Introduction
The search for purely organic molecular magnets, with room temperature ordering is of current interest [
1,
2,
3]. One of the main challenges in organic magnetic materials is to synthesize i) stable organic radicals, and ii) impose higher structural dimensionality. Nitroxide radicals are one of the most promising candidates, due to their stability as well as synthetic flexibility. These radicals have restrictive coordination ability to metal centers because of their weak Lewis basicity. The metal centers are generally used to enforce higher dimensionality along with magnetic moment and hence bulk magnetic properties [
4,
5,
6,
7]. The efforts to use ‘chelating nitroxides’ for better complexations with metal centers also have limited reactivity towards metal centers [
8,
9]. On the other hand,
in-situ radical formation to obtain donor-acceptor compounds (D-A), have already shown room temperature magnetic behavior [
10,
11]. D-A compounds have been studied for many decades as molecule-based materials [
12,
13,
14]. This is based on the reaction between donor and acceptor building blocks, where new candidates for the donor and acceptor roles should be identified by their electrochemical properties and their shape. In particular, acceptors should be planar to facilitate stacking and exhibit reversible electrochemical reduction at potentials that are not too negative for promoting the creation of the radical anion. With the anticipation of finding additional examples of materials exhibiting D-A behaviour, and the promise of improved magnetic properties including higher critical temperatures, new donors and acceptors continue to be the subject of intense study.
N,N,N′,N′-tetramethyl-
p-phenylenediamine (TMPD) has been extensively studied as a donor with various acceptors. Its complex with tetracyano-
p-quinodimethane (TCNQ), a well-known acceptor, has been of intense research lately [
