Molecular mechanics dipole moments
μ are given in
Table 1. In general,
μ increases with the oxidation state of Fe. In particular,
μheme(FeIII)(–His)–CN results the greatest due to the highly polar Fe
δ+–C–N
δ‑ complex. The
μheme(FeIII)(–His)–O2 is relatively large due to the extremely polar Fe
δ+–O–O
δ‑. The inclusion of polarization in MM2 corrects, in general,
μ in the correct direction when compared with MMX+ID. In particular,
μheme(–His) remains almost constant (MM2+polarization). The binding of His in heme increases
μ by a factor of 5. Moreover, the subsequent binding of CN doubles
μ and the binding of O
2 or CO increases
μ 7–16%.
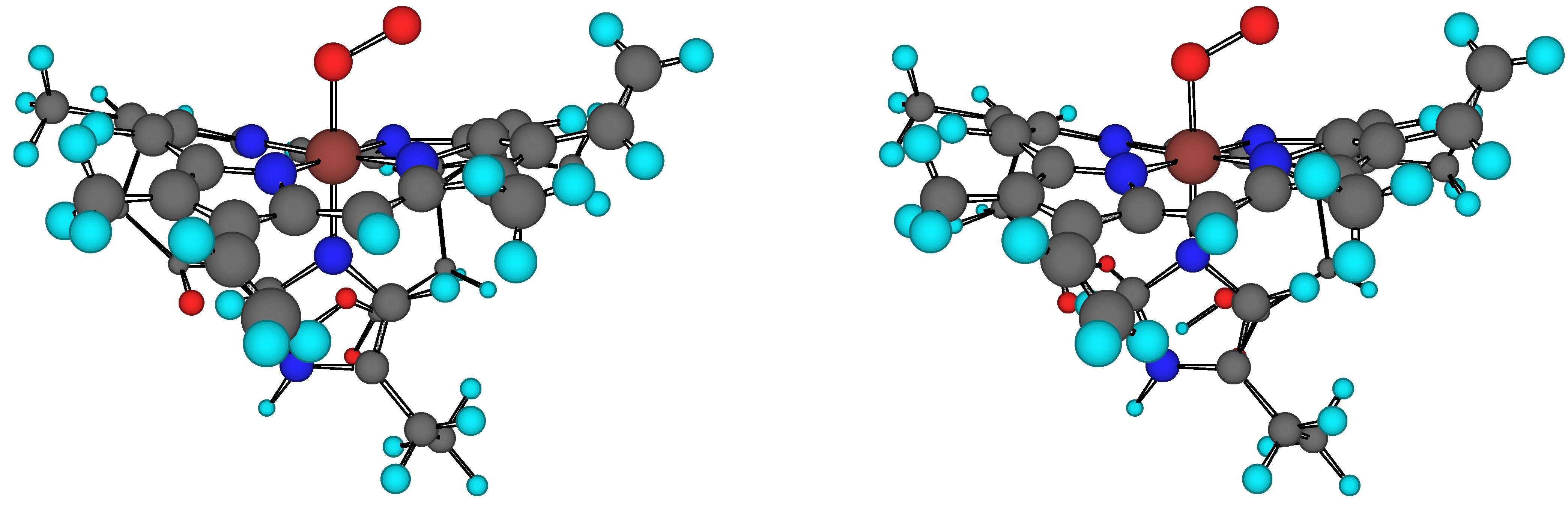
Figure 1.
Stereo view of the heme(–His)–O2 model. Heme is the prosthetic group of oxyhaemoglobin.
The 4-coordinate Fe(P) system
The heme group is a natural starting point for both experimental and theoretical studies. Crystal structures were reported for a number of heme derivatives with different substituents in the ring. In particular, Fe(TPP) (TPP =
meso‑tetraphenylporphyrin) has been chosen. Its electronic state is well known experimentally to correspond to a low spin triplet (
S = 1). Selected structural parameters are listed in
Table 2. The agreement in bond angles between both MM2/MMX+polarization geometries and the X‑ray structure is good, with discrepancies smaller than 5°. Differences in bond distances are larger in a number of cases as for the C–C
bridge, C–C’ and C’–C’’ distances. These have values of 1.395Å, 1.439Å and 1.365Å, respectively, in X‑ray, and ≈1.336Å, 1.340Å and 1.332Å, correspondingly, in MM2/MMX+polarization.
