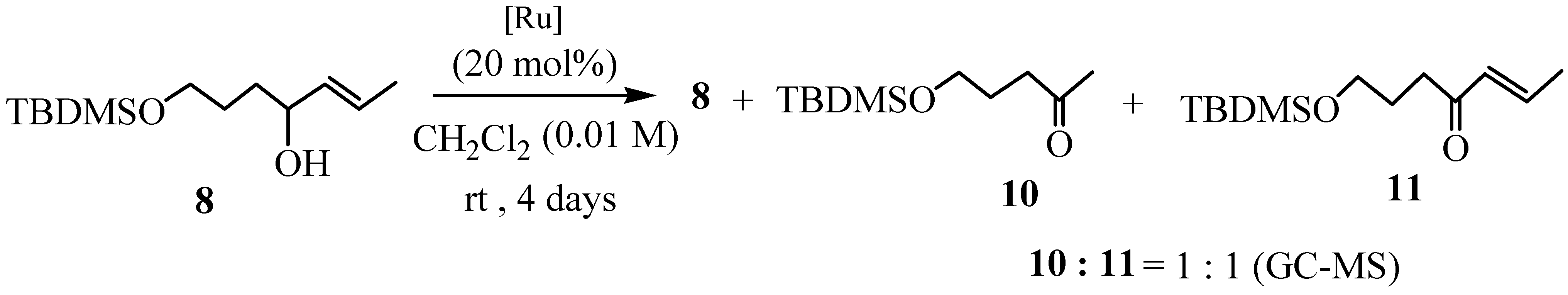Experimental
General
The IR spectra were measured with a JASCO FT/IR-500 spectrophotometer. The 1H- and 13C-NMR spectra were recorded on a Varian Unity 200 or a Varian Mercury 300 spectrometer. Deuteriochloroform was used for NMR and chemical shifts are expressed in ppm and the coupling constants in Hz. The mass spectra including high-resolution mass spectra were taken with a JEOL AX-500 spectrometer. GC-MS was carried out on a HP MS-5973 with GC HP-6890 system. Silica gel BW-300 (200-400 mesh, Fuji silycia) was used for column chromatography, and silica gel 60F254 plate (0.25 mm, Merck) were used for TLC. All reactions were carried out under an argon atmosphere. THF was distilled from LiAlH4 and then from Na-benzophenone prior to use. The Grubbs reagent was purchased from Strem and used as received. The reagent was weighed in the dry box and was used without purification. Anhydrous dichloromethane and benzene used for the reaction were purchased from Kanto Chemical, Japan and were used without further purification.
General procedures for reactions using the Grubbs reagent.
The Grubbs reagent (20 mol%) was weighed in the dry box under an Ar atmosphere and the rubber septum was equipped with or without a reflux condenser. A solution of substrate in CH2Cl2 was introduced into a flask of the Grubbs reagent in one portion. The mixture was kept at the temperature indicated in the table or text for the time indicated. When the reaction was complete, the rubber septum was taken off to expose the reactions mixtures to air under stirring for one hour. The mixture was then directly subjected to column chromatography eluting with an adequate solvent system to afford a crude product. Further purification using column chromatography was carried out to give each pure compound. GC-MS analysis was performed to determine the ratio of the products in each case.
Preparation of 6-t-butyldimethylsilyloxy-1-hexen-3-ol (6).
