related infections due to coagulase-negative staphylococci. J. Invest. Surg 1989, 2, 391–396. [Google Scholar]
- Jansen, B; Schumacher-Perdreau, F; Peters, G; Pulverer, G. New aspects in the pathogenesis and prevention of polymer-associated foreign-body infections caused by coagulase-negative staphylococci. J. Invest. Surg 1989, 2, 361–380. [Google Scholar]
- Hussain, M; Wilcox, MH; White, PJ. The slime of coagulase-negative staphylococci: Biochemistry and relation to adherence. FEMS Microbiol. Rev 1993, 10, 191–207. [Google Scholar]
- Baselga, R; Albizu, I; De La Cruz, M; Del Cacho, E; Barberan, M; Amorena, B. Phase variation of slime production in Staphylococcus aureus: Implications in colonization and virulence. Infect. Immun 1993, 61, 4857–4862. [Google Scholar]
- Christensen, GD; Simpson, WA; Bisno, AL; Beachey, EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun 1982, 37, 318–326. [Google Scholar]
- Kristinsson, KG; Spencer, RC. Slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J. Infect. Dis 1986, 154, 728–729. [Google Scholar]
- Gross, M; Cramton, SE; Gotz, F; Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun 2001, 69, 3423–3426. [Google Scholar]
- Treiman, GS; Copland, S; Yellin, AE; Lawrence, PF; McNamara, RM; Treiman, RL. Wound infections involving infrainguinal autogenous vein grafts: A current evaluation of factors determining successful graft preservation. J. Vasc. Surg 2001, 33, 948–954. [Google Scholar]
- Donlan, RM; Costerton, JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev 2002, 15, 167–193. [Google Scholar]
- Gilbert, P; Maira-Litran, T; McBain, AJ; Rickard, AH; Whyte, FW. The physiology and collective recalcitrance of microbial biofilm communities. Adv. Microb. Physiol 2002, 46, 202–256. [Google Scholar]
- Ito, A; Taniuchi, A; May, T; Kawata, K; Okabe, S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol 2009, 75, 4093–4100. [Google Scholar]
- Aslam, S. Effect of antibacterials on biofilms. Am J Infect Control 2008, 36, S175e9–175e11. [Google Scholar]
- Simões, M; Simões, LC; Vieira, MJ. Species association increases biofilm resistance to chemical and mechanical treatments. Water Res 2009, 43, 229–237. [Google Scholar] [Green Version]
- Anderson, GG; O’Toole, GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol 2008, 322, 85–105. [Google Scholar]
- Lewis, K. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol 2008, 322, 107–131. [Google Scholar]
- Dofferhoff, AS; Nijland, JH; de Vries-Hospers, HG; Mulder, PO; Weits, J; Bom, VJ. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: An in-vitro and in-vivo study. Scand. J. Infect. Dis 1991, 23, 745–754. [Google Scholar]
- Prins, JM; van Deventer, SJ; Kuijper, EJ; Speelman, P. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob. Agents Ch 1994, 38, 1211–1218. [Google Scholar]
- Kirikae, T; Nakano, M; Morrison, DC. Antibiotic-induced endotoxin release from bacteria and its clinical significance. Microbiol. Immunol 1997, 41, 285–294. [Google Scholar]
- Hurley, JC. Antibiotic-induced release of endotoxin: A reappraisal. Clin. Infect. Dis 1992, 15, 840–854. [Google Scholar]
- Nau, R; Eiffert, H. Minimizing the release of proinflammatory and toxic bacterial products within the host: A promising approach to improve outcome in life-threatening infections. FEMS Immunol. Med. Microbiol 2005, 44, 1–16. [Google Scholar]
- Nagase, H; Woessner, F. Matrix metalloproteinases. J. Biol. Chem 1999, 274, 21491–21494. [Google Scholar]
- Young, RM; Cherry, KJ, Jr; Davis, PM; Gloviczki, P; Bower, TC; Panneton, JM; Hallett, JW, Jr. The results of in situ prosthetic replacement for infected aortic grafts. Am. J. Surg 1999, 178, 136–140. [Google Scholar]
- Wilson, SE. New alternatives in management of the infected vascular prosthesis. Surg. Infect 2001, 2, 171–177. [Google Scholar]
- Greco, RS; Harvey, RA; Smilow, PC; Tesoriero, JV. Prevention of vascular prosthetic infection by a benzalkonium-oxacillin bonded polytetrafluoroethylene graft. Surg. Gyn. Obstet 1982, 155, 28–32. [Google Scholar]
- Sobinsky, KR; Flanigan, P. Antibiotic binding to polytetrafluoroethylene via glucosaminoglycan-keratin luminal coating. Surgery 1986, 100, 629–633. [Google Scholar]
- Shenk, JS; Ney, AL; Tsukayama, DT; Olson, ME; Bubrick, MP. Tobramycin-adhesive in preventing and treating PTFE vascular graft infections. J. Surg. Res 1989, 47, 487–492. [Google Scholar]
- Ney, AL; Kelly, PH; Tsukayama, DT; Bubrick, MP. Fibrin glue-antibiotic suspension in the prevention of prosthetic graft infection. J. Trauma 1990, 30, 1000–1006. [Google Scholar]
- Haverich, A; Hirt, S; Karck, M; Siclari, F; Wahlig, H. Prevention of graft infection by bonding of gentamycin to Dacron prostheses. J. Vasc. Surg 1992, 15, 187–193. [Google Scholar]
- Gahtan, V; Esses, GE; Bandyk, DF; Nelson, RT; Dupont, E; Mills, J. Antistaphylococcal activity of rifampin-bonded gelatin-impregnated Dacron grafts. J. Surg. Res 1995, 58, 105–110. [Google Scholar]
- Sago, T; Mori, Y; Takagi, H; Iwata, H; Murase, K; Kawamura, Y; Hirose, H. Local treatment of Dacron patch graft contaminated with Staphylococcus aureus with antibiotic-releasing porous apatite ceramic: An experimental study in the rabbit. J. Vasc. Surg 2003, 37, 169–174. [Google Scholar]
- Darouiche, RO; Mansouri, MD. In vitro activity and in vivo efficacy of antimicrobial-coated vascular grafts. Ann. Vasc. Surg 2004, 18, 497–501. [Google Scholar]
- Kinney, EV; Bandyk, DF; Seabrook, GA; Kelly, HM; Towne, JB. Antibiotic-bonded PTFE vascular grafts: The effect of silver antibiotic on bioactivity following implantation. J. Surg. Res 1991, 50, 430–435. [Google Scholar]
- Hernandez-Richter, T; Schardey, HM; Löhlein, F; Heiss, MM; Redondo-Müller, M; Hammer, C; Schildberg, FW. The prevention and treatment of vascular graft infection with a triclosan (Irgasan)-bonded Dacron graft: An experimental study in the pig. Eur. J. Vasc. Endovasc. Surg 2000, 20, 413–418. [Google Scholar]
- Ghiselli, R; Giacometti, A; Cirioni, O; Mocchegiani, F; Orlando, F; Kamysz, W; Del Prete, MS; Lukasiak, J; Scalise, G; Saba, V. Temporin A as a prophylactic agent against methicillin sodium susceptible and methicillin sodium resistant Staphylococcus epidermidis vascular graft infection. J. Vasc. Surg 2002, 36, 1027–1030. [Google Scholar]
