Experimental
General
Unless otherwise stated, all chemicals were purchased as the highest purity commercially available and were used without further purification. IR spectra (thin film) were recorded on a MATTSON-GENESIS II FT-IR spectrophotometer.
1H- and
13C-NMR spectra were recorded in deuterochloroform and referenced to the residual peak of CHCl
3 at δ 7.26 ppm and δ 77.0 ppm, for
1H- and
13C-, respectively, on a Bruker WP-200 SY or a Bruker DRX 400 MHz instrument. Chemical shifts are reported in δ ppm and coupling constants (J) are given in Hz. MS were performed at a VG-TS 250 spectrometer at 70 eV ionising voltage. Mass spectra are represented at
m/z (% rel. int.). HRMS were recorded on a VG Platform (Fisons) spectrometer using chemical ionisation (ammonia as gas). Optical rotations were determined at a digital ADP 220 polarimeter in 1 dm cells. Diethyl ether, THF and benzene were distilled from sodium, and pyridine and dichloromethane were distilled from calcium hydride under an Ar atmosphere. The raw material
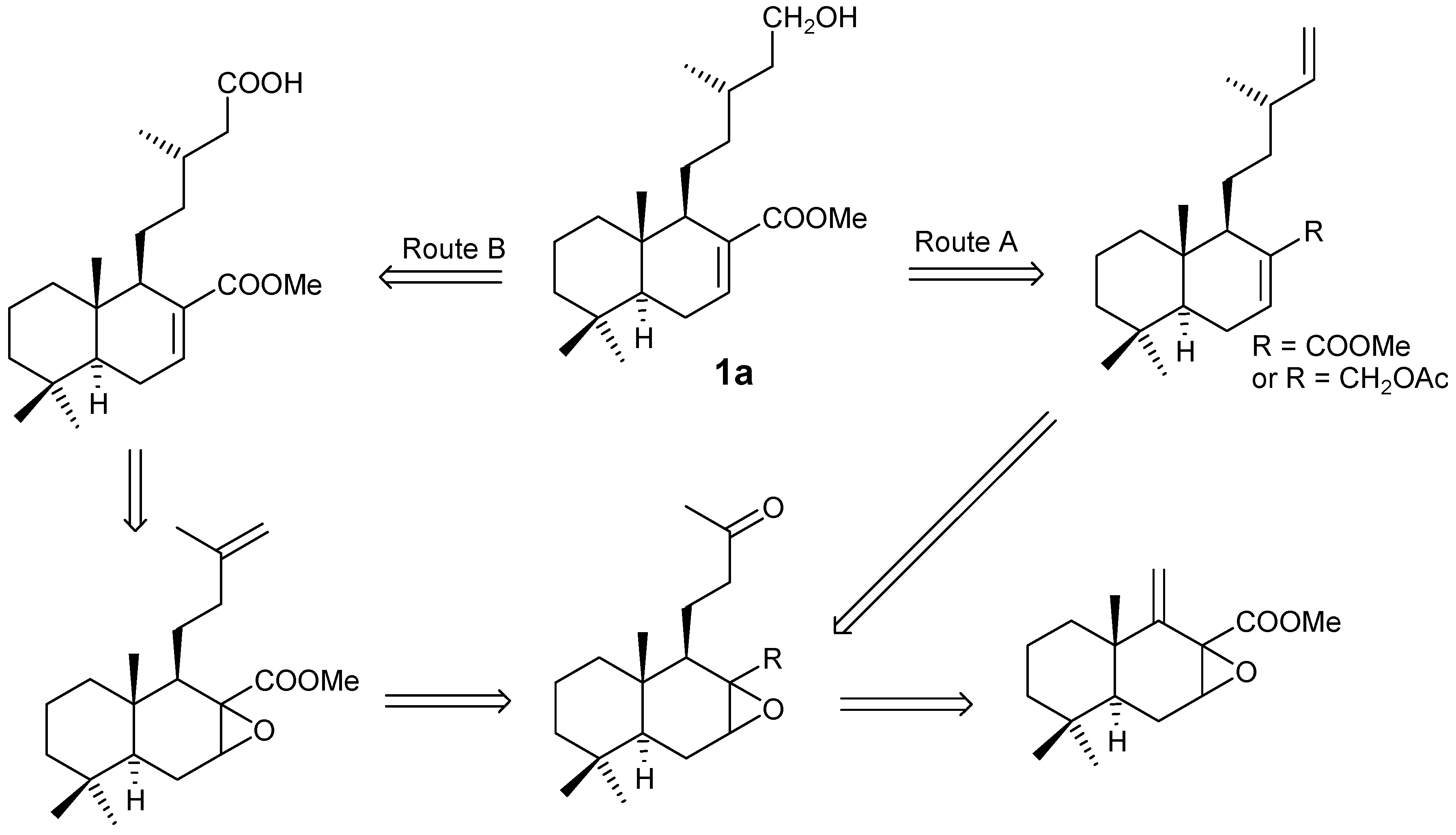
1 was isolated from a hexane extract of
Halimium verticillatum as reported in reference [
11].