To a stirred solution of aldehyde 5 (306 mg, 1.5 mmol) in dry THF (15 mL), vinylmagnesium bromide (0.95M, 3.2 mL, 3.0 mmol) was added at –78˚C and the mixture was kept at the same temperature for 1.5 h. Water and sat. ammonium chloride soln. were added and the mixture was extracted with ether. The organic layer was washed with brine, dried (Na2SO4), and was evaporated to afford a residue, which was purified by silica gel column chromatography (5% hexane-EtOAc) to give alcohol 6 (245.5 mg, 70%); FTIR: 3350, 840, 780 cm-1; 1H-NMR (300 MHz) δ 0.05 (6H, s), 0.88 (9H, s), 1.61 (4H, m), 3.64 (2H, t, J=5.7 Hz), 4.11 (2H, m), 5.07 (1H, dt, J =10.5, 1.8 Hz), 5.22 (1H, dt, J =16.2, 1.5 Hz), 5.85 (1H, ddd, J =16.2, 10.5, 5.7 Hz); 13C NMR (75 MHz, CDCl3) δ –5.4 (CH3×2), 18.3 (C), 25.9 (CH3×3), 28.7 (CH2), 34.4 (CH2), 63.4 (CH2), 72.6 (CH), 114.3 (CH2), 141.1 (CH); MS (CI) m/z 231 [M+H]+, 219, 201, 161, 127, 105, 81 (base), 75; HRMS (CI) Found m/z 231.1777 [M+H]+ C12H27O2Si requires 231.1780.
Preparation of 6-t-butyldimethylsilyloxy-2-methyl-1-hexen-3-ol (7).
The corresponding Grignard reagent was prepared from 2-bromopropene (1.1 mL, 12.4 mmol) and Mg (0.3 g, 12.4 mmol) in dry THF (10 mL) and then a solution of aldehyde 5 (500 mg, 2.5 mmol) in dry THF (10 mL) was added slowly. The mixture was stirred overnight, and then water and sat. ammonium chloride soln. were added. The mixture was extracted with ether and the organic layer was washed with brine, dried (Na2SO4), and was evaporated to afford a residue. The residue was purified by silica gel column chromatography (10% hexane-EtOAc) to give alcohol 7 (210.8 mg, 35%); FTIR: 3400, 830, 780 cm-1; 1H-NMR (300 MHz) δ 0.04 (6H, s), 0.88 (9H, s), 1.60 (4H, m), 1.58 (3H, br s), 2.71 (1H, br s, OH), 3.64 (2H, t, J=6.0 Hz), 4.04 (1H, t, J=5.4 Hz), 4.81 (1H, br s), 4.94 (1H, br s); 13C-NMR (75 MHz) δ –5.0 (CH3×2), 18.2 (CH3), 18.7 (C), 26.3 (CH3×3), 29.2 (CH2), 32.6 (CH2), 63.7 (CH2), 75.7 (CH), 111.1 (CH2), 147.9 (C); MS (CI) m/z 245 [M+H]+, 227, 202, 187, 155, 145, 95 (base), 75, 67; HRMS (CI) Found m/z 245.1960 [M+H]+ C13H29O2Si requires 245.1937.
Preparation of (E)-7-t-butyldimethylsilyloxy-2-hepten-4-ol (8).
The Grignard reagent was prepared from trans-1-bromopropene (0.13 mL, 1.49 mmol) and Mg (36.1 mg, 1.5 mmol) in dry THF (10 mL) and a solution of aldehyde 5 (100 mg, 0.5 mmol) in dry THF (5 mL) was added slowly. The mixture was stirred at room temperature for 30 min. Water and sat. ammonium chloride soln. were added and the mixture was extracted with ether. The organic layer was washed with brine, dried (Na2SO4), and was evaporated to afford a residue, which was purified by silica gel column chromatography (2-100 % hexane-EtOAc) to give alcohol 8 (46.2 mg, 38%); FTIR: 3380 cm-1; 1H-NMR (200 MHz) δ 0.06 (6H, s), 0.89 (9H, s), 1.59-1.83 (8H, m), 3.61-3.67 (3H, m), 5.36-5.74 (2H, m); 13C-NMR (50MHz) δ –5.5 (CH3×2), 17.6 (CH3), 18.3 (C), 25.9 (CH3×3), 28.9 (CH2), 34.5 (CH2), 63.4 (CH2), 72.7 (CH), 126.4 (CH), 134.3 (CH); MS (CI) m/z 245 [M+H]+, 243, 227, 211, 187, 169, 145, 111, 95 (base), 75, 67, 57, 41; HRMS (CI) Found m/z 245.1926 [M+H]+ C13H29O2Si requires 245.1937.