- Ginalska, G; Kowalczuk, D; Osinska, M. A chemical method of gentamicin bonding to gelatine-sealed prosthetic vascular grafts. Int. J. Pharm 2005, 288, 131–140. [Google Scholar]
- Ginalska, G; Osinska, M; Uryniak, A; Urbanik-Sypniewska, T; Belcarz, A; Rzeski, W; Wolski, A. Antibacterial activity of gentamicin-bonded gelatin-sealed polyethylene terephtalate vascular prostheses. Eur. J. Vasc. Endovasc. Surg 2005, 29, 419–424. [Google Scholar]
- Sacar, M; Scar, S; Kaleli, I; Onem, G; Turgut, H; Goksin, I; Ozcan, V; Inan, BK; Duver, H; Baltalari, A. Linezolid alone and in combination with rifampicin prevents experimental vascular graft infection due to methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. J. Surg. Res 2007, 139, 170–175. [Google Scholar]
- Schmacht, D; Armstrong, P; Johnson, B; Pierre, K; Back, M; Honeyman, A; Cuthbertson, D; Bandyk, D. Graft infectivity of rifampin and silver-bonded polyester grafts to MRSA contamination. Vasc. Endo Surg 2005, 39, 411–420. [Google Scholar]
- Hirose, K; Marui, A; Arai, Y; Nomura, T; Inoue, S; Kaneda, K; Kamitani, T; Fujita, M; Mitsuyama, M; Tabata, Y; Komeda, M. Sustained-release vancomycin sheet may help to prevent prosthetic graft methicillin-resistant Staphylococcus aureus infection. J. Vasc. Surg 2006, 44, 377–382. [Google Scholar]
- Hardman, S; Cope, A; Swann, A; Bell, PRF; Naylor, AR; Hayes, PD. An in vitro model to compare the antimicrobial activity of silver-coated versus rifampicin-soaked vascular grafts. Ann. Vasc. Surg 2004, 18, 308–313. [Google Scholar]
- Batt, M; Magne, JL; Alric, P; Muzj, A; Ruotolo, C; Ljungstrom, KG; Garcia-Casas, R; Simms, M. In situ revascularization with silver-coated polyester grafts to treat aortic infection: Early and midterm results. J. Vasc. Surg 2003, 38, 983–989. [Google Scholar]
- Ueberrueck, T; Zippel, R; Tautenhahn, J; Gastinger, I; Lippert, H; Wahlers, T. Vascular graft infections: In vitro and in vivo investigations of a new vascular graft with long-term protection. J. Biomed. Mater. Res. B Appl. Biomat 2005, 74, 601–607. [Google Scholar]
- Hernandez-Richter, T; Schardey, HM; Wittmann, F; Mayr, S; Schmitt-Sody, M; Blasenbreu, S; Heiss, MM; Gabka, C; Angele, MK. Rifampin and triclosan but not silver is effective in preventing bacterial infection of vascular Dacron graft material. Eur. J. Vasc. Endovasc. Surg 2003, 26, 550–557. [Google Scholar]
- Gomez-Lus, R. Evolution of bacterial resistance to antibiotics during the last three decades. Int. Microbiol 1998, 1, 279–284. [Google Scholar]
- Brennan, EP; Reing, J; Chew, D; Myers-Irvin, JM; Young, EJ; Badylak, SF. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng 2006, 12, 2949–2955. [Google Scholar]
- Sarikaya, A; Record, R; Wu, CC; Tullius, B; Badylak, S; Ladisch, M. Antimicrobial activity associated with extracellular matrices. Tissue Eng 2002, 8, 63–71. [Google Scholar]
- Badylak, SF; Coffey, AC; Lantz, GC; Tacker, WA; Geddes, LA. Comparison of the resistance to infection of intestinal submucosa arterial autografts versus polytetrafluoroethylene arterial prostheses in a dog model. J. Vasc. Surg 1994, 19, 465–472. [Google Scholar]
- Badylak, SF; Wu, CC; Bible, M; McPherson, E. Host protection against deliberate bacterial contamination of an extracellular matrix bioscaffold versus Dacron mesh in a dog model of orthopedic soft tissue repair. J. Biomed. Mater. Res. B Appl. Biomater 2003, 67, 648–654. [Google Scholar]
- Steinstraesser, L; Koehler, T; Jacobsen, F; Daigeler, A; Goertz, O; Langer, S; Kesting, M; Steinau, H; Eriksson, E; Hirsch, T. Host defense peptides in wound healing. Mol. Med 2008, 14, 528–537. [Google Scholar]
- Hirsch, T; Metzig, M; Niederbichler, A; Steinau, HU; Eriksson, E; Steinstraesser, L. Role of host defense peptides of the innate immune response in sepsis. Shock 2008, 30, 117–126. [Google Scholar]
- Nuding, S; Zabel, LT; Enders, C; Porter, E; Fellermann, K; Wehkamp, J; Mueller, HA; Stange, EF. Antibacterial activity of human defensins on anaerobic intestinal bacterial species: A major role of HBD-3. Microbes Infect 2009, 11, 384–393. [Google Scholar]
- Brown, KL; Hancock, RE. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol 2006, 18, 24–30. [Google Scholar]
- Gallo, RL; Murakami, M; Ohtake, T; Zaiou, M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol 2002, 110, 823–831. [Google Scholar]
- Alobaid, N; Alnaeb, ME; Sales, KM; Seifalian, AM; Mikhailidis, DP; Hamilton, G. Endothelial progenitor cells and their potential clinical applications in peripheral arterial disease. Endothelium 2005, 12, 243–250. [Google Scholar]
- Miller-Kasprzak, E; Jagodziński, PP. Endothelial progenitor cells as a new agent contributing to vascular repair. Arch. Immunol. Ther. Exp 2007, 55, 247–259. [Google Scholar]
- Hristov, M; Weber, C. Endothelial progenitor cells: Characterization, pathophysiology, and possible clinical relevance. J. Cell Mol. Med 2004, 8, 498–508. [Google Scholar]
- Hristov, M; Erl, W; Weber, PC. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler Thromb. Vasc. Biol 2003, 23, 1185–1189. [Google Scholar]
- Yasu, T. Differentiation of endothelial progenitor cells. Circ. J 2009, 73, 1199–1200. [Google Scholar]
- Pelliccia, F; Pasceri, V; Cianfrocca, C; Vitale, C; Pristipino, C; Speciale, G; Mercuro, G; Rosano, G. Endothelial progenitor cells in patients with coronary artery disease and left ventricular dysfunction. Coron Artery Dis 2009. [Google Scholar]
- Zilla, P; Bezuidenhout, D; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterial 2007, 28, 5009–5027. [Google Scholar]
- Bordenave, L; Fernandez, P; Rémy-Zolghadri, M; Villars, S; Daculsi, R; Midy, D. In vitro endothelialized ePTFE prostheses: Clinical update 20 years after the first realization. Clin. Hemorheol. Microcirc 2005, 33, 227–234. [Google Scholar]
- Bhat, VD; Klitzman, B; Koger, K; Truskey, GA; Reichert, WM. Improving endothelial cell adhesion to vascular graft surfaces: Clinical need and strategies. J. Biomater. Sci. Polym. Ed 1998, 9, 1117–1135. [Google Scholar]