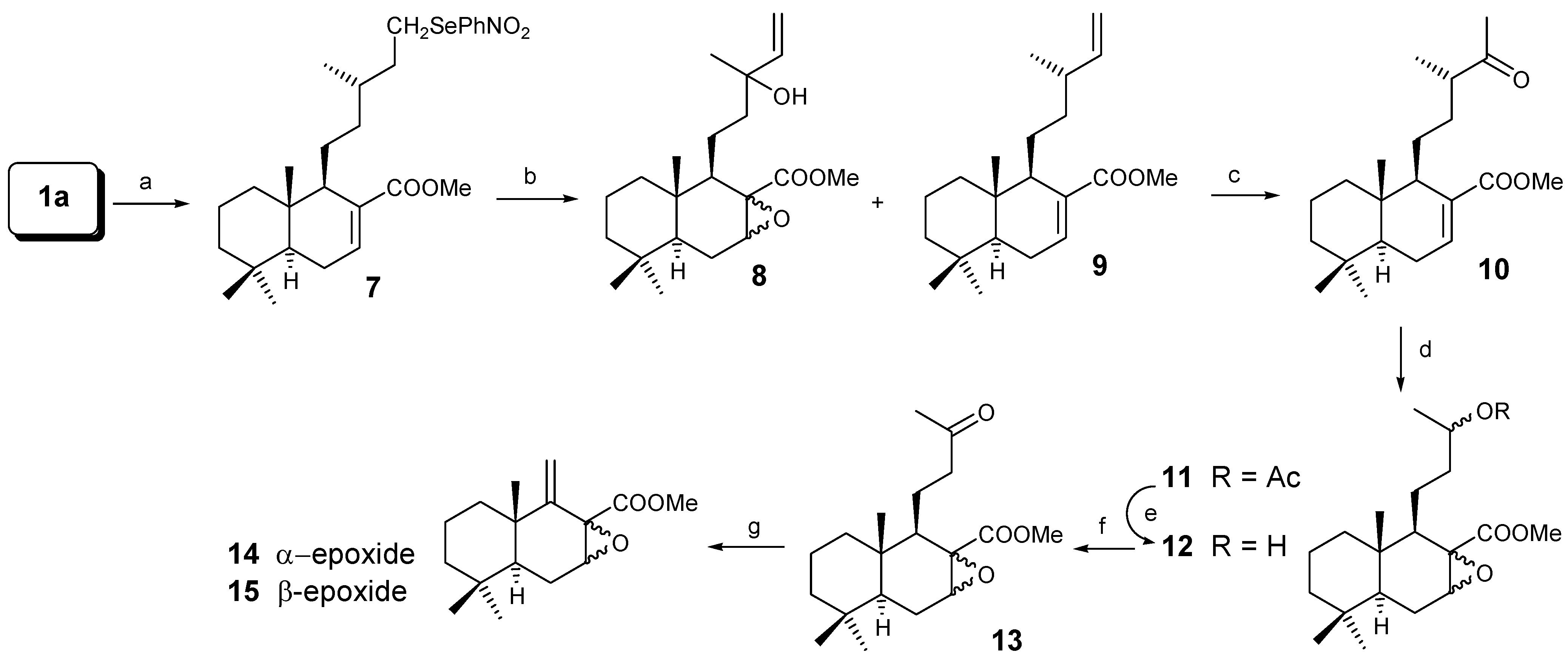
Reaction of 1a: Synthesis of methyl 15-o-nitrophenylseleno-7-labden-17-oate (7).
To a solution of 1a (750 mg, 2.2 mmol) in dry THF (3.3 mL) was added o-nitrophenyl-selenocyanate (635.8 mg, 2.8 mmol) and the mixture was stirred under an inert atmosphere for 20 min. at 48ºC. nBu3P (504.5 mg, 2.5 mmol) was added to the reaction mixture, that was stirred for 48 min., then the solvent was removed and the residue chromatographed (95:5 hexane/EtOAc) yielding 652.5 mg (87%) of 7; 1H-NMR δ: 8.30 (d, 1H, J=8.2, H-3’), 7.47 and 7.27 (both m, 3H, H-4’, H-5’ and H-6’), 6.66 (m, 1H, H-7), 3.70 (s, 3H, MeOOC), 2.95 (m, 2H, H-15), 2.15 – 1.30 (m, 11H), 1.22 – 0.90 (m, 6H), 0.98 (d, 3H, J=5.8 Hz, Me-16), 0.92 (s, 3H, Me-19), 0.89 (s, 3H, Me-18) and 0.84 (s, 3H, Me-20); 13C-NMR δ: 39.4 (C-1), 18.4 (c-2), 41.9 (C-3), 32.6 (C-4), 49.3 (C-5), 23.8 (C-6), 137.0 (C-7), 135.2 (C-8), 50.9 (C-9), 36.8 (C-10), 25.6 (C-11), 37.8 (C-12), 34.3 (C-13), 34.7 (C-14), 23.8 (C-15), 19.3 (C-16), 169.6 (C-17), 33.0 (C-18), 21.8 (C-19), 14.3 (C-20), 51.2 (COOMe), 134.0 (C-1’), 146.3 (C-2’), 126.3 (C-3’), 128.9 (C-4’), 133.4 (C-5’), 125.1 (C-6’); IR cm-1: 2925, 1714, 1644, 1590, 1564, 1515, 1332, 1247,1068, 730.
Oxidation of 7 with H2O2: Synthesis ofmethyl 13-hydroxy-7,14-labdadien-17-oate (8) andmethyl 7,14-labdadien-17-oate (9).
To a solution of 7 (600 mg, 1.15 mmol) in THF (6 mL) was added 30% H2O2 (0.30 mL, 130 mg, 2.0 mmol) and the mixture was stirred for 12 h. The solvent was evaporated at reduced pressure and the crude product chromatographed eluting with a hexane/EtOAc gradient yielding 24 mg (4%) of 8 (95:5 hexane/EtOAc) and 552 mg (92%) of 9 (98:2 hexane/EtOAc).