Table 2.
Selected geometric parameters (Å and degrees) from the geometry optimization of Fe(P) with the pure B3LYP and with the IMOMM(B3LYP:MM3) methods [
37].
a
Table 2.
Selected geometric parameters (Å and degrees) from the geometry optimization of Fe(P) with the pure B3LYP and with the IMOMM(B3LYP:MM3) methods [37].a
| Parameter | MM2 | MM2+NIDb | MM2+IDc | MMXd | MMXd+NIDb | MMXd+IDc |
|---|
| Fe–Ne | 1.881 | 1.878 | 1.877 | 1.881 | 1.878 | 1.877 |
| N–C | 1.353 | 1.352 | 1.350 | 1.353 | 1.352 | 1.350 |
| C–Cbridge | 1.337 | 1.336 | 1.334 | 1.337 | 1.336 | 1.334 |
| C–C’ | 1.341 | 1.340 | 1.339 | 1.341 | 1.340 | 1.339 |
| C’–C’’ | 1.332 | 1.332 | 1.332 | 1.332 | 1.332 | 1.332 |
| Fe–N–C | 128.6 | 128.6 | 128.6 | 128.6 | 128.6 | 128.6 |
| N–Fe–N | 90.2 | 90.2 | 90.2 | 90.2 | 90.2 | 90.2 |
| N–C–Cbridge | 120.8 | 120.8 | 120.9 | 120.8 | 120.8 | 120.9 |
| N–C–C’ | 112.0 | 112.1 | 112.1 | 112.0 | 112.1 | 112.1 |
| Fe-planef | 0.104 | 0.102 | 0.105 | 0.104 | 0.102 | 0.105 |
|
| Parameter | Experiment | Pure QM | QM/MM |
| Fe–N | 1.966 | 2.016 | 1.940 |
| N–C | 1.378e | 1.397 | 1.362 |
| C–Cbridge | 1.395e | 1.402 | 1.369 |
| C–C’ | 1.439e | 1.459 | 1.345 |
| C’–-C’’ | 1.365e | 1.367 | 1.333 |
| Fe–N–C | 127.2e | 127.4 | 127.7 |
| N–Fe–N | 90.0e | 90.0 | 90.0 |
| N–C–Cbridge | 125.3e | 125.5 | 126.2 |
| N–C–C’ | 110.6e | 110.4 | 110.3 |
| Fe-plane | 0.000 | 0.000 | 0.000 |
Agreement between experiment and MM2/MMX+polarization is good (≈0.06Å). All these atoms are, in part, purely described with MM2. The optimized MM2/MMX+polarization values are close to the optimal bond distance for these types of atoms in the applied force field, which is 1.337Å. Another discrepancy in the geometries appears in the Fe–N distance. This is more puzzling, because the calculated distance 1.877–1.881Å is smaller than the experimental value of 1.966Å. The calculated displacement of the Fe atom to the mean plane of the porphyrin N‑atoms ring (0.1Å) is not observed experimentally.
The 5-coordinate Fe(P)(Im) system
Table 3.
Selected geometric parameters (Å and degrees) from the geometry optimization of Fe(P)(NH=CH
2) with the pure B3LYP and of Fe(P)[1‑(Me)Im] with the IMOMM(B3LYP:MM3) methods [
37].
a
Table 3.