- Xiao, L; Shi, D. Role of precoating in artificial vessel endothelialization. Chin. J. Traumatol 2004, 7, 312–316. [Google Scholar]
- Salacinski, HJ; Tiwari, A; Hamilton, G; Seifalian, AM. Cellular engineering of vascular bypass grafts: Role of chemical coatings for enhancing endothelial cell attachment. Med. Biol. Eng. Comput 2001, 39, 609–618. [Google Scholar]
- Shaikh, FM; Callanan, A; Kavanagh, EG; Burke, PE; Grace, PA; McGloughlin, TM. Fibrin: A natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs 2008, 188, 333–346. [Google Scholar]
- Alobaid, N; Salacinski, HJ; Sales, KM; Hamilton, G; Seifalian, AM. Single stage cell seeding of small diameter prosthetic cardiovascular grafts. Clin. Hemorheol. Microcirc 2005, 33, 209–226. [Google Scholar]
- Walluscheck, KP; Steinhoff, G; Haverich, A. Endothelial cell seeding of de-endothelialised human arteries: Improvement by adhesion molecule induction and flow-seeding technology. Eur. J. Vasc. Endovasc. Surg 1996, 12, 46–53. [Google Scholar]
- Walluscheck, KP; Steinhoff, G; Haverich, A. Endothelial cell seeding of native vascular surfaces. Eur. J. Vasc. Endovasc. Surg 1996, 11, 290–303. [Google Scholar]
- Bordenave, L; Rémy-Zolghadri, M; Fernandez, P; Bareille, R; Midy, D. Clinical performance of vascular grafts lined with endothelial cells. Endothelium 1999, 6, 267–275. [Google Scholar]
- Falk, J; Townsend, LE; Vogel, LM; Boyer, M; Olt, S; Wease, GL; Trevor, KT; Seymour, M; Glover, JL; Bendick, PJ. Improved adherence of genetically modified endothelial cells to small-diameter expanded polytetrafluoroethylene grafts in a canine model. J. Vasc. Surg 1998, 27, 902–908. [Google Scholar]
- Rotmans, JI; Heyligers, JM; Stroes, ES; Pasterkamp, G. Endothelial progenitor cell-seeded grafts: Rash and risky. Can. J. Cardiol 2006, 22, 1113–1116. [Google Scholar]
- Reing, JE; Zhang, L; Myers-Irvin, J; Cordero, KE; Freytes, DO; Heber-Katz, E; Bedelbaeva, K; McIntosh, D; Dewilde, A; Braunhut, SJ; Badylak, SF. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng. Part A 2009, 15, 605–614. [Google Scholar]
- Brennan, EP; Tang, XH; Stewart-Akers, AM; Gudas, LJ; Badylak, SF. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J. Tissue Eng. Regen Med 2008, 2, 491–498. [Google Scholar]
- de Mel, A; Jell, G; Stevens, MM; Seifalian, AM. Biofunctionalization of biomaterials for accelerated in situ endothelialization: A review. Biomacromolecules 2008, 9, 2969–2979. [Google Scholar]
- Prasad, CK; Krishnan, LK. Regulation of endothelial cell phenotype by biomimetic matrix coated on biomaterials for cardiovascular tissue engineering. Acta Biomater 2008, 4, 182–191. [Google Scholar]
- Alobaid, N; Salacinski, HJ; Sales, KM; Ramesh, B; Kannan, RY; Hamilton, G; Seifalian, AM. Nanocomposite containing bioactive peptides promote endothelialisation by circulating progenitor cells: An in vitro evaluation. Eur. J. Vasc. Endovasc. Surg 2006, 32, 76–83. [Google Scholar]
- Wijelath, E; Rahman, S; Murray, J; Patel, Y; Savidge, G; Sobel, M. Fibronectin promotes VEGF-induced CD34 cell differentiation into endothelial cells. J. Vasc. Surg 2004, 39, 655–660. [Google Scholar]
- Schneider, A; Chandra, M; Lazarovici, G; Vlodavsky, I; Merin, G; Uretzky, G; Borman, JB; Schwalb, H. Naturally produced extracellular matrix is an excellent substrate for canine endothelial cell proliferation and resistance to shear stress on PTFE vascular grafts. Thromb. Haemost 1997, 78, 1392–1398. [Google Scholar]
- Pollara, P; Alessandri, G; Bonardelli, S; Simonini, A; Cabibbo, E; Portolani, N; Tiberio, GA; Giulini, SM; Turano, A. Complete in vitro prosthesis endothelialization induced by artificial extracellular matrix. J. Invest. Surg 1999, 12, 81–88. [Google Scholar]
- Temple, WJ; Voitk, AJ; Snelling, CF; Crispin, JS. Effect of nutrition, diet and suture material on long term wound healing. Ann. Surg 1975, 182, 93–97. [Google Scholar]
- Say, J. The metabolic changes associated with trauma and sepsis. Nurs. Crit. Care 1997, 2, 83–87. [Google Scholar]
- Wilmore, DW. Metabolic response to severe surgical illness: Overview. World J. Surg 2000, 24, 705–711. [Google Scholar]
- Williams, J; Barbul, A. Nutrition and wound healing. Surg. Clin. North Am 2003, 83, 71–96. [Google Scholar]
- Demling, R. Anticatabolic and anabolic strategies in critical illness. Shock 1998, 10, 155–160. [Google Scholar]
- MacKay, P; Miller, J. Nutritional support for wound healing. Altern. Med. Rev 2003, 8, 359–362. [Google Scholar]
- Demling, RH. Nutrition, anabolism, and the wound healing process: An overview. Eplasty 2009, e9, 65–94. [Google Scholar]
- Trujillo, EB. Effects of nutritional status on wound healing. J. Vasc. Nurs 1993, 11, 12–18. [Google Scholar]
- Kiyama, T; Witte, MB; Thornton, FJ; Barbul, A. The route of nutrition support affects the early phase of wound healing. J. Parenter. Enter. Nutr 1998, 22, 276–279. [Google Scholar]
- Stotts, NA; Washington, DF. Nutrition: A critical component of wound healing. AACN Clin. Issues Crit. Care Nurs 1990, 1, 585–594. [Google Scholar]
- Wray, C; Mammen, J; Hasselgren, P. Catabolic response to stress and potential benefits of nutritional support. Nutrition 2002, 18, 971–977. [Google Scholar]
- Swinburn, BA; Ravussin, E. Energy and macronutrient metabolism. Clin. Endocrinol. Metab 1994, 8, 527–548. [Google Scholar]
- Salacinski, HJ; Goldner, S; Giudiceandrea, A; Hamilton, G; Seifalian, AM; Edwards, A; Carson, RJ. The Mechanical Behaviour of Vascular Grafts: A Review. J. Biomater. Appl 2001, 15, 241–278. [Google Scholar]
- Haruguchi, H; Teraoka, S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: A review. J. Artif. Organs 2003, 6, 227–235. [Google Scholar]
- Arndt, JO; Klauski, J; Hersch, F. The diameter of the intact carotid artery in man. Pfluegers Arch 1968, 301, 230–240. [Google Scholar]
- Patel, DF; Greenfield, JC; Fry, DL. In vitro pressure-length-radius relationships of certain blood vessels in man and dog. In Pulsatile Blood Flow; Attinger, EO, Ed.; Blahiston-McGraw Hill: New York, NY, USA, 1963; pp. 293–305. [Google Scholar]
- Roeder, R; Wolfe, J; Lianakis, N; Hinson, T; Geddes, LA; Obermiller, J. Compliance, elastic modulus and burst pressure of small-intestine submucosa (SIS), small-diameter vascular grafts. J. Biomed. Mater. Res 1999, 47, 65–70. [Google Scholar]