Compound 8: 1H-NMR δ: 6.69 (m, 1H, H-7), 5.86 (dd, 1H, J=17.4, 10.7, H-14), 5.19 (d, 1H, J=17.4, H-15a), 5.02 (d, 1H, J=17.4, H-15b), 3.69 (s, 3H, COOMe), 2.25 – 1.35 (m, 8H), 1.25 – 0.90 (m, 6H), 1.22 (s, 3H, Me-16), 0.89 (s, 3H, Me-19), 0.85 (s, 3H, Me-18) and 0.80 (s, 3H, Me-20); 13C-NMR δ: 39.3 (C-1), 18.4 (C-2), 41.9 (C-3), 32.6 (C-4), 49.3 (C-5), 22.0 (C-6), 137.4 and 137.6 (C-7, epimers), 135.5 and 135.7 (C-8, epimers), 50.7 and 50.8 (C-9, epimers), 36.9 (C-10), 23.9 (C-11), 42.6 and 42.8 (C-12, epimers), 73.1 (C-13), 144.9 and 145.4 (C-14, epimers), 111.3 and 111.6 (C-15, epimers), 169.5 (C-17), 33.0 (C-18), 21.9 (C-19), 14.2 (C-20), 51.3 (COOMe); IR cm-1: 3446, 3086, 2924, 1717, 1646, 1245,1071, 917; MS m/z (EI+): 334 (M+, <1%), 316 (4), 284 (19), 249 (15), 248 (79), 233 (28), 203 (15), 192 (29) 175 (25), 124 (15), 109 (56), 105 (16), 91 (24), 81 (33), 79 (34), 77 (15), 71 (100), 69 (28), 67 (20), 59 (15), 55 (45); HRMS (CI) for C21H34O3 (MH+): Calc 334.2508; Found 334.2519.
Compound 9: 1H-NMR δ: 6.51 (m, 1H, H-7), 5.56 (ddd, 1H, J=17.3, 10.1, 7.3, H-14), 4.82 (dd, 1H, J=17.3 Hz, 2.1 Hz, H-15a), 4.78 (dd, 1H, J=10.1, 2.1 Hz, H-15b), 3.59 (s, 3H, COOMe), 2.15 – 1.65 (m, 4H), 1.60 – 1.25 (m, 6H), 1.20 – 0.90 (m, 5H), 0.87 (d, 3H, J=6.7, Me-16), 0.81 (s, 3H, Me-19), 0.77 (s, 3H, Me-18) and 0.72 (s, 3H, Me-20); 13C-NMR δ: 39.4 (C-1), 18.3 (C-2), 41.9 (C-3), 32.5 (C-4), 49.2 (C-5), 23.6 (C-6), 136.2 (C-7), 135.4 (C-8), 50.8 (C-9), 36.6 (C-10), 25.7 (C-11), 37.8 (C-12), 38.5 (C-13), 144.4 (C-14), 112.2 (C-15), 20.1 (C-16), 169.3 (C-17), 33.0 (C-18), 21.8 (C-19), 14.2 (C-20), 50.9 (COOMe); [α]D20 - 16.7 (c 0.9 in CHCl3); IR cm-1: 3076, 2951, 1722, 1643, 1434, 1246, 1066, 909; MS m/z (EI+) 318 (M+, 1.8%), 248 (10), 195 (3), 194 (9), 165 (3), 162 (13), 137 (8), 135 (12), 124 (79), 109 (100), 95 (36), 81 (41), 69 (50), 67 (34), 55 (83).
Wacker oxidation of 9: Synthesis of methyl 14-oxo-7-labden-17-oate (10).
A solution of PdCl2 (128 mg, 0.72 mmol) and CuCl (3.5 g, 3.6 mmol) in DMF (10 mL) and H2O (1 mL) was activated for 30 min with O2. A solution of 9 (1.1 g, 3.6 mmol) in DMF (8 mL) was added and stirred with O2 atmosphere at room temperature for 36 h. Then to this mixture was added a cooled solution of 3N HCl and the mixture was extracted with CH2Cl2 (3 x 30 mL) and washed successively with 10% NaHCO3 and water, dried, filtered, evaporated and chromatographed (9:1 hexane/EtOAc) to give 910 mg (80%) of 10; 1H-NMR δ: 6.45 (m, 1H, H-7), 3.49 (s, 3H, COOMe), 2.24 (sex, 1H, J=6.8, H-13), 2.00 – 1.60 (m, 4H), 1.91 (s, 3H, Me-15), 1.40 – 0.90 (m, 10H), 0.85 (d, 3H, J=6.8, Me-16), 0.69 (s, 3H, Me-19), 0.65 (s, 3H, Me-18) and 0.60 (s, 3H, Me-20); 13C-NMR δ: 39.0 (C-1), 18.1 (C-2), 41.5 (C-3), 32.3 (C-4), 48.9 (C-5), 23.5 (C-6), 136.8 (C-7), 134.6 (C-8), 50.5 (C-9), 36.4 (C-10), 25.4 (C-11), 34.1 (C-12), 47.6 (C-13), 211.8 (C-14), 27.1 (C-15), 15.6 (C-16), 168.7 (C-17), 32.7 (C-18), 21.5 (C-19), 13.9 (C-20), 50.8 (COOMe); [α]D20 -11.1 (c 0.2 in CHCl3); IR cm-1: 2927, 1713, 1645, 1461, 1365, 1254, 1065; MS m/z (EI+) 334 (M+, <0.8%), 302 (2), 263 (5), 211 (8), 179 (37), 124 (32), 109 (100), 105 (20), 95 (16), 91 (32), 81 (26), 79 (32), 77 (17), 69 (26), 67 (17), 59 (12), 55 (30); HRMS (CI) for C21H34O3 (MH+): Calcd 334.2508; Found 334.2517.
Baeyer-Villiger reaction and epoxidation of 10 with m-CPBA: Synthesis of methyl 13-acetoxy-7,8-epoxy-14,15-dinor-labdan-17-oate (11).