15,
16,
17,
18]. In the present work, we describe the charge-transfer salt comprising the moderate acceptor,
1,2,4,5-tetracyanobenzene (TCNB) and TMPD [
19,
20].
Results and Discussion
The crystal structure of the 1:1 complex formed between TMPD and TCNB has been determined by single crystal X-ray diffraction at room temperature [
20,
21]. The compound crystallizes in the triclinic space group
P-1 with cell dimensions:
a = 7.4986(15) Å,
b = 7.6772(11) Å,
c = 8.0764(15) Å, α = 78.822(12)°, β = 83.3779(19)°, γ = 86.289(17)°.
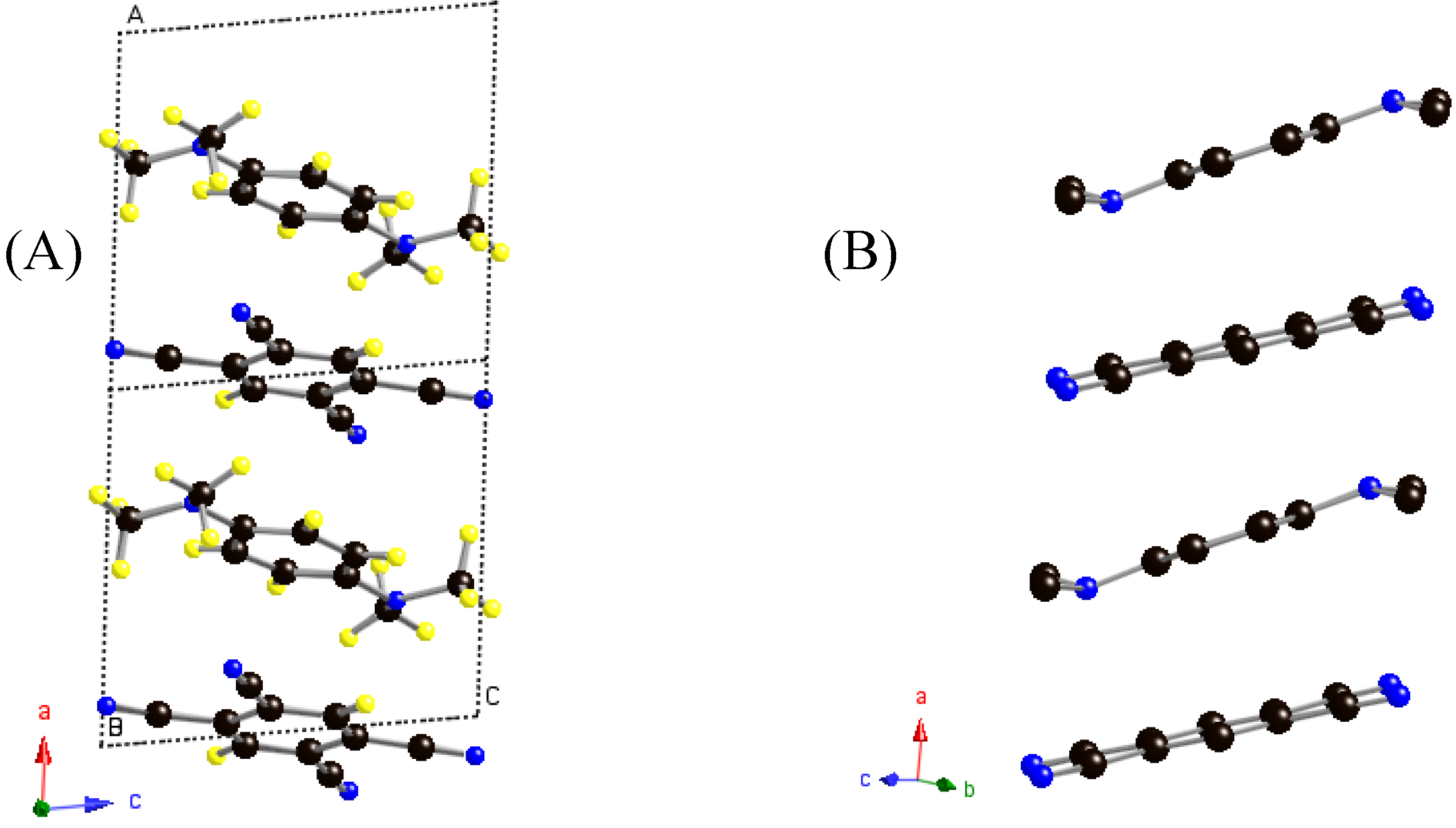
Figure 1.
Stacking of TMPD:TCNB molecules, A: along a-axis; B: showing tetrahedral nature of nitrogen and bending of dimethyl groups attached to it, in TMPD molecule, and planarity of TCNB
Figure 1.
Stacking of TMPD:TCNB molecules, A: along a-axis; B: showing tetrahedral nature of nitrogen and bending of dimethyl groups attached to it, in TMPD molecule, and planarity of TCNB
Both donor and acceptor molecules are approximately planar within experimental error. The TMPD molecule as a whole is not planar, but apart from the carbon atoms of the methyl groups, the rest of the atoms are coplanar. The methyl groups are bent and twisted from the plane comprising the benzene ring and amino-N atoms, as shown in
Figure 1B. Thus, it seems likely that the electronic orbital of the nitrogen atom, N(1), exhibits tetrahedral character and the lone pair electrons of the nitrogen atom do not seem to be perfectly delocalized with
π-electrons of the benzene ring. The C(1)-N(1) distance in TMPD is 1.43Å, equivalent to a single bond, while in the case of the TMPD-TCNQ complex this distance was found to be 1.405(2) Å, which is consistent with double bond character [
10]. The C(6)- C(8) distance in TCNB is shorter by 0.025 Å than the C(6)-C(7) distance [1.4065(19) Å] and C(7)- C(10) is shorter by 0.013Å than C(6)-C(9) [1.438(2) Å].