Selected geometric parameters (Å and degrees) from the geometry optimization of Fe(P)(NH=CH2) with the pure B3LYP and of Fe(P)[1‑(Me)Im] with the IMOMM(B3LYP:MM3) methods [37].a
| Parameter | MM2 | MM2+NIDb | MM2+IDc | MMXd | MMXd+NIDb | MMXd+IDc |
|---|
| Fe–Nporphe | 1.897 | 1.894 | 1.890 | 1.895 | 1.898 | 1.895 |
| Fe–NIm | 1.866 | 1.863 | 1.864 | 1.873 | 1.885 | 1.865 |
| NIm–Cε | 1.327 | 1.327 | 1.326 | 1.398 | 1.388 | 1.389 |
| NIm–Cδ | 1.344 | 1.343 | 1.338 | 1.392 | 1.393 | 1.387 |
| Fe–NIm–Cε | 126.3 | 126.3 | 126.1 | 125.1 | 117.7 | 106.7 |
| Fe–NIm–Cδ | 126.7 | 126.6 | 126.3 | 121.7 | 121.0 | 131.9 |
| Nporph–Fe–NIm–Cε | 115.8 | 115.7 | 114.9 | 135.2 | 100.2 | 99.9 |
| Fe-planef | 0.097 | 0.088 | 0.095 | 0.130 | 0.274 | 0.191 |
|
| Parameter | Experiment | Pure QM | QM/MM |
| Fe–Nporph | 2.075 | 2.101 | 2.029 |
| Fe–NIm | 2.134 | 2.252 | 2.233 |
| NIm–Cε | 1.350 | 1.279 | 1.299 |
| NIm–Cδ | 1.250 | –g | 1.414h |
| Fe–NIm–Cε | 126.5 | 126.1 | 136.8 |
| Fe–NIm–Cδ | 120.4 | 120.6 | 122.6 |
| Nporph–Fe–NIm–Cε | 80.8 | 90.0 | 136.8 |
| Fe-plane | 0.310 | 0.284 | 0.379 |
Coordination of an Im ligand to the heme group leads to a 5‑coordinate species with a square pyramidal geometry. These compounds are good biomimetic models of Mb and Hb, with Im replacing the proximal His of the biological systems. The need to avoid dimerization and formation of 6‑coordinate species with two axial ligands poses serious restrictions on the nature of the porphyrins able to give this kind of complexes. For this study, the species Fe(Piv
2C
8)[1‑(Me)Im] {Piv
2C
8 =
α,
α,5,15‑[2,2’‑(octanediamido)diphenyl]
‑α,
α,10,20‑bis
(o‑pivalamidophenyl)porphyrin} has been chosen. This species has the advantage of having 1‑methylimidazole as axial ligand, in constrast with the more common 2‑methylimidazole, which is more sterically demanding. Neither for Fe(Piv
2C
8)[1‑(Me)Im] nor for other 5‑coordinate derivatives of heme is the electronic state experimentally known. Electronic spectroscopy, magnetic susceptibility and Mössbauer measurements identify it as high spin (
S = 2). Selected parameters are collected in
Table 3. The Fe–N
porph distances are longer than those in the 4‑coordinate system by approximately 0.02Å (MMX+ID). This trend is in agreement with the reference values. The result is fully consistent with the shift from low spin to high spin in the metal. Overall agreement in the geometric parameters is correct. Moreover, one has to view with suspicion the X‑ray parameters of Im, which would make the N=C double bond N
Im–C
ε in Im longer than the N–C single bond N
Im–C
δ. Furthermore, the MM calculations are, in general, in agreement with the QM/MM reference, which provides the expected result.
The sharper discrepancy concerns the Nporph–Fe–NIm–Cε dihedral angle. This angle measures the rotation around the Fe–NIm single bond, and rules the placement of the Im plane with respect to the porphyrin ring. Its sign is arbitrary, because the x and y directions are equivalent in absence of an axial ligand. In this work, a positive sign has been chosen for consistency with data on the 6‑coordinate complexes presented below. An angle of 90° (as in the pure QM reference) means that the Im plane is eclipsing one of the Fe–Nporph bonds, while an angle of 135° (≈135.2° in MMX) indicates a staggered orientation of Im with respect to the Fe–Nporph bonds. Therefore both pure QM and MMX values are just opposite with the experimental value, 126.0°, lying in between, although closer to MMX results. The structural minima lead to structures where the Im ligand is located about the bisector of an angle Nporph–Fe–Nporph. The MMX+polarization results lie, in general, in the range 90–133° of the references. The importance of the large discrepancy between different values is, however, arguable, because there is also a large dispersion in different experimental 5‑coordinate derivatives of heme, as well as in experimental reports of Mb and Hb. The corresponding interpretation is that the rotation around this single bond has a rather low barrier.