To a solution of 10 (334.5 mg, 1.0 mmol) in dry CH2Cl2 (5 mL), m-CPBA (345.0 mg, 2.0 mmol) was added and the mixture stirred at room temperature. After 12 h, the solvent was removed and ether was added. The organic phase was washed with 40% Na2S2O3, 10% Na2CO3 and water until neutrality, dried over Na2SO4, filtered and evaporated to give 11 (317.8 mg, 95%). 1H-NMR δ: 4.60 (sex, 1H, J=6.1, H-13), 3.53 and 3.51 (two s, 2 x 3H, 2 x COOMe), 3.10 (d, 1H, J=6.2, H-7 β-epoxide), 3.00 (sb, 1H, H-7 α-epoxide), 2.00 – 1.78 (m, 1H), 1.81 and 1.79 (each s, 2 x H, 2 x OOCMe), 1.60 – 1.05 (m, 13H), 0.97 (d, 3H, J=6.1, Me-16), 0.66 and 0.62 (each s, 3 x 3H, Me-18, Me-19 and Me-20); 13C-NMR δ: 38.2 and 40.1 (C-1, isomers), 18.3 and 17.8 (C-2), 41.6 (C-3), 32.6 and 32.7 (C-4 isomers), 45.6 (α-isomer) and 47.1 (β-isomer) (C-5), 20.7 (C-6), 57.5 (α-isomer) and 59.4 (β-isomer) (C-7), 58.8 (α-isomer) and 60.2 (β-isomer) (C-8), 49.1 (β-isomer) and 53.5 (α-isomer) (C-9), 34.7 and 35.6 (C-10 isomers), 22.6 (C-11), 34.9 (C-12), 70.4 (C-13), 21.1 (C-16), 170.2 and 170.7 (C-17 isomers), 32.4 and 33.0 (C-18 isomers), 21.7 (C-19), 14.2 and 14.9 (C-20 isomers), 51.9 and 52.1 (2XCOOMe), 170.2 and 170.7 (2XMeCOO), 19.8 (MeCOO); IR cm-1: 2930, 1731, 1715, 1461, 1251, 1142.
Hydrolysis of 11: Synthesis of methyl 13-hydroxy-7α,8α-epoxy-14,15-dinor-labdan-17-oate (12α) and methyl 13-hydroxy-7β,8β-epoxy 14,15-dinor-labdan-17-oate (12β).
To a solution of 11 (250 mg, 0.68 mmol) in methanol (12 mL) was added K2CO3 (150 mg). The reaction mixture was stirred at room temperature for 1 h, water was added and the mixture extracted with ether, washed with 2N HCl and H2O, dried, filtered and evaporated yielding 212 mg (85%) of 12.
Compound 12α: 1H-NMR δ: 3.72 (s, 3H, COOMe), 3.71 (m, 1H, H-13), 3.22 (sb, 1H, H-7), 2.14 (dd, 1H, J=4.3, 14.4, H-6), 1.80 – 1.00 (m, 13H), 1.14 (d, 3H, J=6.1, Me-16), 0.87 (s, 3H,Me-19), 0.86 (s, 3H, 18) and 0.83 (s, 3H, Me-20); 13C-NMR δ: 38.5 (C-1), 18.6 (C-2), 41.8 (C-3), 33.0 (C-4), 45.9 (C-5), 20.1 (C-6), 57.8 (C-7), 59.1 (C-8), 53.8 (C-9), 35.0 (C-10), 21.9 (C-11), 38.3 (C-12), 67.7 (C-13), 23.6 (C-16), 171.1 (C-17), 32.6 (C-18), 21.8 (C-19) 14.6 (C-20), 52.2 (COOMe); [α]D20 + 6.9 (c 0.4 in CHCl3); IR cm-1: 3491, 2981, 1732, 1450, 1375, 1167, 1026.
Compound 12β: 1H-NMR δ: 3.72 (m, 1H, H-13), 3.71 (s, 3H, COOMe), 3.30 (d, 1H, J=6.3, H-7), 2.10 – 0.90 (m, 14H), 1.16 (d, 3H, J=6.2, Me-16); 0.87 (s, 3H, Me-19); 0.84 (s, 3H, Me-18) and 0.82 (s, 3H, Me-20); 13C-NMR δ: 38.5 (C-1), 18.0 (C-2), 41.9 (C-3), 32.9 (C-4), 47.7 (C-5), 21.0 (C-6), 59.7 (C-7), 60.5 (C-8), 49.4 (C-9), 35.9 (C-10), 23.2 (C-11), 40.6 (C-12), 68.1 (C-13), 23.4 (C-16), 172.7 (C-17), 33.2 (C-18), 21.9 (C-19), 15.2 (C-20), 52.3 (COOMe); IR cm-1: 3486, 2985, 1736, 1455, 1371, 1164, 1026.
Oxidation of 12 with CrO3/Py: Synthesis of methyl 7α,8α-epoxy-13-oxo-14,15-dinor-labdan-17-oate (13α) and methyl 7β,8β-epoxy-13-oxo-14,15-dinor-labdan-17-oate (13β).
Pyridine (1 mL) and dry CH2Cl2 (3 mL) were placed in a 50 mL Erlenmeyer flask externally cooled with ice. CrO3 (660 mg, 0.65 mmol) was added in small portions with stirring until a think yellow paste was obtained. This was allowed to reach room temperature and was stirred for 15 min under N2. Following this 12 (324.0 mg, 1.0 mmol) dissolved in dry CH2Cl2 (3 mL) was added and the mixture was stirred vigorously for 1.5 hours. The mixture was filtered and chromatographed on silica-gel, yielding 265.7 mg (82%) of 13. In some fractions the α isomer is the major compound and in other fractions, it is the β isomer.