Molecules of TMPD-TCNB are stacked alternately along the
a-axis (
Figure 1A) with an interplanar spacing of 3.4Å. The molecular plane of the TCNB forms an angle of 7° with that of TMPD (excluding the methyl groups). As shown in
Figure 2, the methyl groups of TMPD lie between the two cyano groups of TCNB, thus the orientation of the TCNB molecule is fixed by the methyl groups. The carbon atoms of the TCNB molecule lie between the two methyl groups attached to the nitrogen atom at 3.502Å and 3.620Å. Whilst the molecules are close-packed along this stacking direction, the two rings of TCNB and TMPD only overlap to a small extent despite Mulliken’s prediction that the extent of overlap of benzene rings should be large [
22], i.e., the structure does not seem to show the usual
π-π interaction between the two aromatic rings. Instead a
n-π interaction between the nitrogen atoms of the donor and the cyano groups of the acceptor is observed. The short contacts between N(1)-C(9) [3.196 Å] and N(1)-C(6) [3.177 Å], strongly suggest that the lone pair electrons that are partially located on these dimethylamino-nitrogen atoms are transferred to the cyano groups. This local charge transfer is further supported by the conclusion drawn from molecular orbital calculation that the carbon atom of the cyano groups in a free molecule of TCNB is deficient in electron density [
23]. The charge transfer interaction seems to make the bond distance C(7)-C(10) shorter than C(6)-C(9) and make C(6)-C(8) shorter than C(6)-C(7) and C(7)-C(8), i.e. the benzene ring of the TCNB would appear to adapt some quinoid structure. On the other hand in the TMPD molecule, the C(1)-N(1) bond distance appears to be elongated by this interaction.
In the related TMPD-TCNQ complex, however, the methyl groups do not affect the relative orientation, because of the geometry of the TCNQ molecule [
15]. Also, the electron affinity of TCNQ is much larger than that of TCNB which facilitates stronger
π-π interaction for TCNQ than in the present complex. These facts indicate that the relative orientation of the two component molecules is mainly determined by the packing of the molecules, especially by those of the methyl groups.
Figure 2.
The stacking of TMPD:TCNB molecules top view along a-axis; showing tight packing of the N,N-dimethyl groups of TMPD and cyanide groups of TCNB.
Figure 2.
The stacking of TMPD:TCNB molecules top view along a-axis; showing tight packing of the N,N-dimethyl groups of TMPD and cyanide groups of TCNB.
The methyl groups hinder the approach and overlap of the benzene rings of the two component molecules, thus inhibiting charge transfer between π-electrons of the donor and acceptor rings of the two components to some extent. Indeed, there is no spectroscopic evidence to support the presence of the anionic radical, TCNB•-, at room temperature; Both Raman and IR spectra do not show any change in cyanide peak observed at 2240 cm-1. Nevertheless, the black color of these crystals suggests some charge transfer.
In
Figure 3 we present the room temperature X-band Electron Paramagnetic Resonance (EPR) spectrum of a solid sample of TMPD:TCNB. This is a classic powder pattern [
24] and can be attributed to
g-tensor anisotropy of an axial S=1/2 system. The main peak, labeled
g⊥, we assign to those crystallites whose magnetic axis is perpendicular to the applied field, and a minor peak, labeled
gll, corresponds to those crystallites whose magnetic axis is aligned parallel to the applied Zeeman field. We obtain
g⊥=2.0009 and
g|| =2.0022. The peak to peak linewidth,
ΔHpp, is 0.06 mT for g
⊥ and the width at half height,
ΔH1/2, is 0.09 mT for
g||. This result compares well to the literature g-values for the radical anions of ~2.002 for similar acceptors like TCNQ and TCNE [
25]. In contrast, TMPD:TCNQ forms a
π-π complex with a diamagnetic ground state. In the present case, the
n-π interaction would appear to generate a paramagnetic system.
Figure 3.
Room temperature EPR spectrum of the solid TMPD:TCNB compound recorded at X-band (ν = 9.8615 GHz).
Figure 3.
Room temperature EPR spectrum of the solid TMPD:TCNB compound recorded at X-band (ν = 9.8615 GHz).