A final number to mention is the displacement of the Fe atom out of the porphyrin N‑atoms plane. This is one of the most important parameters, because it seems to play a critical role in the cooperativity of Hb involved in the allosteric transition. In this case, MMX+NID calculation of 0.274Å lies near the experimental and reference values ca. 0.3Å. The positive sign corresponds to the fact that the displacement is towards Im, and away from the empty site.
The 6-coordinate Fe(P)(Im)(ligand) systems
Coordination of O2 to the 5‑coordinate heme–His species leads to 6‑coordinate species with octahedral geometry. These are the biomimetic forms of Mb–O2 and Hb–O2. X‑ray data are reported only on two complexes: Fe(TpivPP)[1‑(Me)Im](O2) and Fe(TpivPP)[2‑(Me)Im](O2). Both are quite similar, sharing the same porphyrin TpivPP, which is meso‑tetrakis(α,α,α,α‑o‑pivalamidophenyl)-porphyrin. Fe(TpivPP)[1‑(Me)Im](O2), containing the less sterically demanding 1‑methylimidazole ligand, has been chosen for comparison.
Table 4.
Selected geometric parameters (Å and degrees) from the geometry optimization of Fe(P)(NH=CH
2)(O
2) with the pure B3LYP and of Fe(P)[1‑(Me)Im](O
2) with the IMOMM(B3LYP:MM3) methods [
37], and of Fe(T
pivPP)(Im)(O
2) with LSD [
21].
a
Table 4.
Selected geometric parameters (Å and degrees) from the geometry optimization of Fe(P)(NH=CH2)(O2) with the pure B3LYP and of Fe(P)[1‑(Me)Im](O2) with the IMOMM(B3LYP:MM3) methods [37], and of Fe(TpivPP)(Im)(O2) with LSD [21].a
| Parameter | MM2 | MM2+NIDb | MM2+IDc | MMXd | MMXd+NIDb | MMXd+IDc |
|---|
| Fe–Nporphe | 1.880 | 1.876 | 1.870 | 1.899 | 1.891 | 1.899 |
| Fe–NIm | 1.871 | 1.862 | 1.861 | 1.871 | 1.908 | 1.875 |
| Fe–O | 1.847 | 1.841 | 1.845 | 1.866 | 1.903 | 1.866 |
| O–O | 1.211 | 1.210 | 1.210 | 1.256 | 1.261 | 1.244 |
| Fe–O–O | 123.5 | 121.5 | 122.8 | 121.3 | 121.7 | 121.3 |
| O–Fe–NIm | 171.7 | 169.5 | 168.6 | 146.2 | 131.6 | 157.3 |
| Nporph–Fe–NIm–Cε | 179.0 | 178.2 | 176.8 | 177.2 | 160.7 | 173.5 |
| Nporph–Fe–O–O | -3.3 | -5.0 | -16.5 | -25.9 | -11.0 | -24.1 |
| Fe-planef | -0.181 | -0.150 | -0.159 | -0.074 | -0.114 | -0.044 |
|
| Parameter | Experiment | Pure QM | QM/MM | LSD |
| Fe–Nporph | 1.979 | 2.035 | 1.949 | 2.010 |
| Fe–NIm | 2.068 | 2.050 | 2.167 | 2.070g |
| Fe–O | 1.745 | 1.757 | 1.759 | 1.770 |
| O–O | 1.163 | 1.268 | 1.286 | 1.300 |
| Fe–O–O | 129.4 | 121.1 | 117.0 | 121.0 |
| O–Fe–NIm | 180.0 | 175.8 | 179.4 | – |
| Nporph–Fe–NIm–Cε | 159.5 | 177.9 | 137.0 | – |
| Nporph–Fe–O–O | -42.4 | -44.6 | -44.1 | – |
| Fe-plane | -0.030 | -0.034 | -0.076 | – |
The state of this system is a low spin open-shell singlet (
S = 1) resulting in a Fe
III––O
2– charge distribution. Selected parameters are reported in
Table 4. The parameters concerning the coordination of O