- Jacot, JG; Abdullah, I; Belkin, M; Gerhard-Herman, M; Gaccione, P; Polak, JF; Donaldson, MC; Whittemore, AD; Conte, MS. Early adaptation of human lower extremity vein grafts: Wall stiffness changes accompany geometric remodeling. J. Vasc. Surg 2004, 39, 547–555. [Google Scholar]
- Sawyer, PN. Modern Vascular Grafts; McGraw-Hill, Inc.: New York, NY, USA, 1987; p. 326. [Google Scholar]
- Hasegawa, M; Azuma, T. Mechanical properties of synthetic arterial grafts. J. Biomech 1979, 12, 509–517. [Google Scholar]
- Stewart, SFC; Lyman, DJ. Essential physical characteristics of vascular grafts. In Modern Vascular Grafts; Sawyer, PN, Ed.; McGraw-Hill, Inc.: New York, NY, USA, 1987; pp. 117–121. [Google Scholar]
- Freytes, DO; Badylak, SF; Webster, TJ; Geddes, LA; Rundell, AE. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials 2004, 25, 2353–2361. [Google Scholar]
- Herbert, ST; Badylak, SF; Geddes, LA; Hillberry, B; Lantz, GC; Kokini, K. Elastic modulus of prepared canine jejenum, a new vascular graft material. Ann. Biomed. Eng 1993, 21, 727–733. [Google Scholar]
- Freytes, DO; Stoner, RM; Badylak, SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. Mater. Res. Part B: Appl. Biomater 2008, 84B, 408–414. [Google Scholar]
- Callanan, A; Biggins, EM; Badylak, SF; McGloughlin, TM. The influence of endothelial cell attachment to UBM extracellular matrix on the regulation of matrix metalloproteinase’s (MMP’s) and degradation. Proceedings of Biologic Scaffolds for Regenerative Medicine; -Arizona Symposium, Phoenix, AZ, USA, February 15–16th; 2008. [Google Scholar]
- Roeder, RA; Lantz, GC; Geddes, LA. Mechanical remodeling of small-intestine submicosa small–diameter vascular graft—A preliminary report. Biomed. Instrum. Technol 2001, 35, 110–120. [Google Scholar]
- Grimes, M; Pembroke, JT; McGloughlin, TM. The effect of choice of sterilisation method on the biocompatibility and biodegradability of SIS (Small Intestinal Submucosa). Bio.—Med. Mater. Eng 2005, 15, 65–71. [Google Scholar]
- O’Brien, TP; Grace, P; Walsh, M; Burke, P; McGloughlin, T. Computational investigations of a new prosthetic femoral-popliteal bypass graft design. J. Vasc. Surg 2005, 42, 1169–1175. [Google Scholar]
- Rickard, RF; Meyer, C; Hudson, DA. Computational modeling of microarterial anastomoses with size discrepancy (small-to-large). J. Surg. Res 2009, 153, 1–11. [Google Scholar]
- Callanan, A; Morris, L; Badylak, SF; McGloughlin, TM. MMP and VEGF regulation in endothelial cell-seeded UBM extracellular matrix in a bioreactor. Proceeding of European Society of Biomechanics (ESB), Lucerne, Switzerland, 6–9 July, 2008.
- Mitsuoka, H; Kitamura, S; Kuwahara, K; Unno, N. Impact of in vivo ranges of the variances in the flow velocity waveforms and flow split ratio on the hemodynamic effects of the anastomotic cuff at distal end-to-side anastomosis. Surg. Today 2006, 36, 769–774. [Google Scholar]
- Longest, PW; Kleinstreuer, C. Numerical simulation of wall shear stress conditions and platelet localization in realistic end-to-side arterial anastomoses. J. Biomech. Eng 2003, 125, 671–681. [Google Scholar]
- Noori, N; Scherer, R; Perktold, K; Czerny, M; Karner, G; Trubel, M; Polterauer, P; Schima, H. Blood flow in distal end-to-side anastomoses with PTFE and a venous patch: Results of an in vitro flow visualisation study. Eur. J. Vasc. Endovasc. Surg 1999, 18, 191–200. [Google Scholar]
- Lei, M; Archie, JP; Kleinstreuer, C. Computational design of a bypass graft that minimizes wall shear stress gradients in the region of the distal anastomosis. J. Vasc. Surg 1997, 25, 637–646. [Google Scholar]
- Hofer, M; Rappitsch, G; Perktold, K; Trubel, W; Schima, H. Numerical study of wall mechanics and fluid dynamics in end-to-side anastomoses and correlation to intimal hyperplasia. J. Biomech 1996, 29, 1297–1308. [Google Scholar]
- Zhang, L; Moskovitz, M; Piscatelli, S; Longaker, MT; Siebert, JW. Hemodynamic study of different angled end-to-side anastomoses. Microsurgery 1995, 16, 114–117. [Google Scholar]
- Migliavacca, F; Dubini, G. Computational modeling of vascular anastomoses. Biomech. Model. Mechanobiol 2005, 3, 235–250. [Google Scholar]
- Jackson, MJ; Bicknell, CD; Zervas, V; Cheshire, NJ; Sherwin, SJ; Giordana, S; Peiró, J; Papaharilaou, Y; Doorly, DJ; Caro, CG. Three-dimensional reconstruction of autologous vein bypass graft distal anastomoses imaged with magnetic resonance: Clinical and research applications. J. Vasc. Surg 2003, 38, 621–625. [Google Scholar]
- Rickard, RF; Meyer, C; Hudson, DA. Computational modeling of microarterial anastomoses with size discrepancy (small-to-large). J. Surg. Res 2009, 153, 1–11. [Google Scholar]
- Willis, DJ; Kalish, JA; Li, C; Deutsch, ER; Contreras, MA; LoGerfo, FW; Quist, WC. Temporal gene expression following prosthetic arterial grafting. J. Surg. Res 2004, 120, 27–36. [Google Scholar]
- Geary, RL; Wong, JM; Rossini, A; Schwartz, SM; Adams, LD. Expression profiling identifies 147 genes contributing to a unique primate neointimal smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol 2002, 22, 2010–2016. [Google Scholar]
- Orr, AW; Ginsberg, MH; Shattil, SJ; Deckmyn, H; Schwartz, MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol. Biol. Cell 2006, 17, 4686–4697. [Google Scholar]
- Jalali, S; del Pozo, MA; Chen, KD; Miao, H; Li, YS; Schwartz, MA; Shyy, JY; Chien, S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl. Acad. Sci. USA 2001, 98, 1042–1046. [Google Scholar]
- Shyy, JYJ; Chien, S. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res 2002, 91, 769–775. [Google Scholar]
- Li, S; Huang, NF; Hsu, S. Mechanotransduction in endothelial cell migration. J. Cell Biochem 2005, 96, 1110–1126. [Google Scholar]
- Senger, DR; Claffey, KP; Benes, JE; Perruzzi, CA; Sergiou, AP; Detmar, M. Angiogenesis promoted by vascular endothelial growth factor: Regulation through α1β1 and α2β1 integrins. Proc. Natl. Acad. Sci. USA 1997, 94, 13612–13617. [Google Scholar]
- Davis, G; Black, S; Bayless, K. Capillary morphogenesis during human endothelial cell invasion of three-dimensional collagen matrices. In vitro Cell Dev. Biol. An 2000, 36, 513–519. [Google Scholar]