Compound 13α: 1H-NMR δ: 3.62 (s, 3H, COOMe), 3.14 (sb, 1H, H-7), 2.35 – 2.20 (m, 1H, H-6), 2.01 (s, 3H, Me-16), 1.90 – 0.85 (m, 13H), 0.78 (s, 3H, Me-19), 0.77 (s, 3H, Me-18) and 0.73 (s, 3H, Me-20); 13C-NMR δ: 38.1 (C-1), 17.9 (C-2), 41.6 (C-3), 32.7 (C-4), 45.7 (C-5), 18.3 (C-6), 57.5 (C-7), 58.7 (C-8), 53.1 (C-9), 34.8 (C-10), 21.6 (C-11), 42.2 (C-12), 207.6 (C-13), 29.8 (C-16), 170.5 (C-17), 32.4 (C-18), 21.6 (C-19), 14.2 (C-20), 52.2 (COOMe); IR cm-1: 2926, 1732, 1712, 1461, 1282, 1158, 1062, 897.
Compound 13β: 1H-NMR δ: 3.73 (s, 3H, COOMe), 3.31 (d, 1H, J=6.4, H-7), 2.55 – 2.40 (m, 1H, H-6), 2.11 (s, 3H, Me-16), 2.10 – 0.90 (m, 13H), 0.88 (s, 3H, Me-19), 0.85 (s, 3H, Me-18) and 0.82 (s, 3H, Me-20); 13C-NMR δ: 40.5 (C-1), 18.0 (C-2), 41.9 (C-3), 32.9 (C-4), 46.9 (C-5), 20.9 (C-6), 59.9 (C-7), 60.1 (C-8), 49.4 (C-9), 36.0 (C-10), 20.9 (C-11), 42.6 (C-12), 208.2 (C-13), 29.7 (C-16), 172.7 (C-17), 33.3 (C-18), 21.9 (C-19), 15.2 (C-20), 53.5 (COOMe); IR cm-1: 2950, 1733, 1714, 1275, 1163, 1053.
Norrish type II reaction of 13: Synthesis of methyl 7α,8α-epoxy-9-drimen-12-oate (14) and methyl 7β,8β-epoxy-9-drimen-12-oate (15).
A solution of 13 (250.0 mg, 0.77 mmol) in dry hexane (250 mL) was placed in a quartz flask and a stream of dry N2 was bubbled through. The solution was irradiated with UV light (Hanau TQ-150, high pressure) for 90 min. Removal of solvent afforded a yellow oil which was purified by chromatography on silica-gel eluting with 98:2 hexane-EtOAc to yield 23.3 mg (9.3%) of 14, 56.3 mg (22.5%) of 15 and 97.5 mg (39%) of the starting material 13.
Compound 14: 1H-NMR δ: 5.25 (s, 1H, H-11a), 5.13 (s, 1H, H-11b), 3.76 (s, 3H, COOMe), 3.50 (t, 1H, J=1.9, H-7), 2.20 (ddd, 1H, J=1.9, 4.3, 15.1 Hz, H-6a), 1.80 (m, 2H, H-6b and H-1), 1.53 (m, 1H), 1.44 – 1.40 (m, 3H), 1.20 (dd, 1H, J=4.3, 13.1 Hz, H-5), 1.12 (m, 1H, H-3), 1.05 (s, 3H, Me-15), 0.90 (s, 3H, Me-14), and 0.85 (s, 3H, Me-13); 13C-NMR δ: 36.4 (C-1), 18.4 (C-2), 41.8 (C-3), 33.0 (C-4), 41.4 (C-5), 22.2 (C-6), 58.5 (C-7), 57.7 (C-8), 150.8 (C-9), 37.0 (C-10), 114.9 (C-11), 170.0 (C-12), 32.8 (C-13), 22.4 (C-14), 20.2 (C-15), 52.6 (COOMe); [α]D20 + 97.8 (c 0.5 in CHCl3); IR cm-1: 3098, 1743, 1633, 1373, 1242, 1159, 1047, 902; MS m/z (EI+) 264 (M+, 8%), 263 (3), 249 (49), 232 (26), 217 (35), 205 (22), 189 (46), 175 (29), 161 (74), 154 (30), 147 (59), 135 (88), 121 (69), 107 (87), 105 (92), 91 (100), 79 (80); HRMS (CI) for C16H24O3 (MH+): Calcd 264.1725; Found 264.1736.
Compound 15: 1H-NMR δ: 5.33 (s, 1H, H-11a), 5.19 (s, 1H, H-11b), 3.79 (s, 3H, COOMe), 3.57 (d, 1H, J=6.2, H-7), 2.13 (ddd, 1H, J=5.0, 6.2, 15.1 Hz, H-6a), 1.87 (m, 2H, H-6b and H-1), 1.52 – 1.38 (m, 4H), 1.22 (dd, 1H, J=5.0, 13.2, H-5), 1.13 (m, 1H), 1.09 (s, 3H, Me-15), 0.89 (s, 3H, Me-13) and 0.86 (s, 3H, Me-14); 13C-NMR δ: 38.5 (C-1), 18.5 (C-2), 41.8 (C-3), 33.4 (C-4), 48.2 (C-5), 21.3 (C-6), 60.6 (C-7), 60.2 (C-8), 151.1 (C-9), 37.6 (C-10), 115.0 (C-11), 170.0 (C-12), 32.8 (C-13), 21.7 (C-14), 22.7 (C-15), 52.4 (COOMe); [α]D20 + 73.5 (c 0.5 in CHCl3); IR cm-1: 3096, 2923, 1744, 1635, 1275, 1244, 1151, 1043, 902; MS m/z (EI+) 264 (M+, 3%), 263 (3), 249 (49), 232 (26), 217 (35), 205 (22), 189 (46), 175 (29), 161 (74), 154 (30), 147 (59), 135 (88), 121 (69), 107 (87), 105 (92), 91 (100), 79 (80); HRMS (CI) for C16H24O3 (MH+): Calcd 264.1725; Found 264.1737.