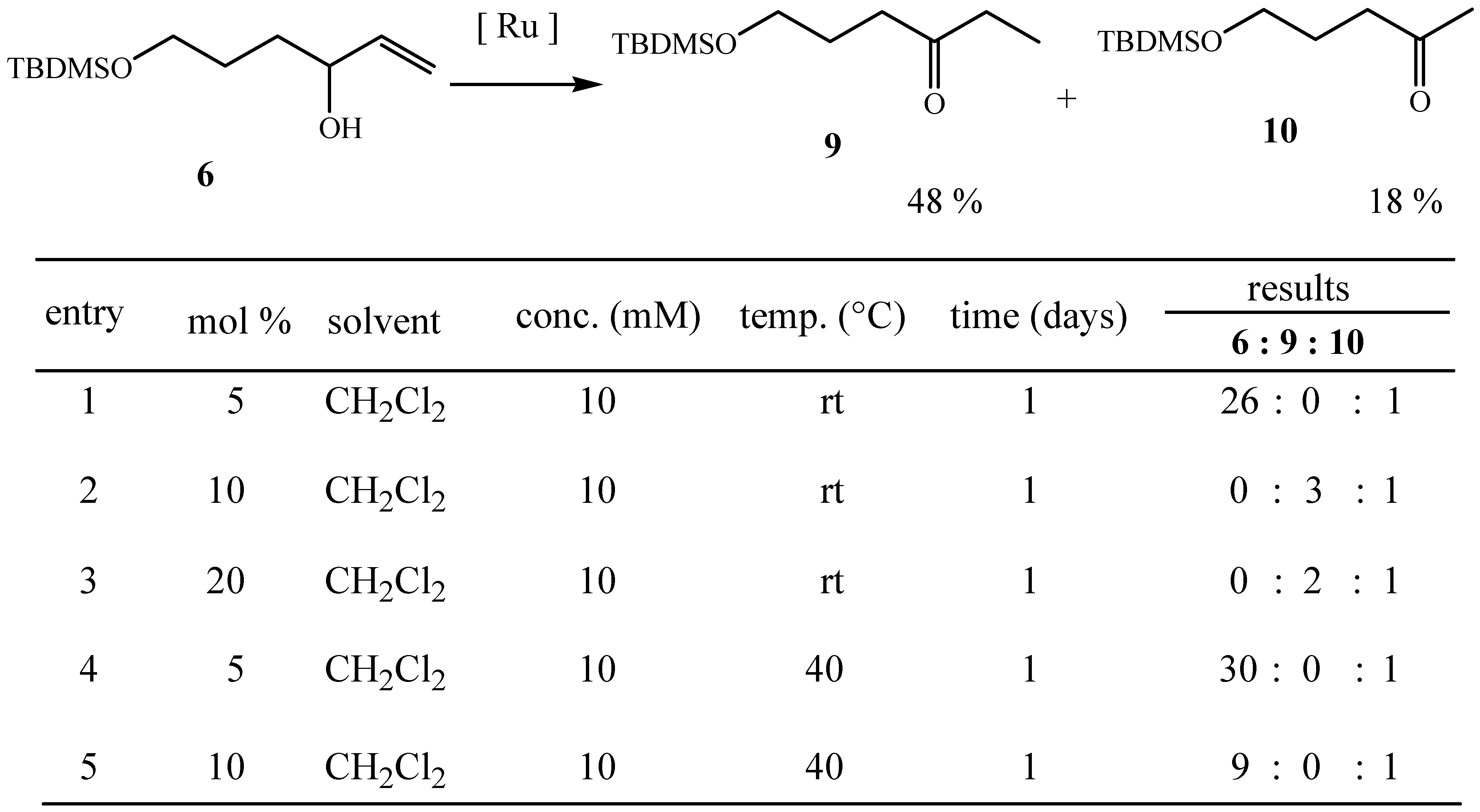
6-t-butyldimethylsilyloxy-3-hexanone (9); FTIR 1720 cm-1; 1H-NMR (300 MHz) δ 0.01 (6H, s), 0.86 (9H, s), 1.03 (3H, t, J=7.4 Hz), 1.76 (2H, quint, J =6.0 Hz), 2.41 (2H, q, J =6.0 Hz), 2.46 (2H, t, J =7.4 Hz), 3.59 (2H, t, J =6.0 Hz); 13C- NMR (75 MHz) δ –5.4 (CH3×2), 7.8 (CH3), 18.3 (C), 25.9 (CH3×3), 26.9 (CH2), 35.9 (CH2), 38.6 (CH2), 62.2 (CH2), 211.5 (C); MS (CI) m/z 231 [M+H]+, 215, 173, 139, 99 (base), 75; HRMS (CI) Found m/z 231.1791 [M+H] + C12H27O2Si requires 231.1790.