- Davis, GE; Bayless, KJ; Mavila, A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat. Record 2002, 268, 252–275. [Google Scholar]
- Sonnenberg, A; Modderman, PW; Hogervorst, F. Laminin receptor on platelets is the integrin VLA-6. Nature 1988, 336, 487–489. [Google Scholar]
- Lim, L; Manser, E; Leung, T; Hall, C. Regulation of phosphorylation pathways by p21 GTPases: The p21 Ras-related Rho subfamily and its role in phosphorylation signaling pathways. Eur. J. Biochem 1996, 242, 171–185. [Google Scholar]
- Monick, MM; Powers, L; Butler, N; Yarovinsky, T; Hunninghake, GW. Interaction of matrix with integrin receptors is required for optimal LPS-induced MAP kinase activation. Am. J. Physiol. Lung Cell Mol. Physiol 2002, 283, 390–402. [Google Scholar]
- Akimoto, S; Mitsumata, M; Sasaguri, T; Yoshida, Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21Sdi1/Cip1/Waf1. Circ. Res 2000, 86, 185–190. [Google Scholar]
- Lin, K; Hsu, PP; Chen, BP; Yuan, S; Usami, S; Shyy, JYJ; Li, YS; Chien, S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc. Natl. Acad. Sci. USA 2000, 97, 9385–9389. [Google Scholar]
- Nagel, T; Resnick, N; Dewey, CF, Jr; Gimbrone, MA, Jr. Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler. Thromb. Vasc. Biol 1999, 19, 1825–1834. [Google Scholar]
- Chiu, JJ; Chen, LJ; Chang, SF; Lee, PL; Lee, CI; Tsai, MC; Lee, DY; Hsieh, HP; Usami, S; Chien, S. Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: Role of NF-{kappa}B. Arterioscler. Thromb. Vasc. Biol 2005, 25, 963–969. [Google Scholar]
- Cenni, E; Granchi, D; Verri, E; Remiddi, G; Cavedagna, D; Di Leo, A. Evaluation of endothelial cell integrins after in vitro contact with polyethylene terephthalate. J. Mater. Sci.—Mater. M 2001, 12, 345–349. [Google Scholar]
- Pu, FR; Williams, RL; Markkula, TK; Hunt, JA. Effects of plasma treated PET and PTFE on expression of adhesion molecules by human endothelial cells in vitro. Biomaterials 2002, 23, 2411–2428. [Google Scholar]
- van der Zijpp, YJ; Poot, AA; Feijen, J. ICAM-1 and VCAM-1 expression by endothelial cells grown on fibronectin-coated TCPS and PS. J. Biomed. Mater. Res. A 2003, 65, 51–59. [Google Scholar]
- O’Keeffe, LM; Muir, G; Piterina, AV; McGloughlin, TM. Vascular cell adhesion molecule-1 expression in endothelial cells exposed to physiological coronary wall shear stresses. J. Biomech. Eng.—T. ASME 2009, 131, 081003–081009. [Google Scholar]
- Aro, H. Effect of nerve injury on fracture healing. Callus formation studied in the rat. Acta Orthop. Scand 1985, 56, 233–237. [Google Scholar]
- Lambiase, A; Manni, L; Bonini, S; Rama, P; Micera, A; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Invest. Ophthalmol. Vis. Sci 2000, 41, 1063–1069. [Google Scholar]
- Cowen, T; Burnstock, G. Quantitative analysis of the density and pattern of adrenergic innervation of blood vessels. A new method. Histochemistry 1980, 66, 19–34. [Google Scholar]
- Crick, SJ; Wharton, J; Sheppard, MN; Royston, D; Yacoub, MH; Anderson, RH; Polak, JM. Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation 1994, 89, 1697–1670. [Google Scholar]
- Cowen, T; MacCormick, DEM; Toff, WD; Burnstock, G; Lumley, JSP. The effect of surgical procedures on blood vessel innervation a fluorescence histochemical study of degeneration and regrowth of perivascular adrenergic nerves. Blood Vessels 1982, 19, 65–78. [Google Scholar]
- Head, RJ. Hypernoradrenergic innervation and vascular smooth muscle hyperplastic change. Blood Vessels 1991, 28, 173–178. [Google Scholar]
- Autiero, M; De Smet, F; Claes, F; Carmeliet, P. Role of neural guidance signals in blood vessel navigation. Cardiovasc. Res 2005, 65, 629–638. [Google Scholar]
- Carmeliet, P; Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 2005, 436, 193–200. [Google Scholar]
- Raab, S; Plate, KH. Different networks, common growth factors: Shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol 2007, 113, 607–626. [Google Scholar]
- Yoshida, K; Okamura, T; Kimura, H; Bredt, DS; Snyder, SH; Toda, N. Nitric oxide synthase-immunoreactive nerve fibers in dog cerebral and peripheral arteries. Brain Res 1993, 629, 67–72. [Google Scholar]
- Auger, FA; D’Orléans-Juste, P; Germain, L. Adventitia contribution to vascular contraction: Hints provided by tissue-engineered substitutes. Cardiovasc. Res 2007, 75, 669–678. [Google Scholar]
- Gutterman, DD. Adventitia-dependent influences on vascular function. Am. J. Physiol 1999, 277, 1265–1272. [Google Scholar]
- van Brummelen, P; Jie, K; van Zwieten, PA. Alpha-adrenergic receptors in human blood vessels. Br. J. Clin. Pharmacol 1986, 21, 33–39. [Google Scholar]
- Vanhoutte, PM. Endothelial adrenoceptors. J. Cardiovasc. Pharm 2001, 38, 796–808. [Google Scholar]
- Tsuru, H; Tanimitsu, N; Hirai, T. Role of perivascular sympathetic nerves and regional differences in the features of sympathetic innervation of the vascular system. Jpn. J. Pharmacol 2002, 88, 9–13. [Google Scholar]
- Tsuru, H. The diversity of autonomic innervation in the vascular system. Autonomic Nervous System 1999, 36, 119–125. [Google Scholar]
- Bevan, JA. Some bases of differences in vascular response to sympathetic activity. Circ. Res 1979, 45, 161–171. [Google Scholar]
- Bevan, RD; Clementson, A; Joyce, E; Bevan, JA. Sympathetic denervation of resistance arteries increases contraction and decreases relaxation to flow. Am. J. Physiol. Heart Circ. Physiol 1993, 264, 490–494. [Google Scholar]
- Meagher, S; McGeachie, J; Prendergast, F. Vein to artery grafts. An experimental study of reinnervation of the graft wall. Ann. Surg 1984, 200, 153–158. [Google Scholar]
- Hoch, JR; Stark, VK; Turnipseed, WD. The temporal relationship between the development of vein graft intimal hyperplasia and growth factor gene expression. J. Vasc. Surg 1995, 22, 51–58. [Google Scholar]
- Reuthebuch, OT; Kadner, A; Lachat, ML; Turina, MI. Graft occlusion after deployment of the symmetry bypass system. Ann. Thorac. Surg 2003, 75, 1626–1629. [Google Scholar]
- Alimi, Y; Juhan, C; Morati, N; Girard, N; Cohen, S. Dilation of woven and knitted aortic prosthetic grafts: CT scan evaluation. Ann. Vasc. Surg 1994, 8, 238–242. [Google Scholar]