Treatment of 1a with m-CPBA: Synthesis of methyl 7,8(α+β)epoxy-15-hydroxylabdan-17-oate (16).
Compound 1a (490 mg, 1.5 mmol) was dissolved in CH2Cl2 (10 mL) and m-CPBA (380 mg, 2.2 mmol) was added. The mixture was stirred at 40º C and monitored by TLC. After 4 h the reaction was complete and the solvent evaporated. Work-up afforded 480 mg of crude product that after chromatography on silica-gel gave 323 mg (67.3%) of 16 in the 7:3 hexane/EtOAc fractions. 1H-NMR δ: 3.68 (s, 3H, COOMe), 3.64 (m, 2H, H-15), 3.26 (d, 1H, J=6.3, H-7 β-epoxide), 3.18 (sb, 1H, H-7 α-epoxide), 2.09 – 1.88 (m, 3H), 1.82 – 0.90 (m, 14H), 0.89 (d, 3H, J=6.7, Me-16), 0.84 (s each, 2 x 3H, Me-18 and Me-19) and 0.80 (s, 3H, Me-20); 13C-NMR δ (α-epoxide): 38.4 (C-1), 18.0 (C-2), 41.8 (C-3), 32.8 (C-4), 45.7 (C-5), 20.9 (C-6), 57.7 (C-7), 59.0 (C-8), 54.2 (C-9), 35.0 (C-10), 24.6 (C-11), 36.2 (C-12), 30.1 (C-13), 40.5 (C-14), 60.9 (C-15), 19.6 (C-16), 171.4 (C-17), 32.6 (C-18), 21.9 (C-19), 15.2 (C-20), 52.1 (COOMe); 13C-NMR δ (β-epoxide): 39.2 (C-1), 18.6 (C-2), 41.9 (C-3), 33.2 (C-4), 48.4 (C-5), 21.5 (C-6), 59.6 (C-7), 60.7 (C-8), 49.4 (C-9), 35.9 (C-10), 24.6 (C-11), 36.6 (C-12), 29.9 (C-13), 40.5 (C-14), 60.9 (C-15), 19.7 (C-16), 172.7 (C-17), 32.8 (C-18), 21.8 (C-19), 14.6 (C-20), 52.1 (COOMe); IR cm-1: 3445, 1732, 1457, 1283, 1063, 758; HRMS (CI) for C21H36O4 (MH+): Calcd 352.2614; Found 352.2625.
Reaction of 16: Synthesis of methyl 15-o-nitrophenylseleno-7α,8α-epoxylabdan-17-oate (17α) and methyl 15-o-nitrophenylseleno-7β,8β-epoxylabdan-17-oate (17β)
To a solution of 16 (450 mg, 1.3 mmol) in dry THF (3.0 mL) was added o-nitrophenylselenocyanate (363.3 mg, 1.6 mmol) and stirred under inert atmosphere for 20 min at 48º C. n-Bu3P (322.9 mg, 1.6 mmol) was added to reactional mixture, stirred for 48 min, the solvent was removed at reduced pressure and the residue chromatographed yielding with hexane/EtOAc 95:5, 338.0 mg (75%) of 17.
Compound 17α: 1H-NMR δ: 8.27 (d, 1H, J=8.1, H-3’), 7.60 – 7.18 (m, 3H, H-4’, H-5’ and H-6’), 3.67 (s, 3H, COOMe), 3.21 (sb, 1H, H-7), 2.89 (m, 2H, H-15), 2.24 – 1.90 (m, 3H), 1.85 – 0.90 (m, 14H), 0.94 (d, 3H, J=6.1, Me-16), 0.86 (s each, 2 x 3H, Me-19 and Me-18) and 0.83 (s, 3H, Me-20); 13C-NMR δ: 38.7 (C-1), 18.7 (C-2), 42.0 (C-3), 33.1 (C-4), 46.2 (C-5), 21.7 (C-6), 57.9 (C-7), 59.2 (C-8), 54.7 (C-9), 35.2 (C-10), 22.1 (C-11), 35.0 (C-12), 34.1 (C-13), 35.9 (C-14), 23.9 (C-15), 19.6 (C-16), 171.0 (C-17), 32.7 (C-18), 21.9 (C-19), 14.6 (C-20), 52.2 (COOMe), 134.8 (C-1’), 146.9 (C-2’), 126.5 (C-3’), 129.1 (C-4’), 133.6 (C-5’), 125.3 (C-6’); IR cm-1: 2930, 1740, 1596, 1514, 1331, 715, 711.
Compound 17β: 1H-NMR δ: 8.29 (d, 1H, J=8.2, H-3’), 7.40 – 7.30 (m, 3H,H-4’, H-5’ and H-6’), 3.71 (s, 3H, COOMe), 3.32 (d, 1H, J=6.4, H-7), 2.90 (m, 2H, H-15), 2.10 – 1.90 (m, 2H), 1.85 – 0.90 (m, 15H), 1.06 (d, 3H, J=6.1, Me-16), 0.90 (s, 3H, Me-19), 0.85 (s, 3H, Me-18) and 0.85 (s, 3H, Me-20); 13C-NMR (CDCl3) δ: 40.7 (C-1), 18.1 (C-2), 42.0 (C-3), 33.3 (C-4), 48.4 (C-5), 21.1 (C-6), 59.8 (C-7), 60.7 (C-8), 49.5 (C-9), 35.9 (C-10), 24.8 (C-11), 34.9 (C-12), 34.1 (C-13), 36.1 (C-14), 23.9 (C-15), 19.4 (C-16), 172.7 (C-17), 32.9 (C-18), 22.0 (C-19), 15.4 (C-20), 52.3 (COOMe), 134.4 (C-1’), 146.5 (C-2’), 126.5 (C-3’), 129.0 (C-4’), 133.6 (C-5’), 125.3 (C-6’); IR cm-1: 3062, 2929, 1733, 1590, 1513, 1332, 1037, 754.