5-t-butyldimethylsilyloxy-2-pentanone (10); FTIR 1720 cm-1; 1H-NMR (300 MHz) δ -0.02 (6H, s), 0.83 (9H, s), 1.73 (2H, quint, J =6.0 Hz), 2.09 (3H, s), 2.45 (2H, t, J =6.0 Hz), 3.56 (2H, t, J =6.0 Hz); 13C-NMR (75 MHz) δ –5.4 (CH3×2), 18.2 (C), 25.9 (CH3×3), 26.8 (CH2), 29.9 (CH2), 40.0 (CH3), 62.0 (CH2), 208.8 (C); MS (CI) m/z 217 [M+H]+, 201, 159, 125, 85 (base); HRMS (CI) Found m/z 217.1625 [M+H] + C11H25O2Si requires 217.1624.
7-t-butyldimethylsilyloxy-2-hepten-4-one (11); FTIR 1680, 1640 cm-1; 1H-NMR (300 MHz) δ 0.03 (6H, s), 0.88 (9H, s), 1.81 (2H, quint, J =6.0 Hz), 1.89 (3H, dd, J =6.9, 1.7 Hz), 2.61 (2H, t, J =6.0 Hz), 3.62 (2H, t, J=6.0 Hz), 6.12 (1H, dq, J=15.9, 1.7 Hz), 6.85 (1H, dq, J=15.9, 6.9 Hz); 13C-NMR (75 MHz) δ –5.3 (CH3×2), 18.2 (CH3), 18.3 (C), 25.9 (CH3×3), 27.3 (CH2), 36.2 (CH2), 62.2 (CH2), 132.0 (CH), 142.4 (CH), 200.4 (C); MS (CI) m/z 243 [M+H]+, 228, 201, 185, 151, 111 (base), 89, 75; HRMS (CI) Found m/z 243.1782 [M+H] + C13H27O2Si requires 243.1780.
Preparation of 4-bromo-1-t-butyldimethylsilyloxybutane (13)[8].
To a stirred solution of alcohol 4 (2.0 g, 9.8 mmol) in dichloromethane (40 mL) was added triethylamine (1.5 mL, 10.8 mmol) and methanesulfonyl chloride (0.83 mL, 10.7 mmol) at 0˚C. The mixture was stirred for 1 h and sat. NaHSO4 soln. was added. The mixture was extracted with ether. The organic layer was washed with brine, dried (Na2SO4), and was evaporated to afford mesylate 12 (2.4 g, 88%). To a stirred solution of mesylate 12 (2.4 g, 8.6 mmol) in dry THF (60 mL) was added lithium bromide (1.13 g, 13.0 mmol) and the mixture was refluxed overnight. Sat. NaHSO4 was added and the mixture was extracted with pentane. The organic layer was washed with brine, dried (Na2SO4), and was evaporated to afford a residue, which was purified by silica gel column chromatography (5-100 % hexane-EtOAc) to give bromide 13 (1.7 g, 66%); 1H-NMR (300 MHz) δ 0.04 (6H, s), 0.89 (9H, s), 1.65 (2H, quint, J=6.3 Hz), 1.94 (2H, quint, J=6.6 Hz), 3.44 (2H, t, J=6.6 Hz), 3.64 (2H, t, J=6.3 Hz); 13C-NMR (75 MHz) δ –5.3 (CH3×2), 18.3 (C), 26.0 (CH3×3), 29.5 (CH2), 31.3 (CH2), 34.0 (CH2), 62.2 (CH2); MS (CI) m/z 267 [M+H]+, 209, 187 (base), 169, 135, 89; HRMS (CI) Found m/z 267.0792 [M+H]+ C10H24OBrSi requires 267.0780.