- Kropp, BP; Sawyer, BD; Shannon, HE; Rippy, MK; Badylak, SF; Adams, MC; Keating, MA; Rink, RC; Thor, KB. Characterization of small intestinal submucosa regenerated canine detrusor: assessment of reinnervation, in vitro compliance and contractility. J. Urol 1996, 156, 599–607. [Google Scholar]
- Kropp, BP; Rippy, MK; Badylak, SF; Adams, MC; Keating, MA; Rink, RC; Thor, KB. Regenerative urinary bladder augmentation using small intestinal submucosa: Urodynamic and histopathologic assessment in long-term canine bladder augmentations. J. Urol 1996, 155, 2098–2104. [Google Scholar]
- Vaught, JD; Kropp, BP; Sawyer, BD; Rippy, MK; Badylak, SF; Shannon, HE; Thor, KB. Detrusor regeneration in the rat using porcine small intestinal submucosal grafts: Functional innervation and receptor expression. J. Urol 1996, 155, 374–378. [Google Scholar]
- Ciardelli, G; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci 2006, 6, 13–26. [Google Scholar]
- Crouzier, T; McClendon, T; Tosun, Z; McFetridge, PS. Inverted human umbilical arteries with tunable wall thicknesses for nerve regeneration. J. Biomed. Mater. Res. A 2009, 89, 818–828. [Google Scholar]
- Hadlock, TA; Sundback, CA; Hunter, DA; Vacanti, JP; Cheney, ML. A new artificial nerve graft containing rolled Schwann cell monolayers. Microsurgery 2001, 21, 96–101. [Google Scholar]
- Su, Y; Zeng, BF; Zhang, CQ; Zhang, KG; Xie, XT. Study of biocompatibility of small intestinal submucosa (SIS) with Schwann cells in vitro. Brain Res 2007, 11, 41–47. [Google Scholar]
- Aumailley, M; El Khal, A; Knöss, N; Tunggal, L. Laminin 5 processing and its integration into the ECM. Matrix Biol 2003, 22, 49–54. [Google Scholar]
- Armstrong, SJ; Wiberg, M; Terenghi, G; Kingham, PJ. ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng 2007, 13, 2863–2870. [Google Scholar]
- Matesz, C; Modis, L; Halasi, G; Szigeti, ZM; Felszeghy, S; Bacskai, T; Szekely, G. Extracellular matrix molecules and their possible roles in the regeneration of frog nervous system. Brain Res. Bull 2005, 66, 526–531. [Google Scholar]
- Ranieri, JP; Bellamkonda, R; Bekos, EJ; Gardella, JA, Jr; Mathieu, HJ; Ruiz, L; Aebischer, P. Spatial control of neuronal cell attachment and differentiation on covalently patterned laminin oligopeptide substrates. Int. J. Dev. Neurosci 1994, 12, 725–735. [Google Scholar]
- Reichardt, LF; Tomaselli, KJ. Extracellular matrix molecules and their receptors: Functions in neural development. Annu. Rev. Neurosci 1991, 14, 531–570. [Google Scholar]
- McFetridge, PS; Daniel, JW; Bodamyali, T; Horrocks, M; Chaudhuri, JB. Preparation of porcine carotid arteries for vascular tissue engineering applications. J. Biomed. Mater. Res. A 2004, 70, 224–234. [Google Scholar]
- Grauss, RW; Hazekamp, MG; Oppenhuizen, F; van Munsteren, CJ; Gittenberger-de Groot, AC; DeRuiter, MC. Histological evaluation of decellularised porcine aortic valves: Matrix changes due to different decellularisation methods. Eur. J. Cardiothorac Surg 2005, 27, 566–571. [Google Scholar]
- Schenke-Layland, K; Vasilevski, O; Opitz, F; König, K; Riemann, I; Halbhuber, KJ; Wahlers, TH; Stock, UA. Impact of decellularisation of xenogenetic tissue on extracellular matrix integrity for tissue engineering of heart valves. J. Struct. Biol 2003, 143, 201–208. [Google Scholar]
- Spark, JI; Yeluri, S; Derham, C; Wong, YT; Leitch, D. Incomplete cellular depopulation may explain the high failure rate of bovine ureteric grafts. Br. J. Surg 2008, 95, 582–585. [Google Scholar]
- Gratzer, PF; Harrison, RD; Woods, T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng 2006, 12, 2975–2983. [Google Scholar]
- Korossis, SA; Wilcox, HE; Watterson, KG; Kearney, JN; Ingham, E; Fisher, J. In-vitro assessment of the functional performance of the decellularized intact porcineaortic root. J. Heart Valve Dis 2005, 14, 408–421. [Google Scholar]
- Gilbert, TW; Freund, JM; Badylak, SF. Quantification of DNA in biologic scaffold materials. J. Surg. Res 2008, 152, 135–139. [Google Scholar]
- Daly, K; Stewart-Akers, A; Hara, H; Ezzelarab, M; Long, C; Cordero, K; Johnson, S; Ayares, D; Cooper, D; Badylak, SF. Effect of the αGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng Part A 2009. [Google Scholar]
- Seebacher, G; Grasl, C; Stoiber, M; Rieder, E; Kasimir, MT; Dunkler, D; Simon, P; Weigel, G; Schima, H. Biomechanical properties of decellularized porcine pulmonary valve conduits. Artif. Organs 2008, 32, 28–35. [Google Scholar]
- Herijgers, P; Ozaki, S; Verbeken, E; van Lommel, A; Ràcz, R; Zietkiewicz, M; Perek, B; Flameng, W. Calcification characteristics of porcine stentless valves injuvenile sheep. Eur. J. Cardiothorac Surg 1999, 15, 134–142. [Google Scholar]
- Hopkins, RA; Jones, AL; Wolfinbarger, L; Moore, MA; Bert, AA; Lofland, GK. Decellularization reduces calcification while improving both durability and 1-year functional results of pulmonary homograft valves in juvenile sheep. J. Thorac. Cardiovasc. Surg 2009, 137, 907–913. [Google Scholar]
- Woods, AM; Rodenberg, EJ; Hiles, MC; Pavalko, FM. Improved biocompatibility of small intestinal submucosa (SIS) following conditioning by human endothelial cells. Biomaterials 2004, 25, 515–525. [Google Scholar]
- Ball, P. Natural strategies for the molecular engineer. Nanotechnology 2002, 13, 15–28. [Google Scholar]
- Hu, L; Hu, Z; Wang, S. Progress in genetic modification of vascular prostheses and its significance in molecular reconstruction. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2008, 22, 1501–1504. [Google Scholar]
- Baker, AH. Designing gene delivery vectors for cardiovascular gene therapy. Prog. Biophys. Mol. Biol 2004, 84, 279–299. [Google Scholar]
- Hay, CM; De Leon, H; Jafari, JD; Jakubczak, JL; Mech, CA; Hallenbeck, PL; Powell, SK; Liau, G; Stevenson, SC. Enhanced gene transfer to rabbit jugular veins by an adenovirus containing a cyclic RGD motif in the HI loop of the fiber knob. J. Vasc. Res 2001, 38, 315–323. [Google Scholar]
- Tiera, MJ; Winnik, FO; Fernandes, JC. Synthetic and natural polycations for gene therapy: State of the art and new perspectives. Curr. Gene. Ther 2006, 6, 59–71. [Google Scholar]