Oxidation of 17 with H2O2: Synthesis of methyl 7,8(α+β)-epoxy-14-labden-17-oate (18).
To a solution of 17 (330.0 mg, 0.61 mmol) in THF (5 mL) was added 30% H2O2 (0.07 mL, 42 mg, 1.2 mmol) and the mixture was stirred for 12 h. The solvent was evaporated at reduced pressure and the crude product chromatographed yielding with 98:2 hexane/EtOAc, 284 mg (86%) of 18 as a mixture of epoxides. Data is given for a fraction in which the β-isomer predominates.
Compound 18β: 1H-NMR δ: 5.63 (ddd, 1H, J=7.7, 10.2, 17.5, H-14), 4.93 (dd, 1H, J=3.1, 17.5, H-15a), 4.91 (dd, 1H, J=3.1, 10.2, H-15b), 3.72 (s, 3H, COOMe), 3.29 (d, 1H, J=6.3, H-7), 2.15 – 1.90 (m, 2H, H-6), 1.85 – 0.98 (m, 13H), 0.96 (d, 3H, J=6.7, Me-16), 0.88 (s, 3H, Me-19) and 0.83 (s each, 2X3H, Me-18 and Me-20); 13C-NMR δ: 40.7 (C-1), 18.1 (C-2), 42.0 (C-3), 32.9 (C-4), 48.0 (C-5), 21.0 (C-6), 59.7 (C-7), 60.6 (C-8), 49.5 (C-9), 35.9 (C-10), 24.9 (C-11), 36.0 (C-12), 38.2 (C-13), 144.3 (C-14), 112.9 (C-15), 20.4 (C-16), 172.7 (C-17), 33.3 (C-18), 22.0 (C-19), 15.3 (C-20), 52.3 (COOMe); IR cm-1: 3074, 2926, 1733, 1649,1460, 1241.
Wacker oxidation of 18: Synthesis of methyl 7,8(α+β)-epoxy-14-oxolabdan-17-oate (19).
To a solution of PdCl2 (64 mg, 0.36 mmol) and CuCl (1.7 g, 1.8 mmol) in DMF (8 ml) and H2O (1 mL) was activated for 30 min with O2. A solution of 18 (550 mg, 1.8 mmol) in DMF (7 mL) was added and stirred with O2 atmosphere at room temperature for 36 h. Then the reaction mixture was added a cooled solution of 3N HCl, extracted with CH2Cl2 (3 x 30 mL) and washed successively with 10% NaHCO3 and water, dried, filtered, evaporated and chromatographed (eluting with 9:1 hexane/EtOAc) to give 440 mg (80%) of 19. A fraction collected during this CC was purified to give 4% of the α-isomer of 19, which was used for characterization purposes.
Compound 19α: 1H-NMR δ: 3.76 (s, 3H,COOMe), 3.23 (sb, 1H, H-7), 2.47 (sex, 1H, J=6.6, H-13), 2.11 (s, 3H, Me-15), 1.84 – 0.90 (m, 14H), 1.06 (d, 3H, J=6.6, Me-16), 0.86 (s each, 2X3H, Me-19 and Me-18) and 0.84 (s, 3H, Me-20); 13C-NMR δ: 38.5 (C-1), 18.6 (C-2), 41.9 (C-3), 33.0 (C-4), 46.0 (C-5), 21.9 (C-6), 57.8 (C-7), 59.0 (C-8), 54.4 (C-9), 35.1 (C-10), 21.9 (C-11), 32.3 (C-12), 47.4 (C-13), 212.1 (C-14), 28.0 (C-15), 16.4 (C-16), 170.8 (C-17), 32.6 (C-18), 21.9 (C-19), 14.5 (C-20), 52.3 (COOMe); m/z (EI+) 350 (M+, 10%), 318 (24), 300 (55), 285 (55), 261 (73), 257 (100), 233 (59), 229 (27), 219 (23), 201 (67), 177 (15), 163 (27), 151 (15), 141 (16), 123 (34), 109 (43), 95 (19), 79 (22), 69 (23), 55 (36); [α]D20 + 11.7 (c 0.5 in CHCl3); IR cm-1: 2926, 1731, 1718, 1461, 1282, 1158, 769; HRMS (CI) for C21H34O4 (MH+): Calcd 350.2457; Found 350.2468.
Reaction of 19 with m-CPBA: Synthesis of 11.
Compound 19 (300 mg, 0.86 mmol) was dissolved in anhydrous CH2Cl2 (10 mL) and m-CPBA (276 mg, 1.6 mmol) was added. The mixture was stirred at 30º C and monitored by TLC. After 5 h the reaction was complete and the solvent evaporated. Work-up afforded 290 mg of crude product that after chromatography on silica-gel gave in the 9:1 hexane/EtOAc fractions 264 mg (88%) of 11 as an epoxide mixture.
Reaction of 20: Synthesis of 17-acetoxy-15-o-nitrophenylseleno-7-labdene (21).