2, which are probably the most critical for the biochemical activity of Hb, are well reproduced. The computed values for the Fe–O distance, 1.8–1.9Å, are close to the experimental value of 1.746Å. The calculated values for the O–O distance, 1.21–1.26Å, on the other hand, are far from the experimental value of 1.163Å, but the experimental value, even shorter than the 1.21Å for free O
2, is suspect, because of the disorder on the placement of the ending O atom within the crystal, as admitted by the authors of the X‑ray experiment [
34]. The O–O interatomic distance increases from 1.21Å in free O
2 to 1.261Å (MMX+NID), suggesting that electronic charge is transferred from FeP to O
2, in agreement with the experimental observation that the Fe–O
2 bond can be formally described as Fe
III–O
2– [
9].
The most significant feature of the present structure is the bent Fe-O-O bond. The Fe-O-O bond angles are indicative of a bent
η1 coordination mode, where only one O atom is directly attached to the metal. These calculations are in agreement with the experiment and calculation references. Atomic net charges have been calculated with our program POLAR [
51]. The results,
qFe = 3.078,
qO binding = –0.412 and
qO end = –0.531e are in agreement with Weiss’ model for the Fe–O
2 bond in which the bond might be ionic between a Fe
3+ and a superoxide ion, net charge being transferred from Fe to O
2 (Fe
3+O
2–) [
52]. Experimental support for Weiss’ model was advanced by Misra and Fridovich [
53]. The geometry agrees with Pauling’s prediction of a bent FeO
2 bond, and the O–O distance is close to that of 1.27Å, predicted by him [
54]. It is slightly shorter than that of 1.34Å in O
2– close to the electron spin resonance results, which show that no more than 2/3 of the density of one electron is transferred from the metal to the antibonding
π* orbitals of O
2.
The greatest discrepancy concerns the Nporph–Fe–O–O dihedral angle. This angle measures the rotation around the Fe–O single bond, and rules the placement of the Fe–O–O plane with respect to the porphyrin ring. An angle of 0° (approximately the –3.3° in MM2) means that the Fe–O–O plane is eclipsing one of the Fe–Nporph bonds, while an angle of –45° (approximately the –44.6° in pure QM) indicates a staggered orientation of the O2 with respect to the Fe–Nporph bonds. The structural minima lead to structures where the O2 ligand is located about the bisector of an angle Nporph–Fe–Nporph. The MMX/MMX+NID results lie near the reference results. The structural minimum correspond to a trans isomer, where the ending O is placed above the opposite quadrant where the Nδ is located. Finally, the Fe atom is computed by MMX+ID to lie in the energy minimum rather close to the porphyrin N‑atoms plane, with a deviation of 0.044Å, always towards the O atom. This is again in agreement with the experiment and reference calculations, and rather different from the behaviour of the 5‑coordinate system.