- Li, JM; Collins, L; Zhang, X; Gustafsson, K; Fabre, JW. Efficient gene delivery to vascular smooth muscle cells using a nontoxic, synthetic peptide vector system targeted to membrane integrins: A first step toward the gene therapy of chronic rejection. Transplantation 2000, 70, 1616–1624. [Google Scholar]
- Corchero, JL; Villaverde, A. Biomedical applications of distally controlled magnetic nanoparticles. Trends Biotechnol 2009, 8, 468–476. [Google Scholar]
- Zhao, X; Pan, F; Holt, CM; Lewis, AL; Lu, JR. Controlled delivery of antisense oligonucleotides: A brief review of current strategies. Expert Opin. Drug Deliv 2009, 6, 673–686. [Google Scholar]
- Xu, G; Zhang, N. Nanoparticles for gene delivery: A brief patent review. Recent Pat. Drug Deliv. Formul 2009, 3, 125–136. [Google Scholar]
- Nam, HY; Park, JH; Kim, K; Kwon, IC; Jeong, SY. Lipid-based emulsion system as non-viral gene carriers. Arch. Pharm. Res 2009, 32, 639–646. [Google Scholar]
- Singh, R; Kostarelos, K. Designer adenoviruses for nanomedicine and nanodiagnostics. Trends Biotechnol 2009, 27, 220–229. [Google Scholar]
- Paleos, CM; Tziveleka, LA; Sideratou, Z; Tsiourvas, D. Gene delivery using functional dendritic polymers. Expert Opin. Drug Deliv 2009, 6, 27–38. [Google Scholar]
- Glickson, JD; Lund-Katz, S; Zhou, R; Choi, H; Chen, IW; Li, H; Corbin, I; Popov, AV; Cao, W; Song, L; Qi, C; Marotta, D; Nelson, DS; Chen, J; Chance, B; Zheng, G. Lipoprotein nanoplatform for targeted delivery of diagnostic and therapeutic agents. Adv. Exp. Med. Biol 2009, 645, 227–239. [Google Scholar]
- Wang, V; Wu, W. MicroRNA-based therapeutics for cancer. BioDrugs 2009, 23, 15–23. [Google Scholar]
- Mykhaylyk, O; Zelphati, O; Hammerschmid, E; Anton, M; Rosenecker, J; Plank, C. Recent advances in magnetofection and its potential to deliver siRNAs in vitro. Methods Mol. Biol 2009, 487, 111–146. [Google Scholar]
- Stone, PJ; Morris, SM; Griffin, S; Mithieux, S; Weiss, AS. Building Elastin. Incorporation of recombinant human tropoelastin into extracellular matrices using nonelastogenic rat-1 fibroblasts as a source for lysyl oxidase. Am. J. Respir. Cell Mol. Biol 2001, 24, 733–739. [Google Scholar]
- Kallenbach, K; Salcher, R; Heim, A; Karck, M; Mignatti, P; Haverich, A. Inhibition of smooth muscle cell migration and neointima formation in vein grafts by overexpression of matrix metalloproteinase-3. J. Vasc. Surg 2009, 49, 750–758. [Google Scholar]
- Li, SF; Meng, QH; Yao, WC; Hu, GJ; Li, GL; Li, ZJ; Wei, JJ; Bo, YL; Zhang, ZH; Wang, RZ. Recombinant AAV1 mediated vascular endothelial growth factor gene expression promotes angiogenesis and improves neural function: Experiment with rats. Zhonghua Yi Xue Za Zhi 2009, 89, 167–170. [Google Scholar]
- Callanan, A; Morris, L; McGIoughlin, TM; Gilbert, TW; Badylak, SF. Regulation of MMP‘s in endothelial cell-seeded UBM extracellular matrix under shear. Proceedings of the Tissue Engineering Regenerative Medicine International Society (TERMIS) EU Chapter Meeting, London, UK, September 4–7, 2007.
- Gilbert, TW; Stewart-Akers, AM; Sydeski, J; Nguyen, TD; Badylak, SF; Woo, SLY. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Eng 2007, 13, 1313–1323. [Google Scholar]
- Shaikh, FM; O’Brien, TP; Callanan, A; Kavanagh, EG; Burke, PE; Grace, PA; McGloughlin, TM. New pulsatile hydrostatic pressure bioreactor for vascular tissue-engineered constructs. Artif Organs 2009. [Google Scholar]
- Isenberg, BC; Williams, C; Tranquillo, RT. Small-diameter Artificial arteries engineered in vitro. Circ. Res 2006, 98, 25–35. [Google Scholar]
- Stankus, JJ; Freytes, DO; Badylak, SF; Wagner, WR. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J. Biomater. Sci. Polym. Ed 2008, 19, 635–652. [Google Scholar]
- Courtney, T; Sacks, MS; Stankus, J; Guan, J; Wagner, WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials 2006, 27, 3631–3638. [Google Scholar]
- Freytes, DO; Rundell, AE; vande Geest, J; Vorp, DA; Webster, TJ; Badylak, SF. Analytically derived material properties of multilaminated extracellular matrix devices using the ball-burst test. Biomaterials 2005, 26, 5518–5531. [Google Scholar]
- Zeugolis, DI; Khew, ST; Yew, ESY; Ekaputra, AK; Tong, YW; Yung, L-YL; Hutmacher, DW; Sheppard, C; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin. Biomaterials 2008, 29, 2293–2305. [Google Scholar]
- Min, L; Paul, CYC; James, CYD. Evaluation of small intestinal submucosa as scaffoldsfor intestinal tissue engineering. J. Surg. Res 2008, 147, 168–171. [Google Scholar]
- Lutolf, MP; Hubbell, JA. Synthetic biomaterials as cell-responsive artificial extracellular matrices. In Advances in Tissue Engineering, 2nd ed; Polak, J, Mantalaris, S, Harding, SE, Eds.; Imperial College Press: London, UK, 2007; pp. 154–196. [Google Scholar]
- Ruoslahti, E; Reed, J. Cell adhesion: New way to activate caspases. Nature 1999, 397, 479–480. [Google Scholar]
- Lauer-Fields, J; Nagase, H; Fields, G. Selective hydrolysis of triple-helical peptides by matrix metalloproteinases. In Peptides for the New Millennium; Kluwer Academic, Publisher, Springer: New York, NY, USA, 2000; pp. 342–343. [Google Scholar]
- Zhang, Y; He, Y; Bharadwaj, S; Hammam, N; Carnagey, K; Myers, R; Atala, A; van Dyke, M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009, 30, 4021–4028. [Google Scholar]
- Thomas, AC; Campbell, GR; Campbell, JH. Advances in vascular tissue engineering. Cardiovasc. Pathol 2003, 12, 271–276. [Google Scholar]
- Swartz, DD; Russell, JA; Andreadis, ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. Heart Circ. Physiol 2005, 288, 1451–1460. [Google Scholar]
- Lamm, P; Adelhard, K; Juchem, G; Weitkunatb, R; Milzc, S; Kilgerd, E; Gotzd, A; Reicharta, B. Fibrin glue in coronary artery bypass grafting operations: Casting out the Devil with Beelzebub? Eur. J. Cardiothorac Surg 2007, 32, 567–572. [Google Scholar]
- Aldenhoff, YB; van Der Veen, FH; ter Woorst, J. Performance of a polyurethane vascular prosthesis carrying a dipyridamole (Persantin) coating on its lumenal surface. J. Biomed. Mater. Res 2001, 54, 224–233. [Google Scholar]