Preparation of 8-t-butyldimethylsilyloxy-2-methyl-2-octen-4-ol (15).
A solution of aldehyde 14 (200 mg, 2.38 mmol) in dry THF (5 mL) was added slowly to the Grignard reagent prepared from 13 (1.27 g, 4.76 mmol) and Mg (120 mg, 4.76 mmol) in dry THF (5 mL). The mixture was stirred overnight at room temperature. Water and sat. ammonium chloride soln. were added and the mixture was extracted with ether. The organic layer was washed with brine, dried (Na2SO4), and was evaporated to afford a residue, which was purified by silica gel column chromatography (5-100 % hexane-EtOAc) to give alcohol 15 (399 mg, 62%); FTIR: 3350, 840, 780 cm-1; 1H-NMR (300 MHz) δ 0.02 (6H, s), 0.86 (9H, s), 1.40 (6H, m), 1.65 (3H, d, J=1.5 Hz), 1.69 (3H, d, J=1.2 Hz), 3.58 (2H, t, J=6.6 Hz), 4.31 (1H, dt, J=8.7, 6.6 Hz), ), 5.12 (1H, br d, J=8.7 Hz); 13C-NMR (75 MHz) δ –5.3 (CH3×2), 18.2 (CH3), 18.3 (C), 21.7 (CH2), 25.8 (CH3), 25.9 (CH3×3), 32.7 (CH2), 37.4 (CH2), 63.1 (CH2), 68.6 (CH), 128.2 (CH), 134.8 (C); MS (CI) m/z 272 [M-H+H]+, 255, 215, 197, 139, 123 (base), 85, 81; HRMS (CI) Found m/z 272.2167 [M-H+H]+ C15H32O2Si requires 272.2172.
8-t-butyldimethylsilyloxy-2-methyl-2-octen-4-ol (16); FTIR: 1690 cm-1; 1H-NMR (200 MHz) δ 0.04 (6H, s), 0.89 (9H, s), 1.57 (5H, s), 1.70 (2H, dd, J=6.4, 1.2 Hz), 1.88 (2H, d, J=1.2 Hz), 2.14 (2H, d, J=1.2 Hz), 2.42 (1H, t, J=6.8 Hz), 3.61 (2H, t, J=6.4 Hz), 6.07 (1H, t, J=1.4 Hz); 13C-NMR (50MHz) δ –5.3 (CH3×2), 10.0, 18.4 (C), 20.7, 26.0 (CH3×3), 27.6, 32.4, 44.0, 63.0, 123.8; MS (CI) m/z 271 [M+H]+, 255, 239, 213, 197, 171, 155, 139 (base), 123, 101, 95, 83, 75, 67, 59, 41; HRMS (CI) Found m/z 271.2105 [M+H]+ C15H31O2Si requires 271.2093.
8-t-butyldimethylsilyloxy-2-methyl-1,3-octadiene (17); 1H-NMR (200 MHz) δ 0.05 (6H, s), 0.90 (9H, s), 1.14-1.83 (9H, m), 2.13 (2H, q, J=6.8 Hz), 4.86 (2H, s), 5.58-5.72 (1H, m), 6.14 (1H, d, J=15.6 Hz); 13C-NMR (50 MHz) δ –5.3 (CH3×2), 18.4 (C), 18.7 (CH2), 21.7 (CH3), 25.6 (CH2), 26.0 (CH3× 3), 32.4 (CH2), 63.1 (CH2), 114.2 (CH2), 130.8 (CH), 133.0 (CH), 142.2 (C); MS (CI) m/z 255 [M+H]+, 239, 211, 197, 155, 139, 123(base), 95, 83, 75, 67, 41; HRMS (CI) Found m/z 255.2136 [M+H]+ C15H31OSi requires 255.2144.