Structural parameters of Fe(P)(Im)(CO) are summarized in
Table 5. The geometric parameters concerning the coordination of CO, which are probably the most critical for the biochemical activity of Hb, are well reproduced. The computed values for the Fe–C distance, 1.97–2.01Å, are close to the experimental value of 1.770Å. The calculated values for the C–O distance, 1.11–1.14Å, are also close to the experimental report of 1.120Å. The C–O distance is similar in the free molecule, 1.171Å, calculated with AM1 [
55] and in Fe(P)(Im)(CO), 1.143Å (MMX). The interpretation is that electronic charge is not transferred from FeP to CO. This is in agreement with the experimental observation that the Fe–CO bond can be formally described as Fe
II–CO [
9]. The most significant feature of the present structure is the linear Fe–C–O bond. While such a linear bond is to be expected based on the extensive literature on transition metal CO complexes, the result is nonetheless highly significant since bent Fe–C–O bonds with bond angles of 135–145º appear to be the rule in various CO complexes of hemoproteins [
56,
57,
58].
Table 5.
Selected geometric parameters (Å and degrees) from the complete geometry optimization of Fe(P)(Im)(CO) with LDA [
39], partial geometry optimization of Fe(T
pivPP)[1‑(Me)Im](CO) with DFT (B3LYP and BPW91) [
38], and complete geometry optimization of Fe(mdi)
2(py)(CO) with NLDFT [
16], of Fe(P)(Im)(CO) with LDFT [
19] and of Fe(T
pivPP)(Im)(CO) with LSD [
21].
a
Table 5.
Selected geometric parameters (Å and degrees) from the complete geometry optimization of Fe(P)(Im)(CO) with LDA [39], partial geometry optimization of Fe(TpivPP)[1‑(Me)Im](CO) with DFT (B3LYP and BPW91) [38], and complete geometry optimization of Fe(mdi)2(py)(CO) with NLDFT [16], of Fe(P)(Im)(CO) with LDFT [19] and of Fe(TpivPP)(Im)(CO) with LSD [21].a
| Parameter | MM2 | MM2+NIDb | MM2+IDc | MMXd | MMXd+NIDb | MMXd+IDc |
|---|
| Fe–Nporphe | 1.878 | 1.873 | 1.871 | 1.900 | 1.893 | 1.896 |
| Fe–NIm | 1.868 | 1.863 | 1.862 | 1.876 | 1.877 | 1.886 |
| Fe–C | 1.971 | 1.970 | 1.970 | 1.998 | 1.989 | 2.008 |
| C–O | 1.110 | 1.109 | 1.110 | 1.143 | 1.137 | 1.138 |
| Fe–C–O | 180.0 | 180.0 | 180.0 | 179.6 | 179.8 | 177.4 |
| C–Fe–NIm | 179.7 | 174.6 | 174.3 | 158.3 | 172.7 | 149.3 |
| Nporph–Fe–NIm–Cε | 136.0 | 178.9 | 176.0 | 135.7 | 161.4 | 169.0 |
| Fe-planef | -0.182 | -0.180 | -0.159 | -0.037 | -0.052 | -0.064 |
|
| Parameter | Experiment | LDA | B3LYP | BPW91 | NLDFT | LDFT | LSD |
| Fe–Nporph | 2.015 | 1.990 | – | – | 1.961 | 1.983 | 2.020 |
| Fe–NIm | 2.100 | 1.960 | – | – | 2.139 | 1.966 | 2.070g |
| Fe–C | 1.770 | 1.790 | 1.801 | 1.743 | 1.739 | 1.733 | 1.720 |
| C–O | 1.120 | 1.160 | 1.147 | 1.167 | 1.166 | 1.165 | 1.170 |
| Fe–C–O | 179.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 |
| C–Fe–NIm | 177.5 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | – |
| Nporph–Fe–NIm–Cε | – | 174.2 | – | – | – | – | – |
| Fe-plane | -0.020 | – | – | – | – | – | – |
The global energy minimum is linear for Fe–C–O (MM2/MMX+polarization). These calculations are in agreement with the experiment and calculation references. The Im ring is rotated so that the N atom is directed toward the Fe atom, and the rotation angle Nporph–Fe–NIm–Cε reaches approximately 176° (MM2+polarization) in agreement with the LDA reference (174.2°). The Fe atom is computed by MMX to lie in the energy minimum rather close to the porphyrin N‑atoms plane, with a deviation of 0.037Å, always towards the C atom. This is again in agreement with the experiment and reference calculations.