- Walpoth, BH; Rogulenko, R; Tikhvinskaia, E; Gogolewski, S; Schaffner, T; Hess, OM; Althaus, U. Improvement of patency rate in heparin-coated small synthetic vascular grafts. Circulation 1998, 98, 319–323. [Google Scholar]
- Nerem, RM; Seliktar, D. Vascular tissue engineering. Annu. Rev. Biomed. Eng 2001, 3, 225–243. [Google Scholar]
- Stitzel, J; Liu, J; Lee, SJ; Komura, M; Berry, J; Soker, S; Lim, G; van Dyke, M; Czerw, R; Yoo, JJ; Atala, A. Controlled fabrication of a biological vascular substitute. Biomaterials 2006, 27, 1088–1094. [Google Scholar]
- Tillman, BW; Yazdani, SK; Lee, SJ; Geary, RL; Atala, A; Yoo, JJ. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar]
| Product Company | Material | Processing | Form | |
|---|---|---|---|---|
| AlloDerm | Lifecell | Human skin | Natural | Dry sheet |
| AlloPatch® | Musculoskeletal Transplant Foundation | Human fascia lata | Natural | Dry sheet |
| Axis™ dermis | Mentor | Human dermis | Natural | Dry sheet |
| Bard® Dermal Allograft | Bard | Cadaveric human dermis | Natural | Dry sheet |
| CuffPatch™ | Arthrotek | Porcine SIS | Cross-linked | Hydrated sheet |
| DurADAPT™ | Pegasus Biologicals | Horse pericardium | Cross-linked | Dry sheet |
| Dura-Guard® | Synovis Surgical | Bovine pericardium | Cross-linked | Hydrated sheet |
| Durasis® | Cook SIS | Porcine SIS | Natural | Dry sheet |
| Durepair® | TEI Biosciences | Fetal bovine skin | Natural | Dry sheet |
| FasLata® | Bard | Cadaveric fascia lata | Natural | Dry sheet |
| Graft Jacket® | Wright Medical Tech | Human skin | Natural | Dry sheet |
| Oasis® | Healthpoint | Porcine SIS | Natural | Dry sheet |
| OrthADAPT™ | Pegasus Biologicals | Horse pericardium | Cross-linked | Dry sheet |
| Pelvicol® | Bard | Porcine dermis | Cross-linked | Hydrated sheet |
| Peri-Guard® | Synovis Surgical | Bovine pericardium | Cross-linked | Dry sheet |
| Permacol ™ | Tissue Science Laboratories | Porcine skin | Cross-linked | Hydrated sheet |
| PriMatrix™ | TEI Biosciences | Fetal bovine skin | Natural | Dry sheet |
| Restore ™ | DePuy | Porcine SIS | Natural | Dry sheet |
| Stratasis® | Cook SIS | Porcine SIS | Natural | Dry sheet |
| SurgiMend ™ | TEI Biosciences | Fetal bovine skin | Natural | Dry sheet |
| Surgisis® | Cook SIS | Porcine SIS | Natural | Dry sheet |
| Suspend ™ | Mentor | Human fascia lata | Natural | Dry sheet |
| TissueMend® | TEI Biosciences | Fetal bovine skin | Natural | Dry sheet |
| Vascu-Guard® | Synovis Surgical | Bovine pericardium | Cross-linked | Dry sheet |
| Veritas® | Synovis Surgical | Bovine pericardium | Cross-linked | Hydrated sheet |
| Xelma ™ | Mölnlycke Health Care | ECM protein, PGA, water | Gel | |
| Xenform ™ | TEI Biosciences | Fetal bovine skin | Natural | Dry sheet |
| Zimmer Collagen Patch® | Tissue Science Laboratories | Porcine dermis | Cross-linked | Hydrated sheet |
| Product Com | pany Material | Processing Form | ||
|---|---|---|---|---|
| Anginera ™(i) | Theregen | Seeded (ii) Dexon or Vicryl | Sheet | |
| Apligraf® | Apligraf | Seeded (iii) Bovine Collagen I | Cross-linked | Fibrous sheet |
| Biobrane® | Bertek Pharmaceuticals Inc. | Silicone, Porcine Dermal Collagen I coated Nylon | Covalently bonded | Bi-layered sheet |
| Biostite® | Vebas S.r.l | Collagen I, HA (iv), CS (v) | Powder | |
| Collagraft™ | Zimmer | Bovine dermis, HA, TCP(vi) | Cross-linked | Granules |
| Collapat II® | Biomet | Calf skin collagen, HA | Cross-linked | Sponge |
| Healos FX® | DePuy Spine, Inc. | Collagen with HA coating | Cross-linked | Fibrous material |
| Integra® | Integra LifeSciences | Silicone, Collagen I, GAGs(vii) | Cross-linked | Fibrous sheet |
| OrCel® | Ortec International Inc. | Collagen I | Cross-linked | Sponge/Gel |
| TransCyte™ | Smith & Nephew | Silicone, Seeded Porcine Dermal Collagen coated Nylon | Bi-layered sheet |
| Vessel type | Compliance | Modulus of Elasticity (E) | Reference |
|---|---|---|---|
| Carotid (man) | 14.7% | 0.4 × 106 dynes/cm2 | [175] |
| Carotid (man) | - | 6.07 × 106 dynes/cm2 | [176] |
| Asc. A (man) | - | 0.76 × 106 dynes/cm2 | [176] |
| SIS, 3-layer (pig) | 4.6–8.7% | 8.03 × 106 dynes/cm2 | [177] |
| Saphenous Vein | 1.96–0.64% | 5.5 × 106 dynes/cm2 | [178,179] |
| Dacron® | 0.76% | 56.49 × 106 dynes/cm2 | [178,180] |
| ePTFE | 0.2% | 39.07 × 106 dynes/cm2 | [180,181] |
| SIS UBM | ||
|---|---|---|
| 2-layer | 42 ± 9 N | 19 ± 7 N |
| 4-layer | 130 ± 29 N | 35 ± 2 N |
| 8-layer | 325 ± 53 N |
| Graft Material | Location | Biological Response in anastomosis site | Reference | ||
|---|---|---|---|---|---|
| Methodology | Up-Regulated Biomarkers | Down-Regulated Biomarkers | |||
| PTFE | Carotid artery (Dog) | Microarray, RT-PCR and immunohistochemistry. | (α1) collagen -I, (α2) collagen-I, 80K-L protein (MARCKS), osteopontin, NAP-22, VESPR. | Smoothelin-B, tropomyosin 2 (β), calcium/calmodulin-dependent protein kinase II, RBP-MS types 4 and 5, cysteinerich motor neuron 1 | [200] |
| Aorta (monkey) | Immunohistochemistry | Osteoblast-specific factor-2 (OSF2)/Cbfα1, (α2)collagen-I, (α1)collagen-III, versican, (α3)collagen-VI, (α2)collagen-V, (α1) collagen-V. | SPARClike-1 (SPARCL1)/hevin, RGS5. | [201] | |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Piterina, A.V.; Cloonan, A.J.; Meaney, C.L.; Davis, L.M.; Callanan, A.; Walsh, M.T.; McGloughlin, T.M. ECM-Based Materials in Cardiovascular Applications: Inherent Healing Potential and Augmentation of Native Regenerative Processes. Int. J. Mol. Sci. 2009, 10, 4375-4417. https://doi.org/10.3390/ijms10104375
Piterina AV, Cloonan AJ, Meaney CL, Davis LM, Callanan A, Walsh MT, McGloughlin TM. ECM-Based Materials in Cardiovascular Applications: Inherent Healing Potential and Augmentation of Native Regenerative Processes. International Journal of Molecular Sciences. 2009; 10(10):4375-4417. https://doi.org/10.3390/ijms10104375
Chicago/Turabian StylePiterina, Anna V., Aidan J. Cloonan, Claire L. Meaney, Laura M. Davis, Anthony Callanan, Michael T. Walsh, and Tim M. McGloughlin. 2009. "ECM-Based Materials in Cardiovascular Applications: Inherent Healing Potential and Augmentation of Native Regenerative Processes" International Journal of Molecular Sciences 10, no. 10: 4375-4417. https://doi.org/10.3390/ijms10104375