Table 6.
Selected geometric parameters (Å and degrees) from the geometry optimization of Fe(mdi)
2(py)(CN) with NLDFT [
16].
a
Table 6.
Selected geometric parameters (Å and degrees) from the geometry optimization of Fe(mdi)2(py)(CN) with NLDFT [16].a
| Parameter | MM2 | MM2+NIDb | MM2+IDc | MMXd | MMXd+NIDb | MMXd+IDc |
|---|
| Fe–Nporphe | 1.878 | 1.871 | 1.867 | 1.897 | 1.893 | 1.894 |
| Fe–NIm | 1.868 | 1.860 | 1.858 | 1.874 | 1.901 | 1.883 |
| Fe–C | 1.944 | 1.942 | 1.941 | 1.981 | 1.963 | 1.977 |
| C–N | 1.164 | 1.163 | 1.164 | 1.190 | 1.202 | 1.193 |
| Fe–C–N | 179.8 | 179.8 | 179.6 | 172.9 | 176.8 | 179.4 |
| Fe-planef | -0.318 | -0.246 | -0.318 | -0.188 | -0.202 | -0.199 |
|
| Parameter | Experiment | NLDFT |
| Fe–Nporph | 1.980 | 1.917 |
| Fe–NIm | 2.087 | 2.159 |
| Fe–C | 1.934 | 1.903 |
| C–N | 1.150 | 1.173 |
| Fe–C–N | 179.1 | 180.0 |
| Fe-plane | -0.010 | – |
Selected bond lengths and angles of Fe(P)(Im)(CN) are indicated in
Table 6. The geometric parameters concerning the coordination of CN are well reproduced. The computed values for the Fe–C distance, 1.9–2.0Å, are in agreement with the experimental value of 1.934Å. The calculated values for the C–N distance, 1.16–1.20Å, are close to the experimental report of 1.150Å. The C–N distance increases from 1.147Å in the free molecule (AM1) to 1.202Å in Fe(P)(Im)(CN) (MMX+NID). The interpretation is that electronic charge is transferred from FeP to CN. This is in agreement with the experimental observation that the Fe–CN bond can be formally described as Fe
III–(CN)
– [
9]. The most significant feature of the present structure is the linear Fe–C–N bond. The global energy minimum is linear for Fe–C–N (MM2/MMX+polarization). These calculations are in agreement with the experiment and calculation references. The Fe atom is computed by MMX to lie in the energy minimum rather close to the porphyrin N‑atoms plane, with a deviation of 0.188Å, always towards the C atom. This distance is hardly observed in the experiment.
Table 7.
Molecular mechanics (MMX+ID) results for heme‑IX adducts: distance of the Fe atom to the porphyrin N‑atoms plane.
Table 7.
Molecular mechanics (MMX+ID) results for heme‑IX adducts: distance of the Fe atom to the porphyrin N‑atoms plane.
| Adduct | D = 0.2 | D = 0.1 | D = 0.05 | Experiment | Pure QM | QM/MM |
|---|
| heme | 0.105 | 0.105 | 0.105 | 0.000 | 0.000 | 0.000 |
| heme(–His) | 0.269 | 0.728 | 0.191 | 0.310 | 0.284 | 0.379 |
| heme(–His)–O2 | -0.105 | -0.323 | -0.044 | -0.030 | -0.034 | -0.076 |
| heme(–His)–CO | -0.199 | -0.178 | -0.064 | -0.020 | – | – |
| heme(–His)–CN | -0.174 | -0.334 | -0.199 | -0.010 | – | – |
The MMX+ID displacement of the Fe atom to the porphyrin N‑atoms plane is shown in
Table 7 for the heme-IX adducts as a function of different scaling factors
D. The average unsigned error for
D = 0.05 (0.094Å) is smaller than for
D = 0.1 (0.260Å) and
D = 0.2 (0.113Å). The results for
D = 0.05 lie in or are closer to the range of the three references.