To a solution of 20 (450 mg, 1.3 mmol) in dry THF (3.0 mL) was added o-nitrophenylselenocyanate (363.3 mg, 1.6 mmol) and the mixture was stirred under an inert atmosphere for 20 min at 48º C. Then n-Bu3P (322.9 mg, 1.6 mmol) was added to the mixture, which ws stirred for 48 min, then the solvent was removed under reduced pressure and the residue chromatographed (95:5 hexane/EtOAc) yielding 382.5 mg (85%) of 21. 1H-NMR δ: 8.29 (d, 1H, J=8.2, H-3’), 7.55 – 7.26 (m, 3H, H-4’, H-5’ and H-6’), 4.57 (d, 1H, J=12.1, H-17a), 4.38 (d, 1H, J=12.1, H-17b), 2.85 (m, 2H, H-15), 2.03 (s, 3H, OOCMe), 2.00 – 0.95 (m, 17H), 0.97 (d, 3H, J=5.7, Me-16), 0.88 (s, 3H, Me-19), 0.85 (s, 3H, Me-18) and 0.75 (s, 3H, Me-20); 13C-NMR δ: 39.0 (C-1), 18.7 (C-2), 42.1 (C-3), 32.9 (C-4), 49.6 (C-5), 23.8 (C-6), 129.1 (C-7), 134.0 (C-8), 52.5 (C-9), 36.7 (C-10), 23.9 (C-11), 38.3 (C-12), 34.4 (C-13), 34.8 (C-14), 23.9 (C-15), 19.4 (C-16), 67.8 (C-17), 33.0 (C-18), 21.8 (C-19), 13.6 (C-20), 170.6 (MeCOO), 21.2 (Me-COO), 134.7 (C-1’), 146.7 (C-2’), 126.4 (C-3’), 129.1 (C-4’), 133.6 (C-5’), 125.2 (C-6’); IR cm-1: 2930, 1733, 1591, 1515, 1332, 1247, 737.
Oxidation of 21 with H2O2: Synthesis of 17-acetoxy-7,14-labdadiene (22).
To a solution of 21 (330 mg, 0.61 mmol) in THF (5 mL) was added 30% H2O2 (0.07 mL, 42 mg, 1.2 mmol) and this mixure was stirred for 12 h. The solvent was evaporated under reduced pressure and the crude product chromatographed (95:5 hexane/EtOAc) yielding 297 mg (90%) of 22. 1H-NMR δ: 5.71 (m, 1H, H-7), 5.58 (ddd, 1H, J=7.7, 10.3, 17.1, H-14), 4.89 (dd, 1H, J=2.1, 17.1, H-15a), 4.86 (dd, 1H, J=2.1, 17.1, H-15b), 4.50 (d, 1H, J=12.1, H-17a), 4.30 (d, 1H, J=12.1, H-17b), 1.99 (s, 3H, OOCMe), 1.95 – 0.80 (m, 15H), 0.92 (d, 3H, J=6.7, Me-16), 0.83 (s, 3H, Me-19), 0.80 (s, 3H, Me-18) and 0.69 (s, 3H, Me-20); 13C-NMR δ: 38.9 (C-1), 18.6 (C-2), 42.0 (C-3), 32.8 (C-4), 49.5 (C-5), 23.6 (C-6), 128.5 (C-7), 134.1 (C-8), 52.3 (C-9), 36.6 (C-10), 24.0 (C-11), 38.3 (C-12), 38.5 (C-13), 144.1 (C-14), 112.8 (C-15), 20.4 (C-16), 67.7 (C-17), 32.9 (C-18), 21.7 (C-19), 13.4 (C-20), 170.5 (MeCOO), 21.0 (Me-COO); [α]D20 - 3.9 (c 0.5 in CHCl3); IR cm-1: 3076, 1742, 1640, 1240, 911.
Wacker oxidation of 22: Synthesis of 23.
A solution of PdCl2 (64 mg, 0.34 mmol) and CuCl (1.6 g, 1.6 mmol) in DMF (8 mL) and H2O (1 mL) was activated for 30 min with O2. A solution of 22 (520 mg, 1.6 mmol) in DMF (7 mL) was then added and the mixture stirred with an O2 atmosphere at room temperature for 36 h, then it was added to a cooled solution of 3N HCl, extracted with CH2Cl2 (3 x 30 mL) and washed successively with 10% NaHCO3 and water, dried, filtered, evaporated and chromatographed (9:1 hexane/EtOAc) to give 416 mg (80%) of 23.
Treatment of 23 with m-CPBA: Synthesis of 13,17-diacetoxy-7,8-epoxy-14,15-dinor-labdane (24).
Compound 23 (300 mg, 0.86 mmol) was dissolved in anhydrous CH2Cl2 (10 mL) and m-CPBA (276 mg, 1.6 mmol) was added. The mixture was stirred at 40º C and monitored by TLC. After 4 h the reaction was complete and the solvent evaporated. Work-up afforded 290 mg of crude product that after chromatography on silica-gel giving 264 mg (88%) of 24 (as a mixture of epoxides) in the 9:1 hexane/EtOAc fractions. 1H-NMR δ: 4.95 – 4.71 (m, 1H, H-13), 4.20 (d, 1H, J=12.1, H-17a), 4.03 (d, 1H, J=12.1, H-17b), 3.22 (d, 1H, J=6.1, H-7 β-isomer), 3.14 (sb, 1H, H-7 α-isomer), 2.04 and 1.99 (s each, 2X3H, 2X-OOCMe), 1.85 – 0.75 (m, 14H), 1.19 (d, 3H, J=6.2, Me-16), 0.83 (s, 3H, Me-19), 0.82 (s, 3H, Me-18) and 0.72 (s, 3H, Me-20); 13C-NMR δ: 38.0 and 38.4 (C-1, isomers), 18.5 (C-2), 41.9 (C-3), 32.9 (C-4), 45.6 (C-5), 20.3 (C-6), 57.7 (C-7), 58.6 (C-8), 54.1 (C-9), 35.6 (C-10), 22.1 (C-11), 38.0 and 38.4 (C-12, isomers), 70.8 (C-13), 20.8 (C-16), 67.1 (C-17), 32.5 (C-18), 21.8 (C-19), 14.0 (C-20), 170.3 and 170.9 (2 x MeCOO), 21.2 (2 x Me-COO); IR cm-1: 2925, 1724, 1246, 1024.
Hydrolysis of 24: Synthesis of 13,17-dihydroxy-7,8-epoxy-14,15-dinor-labdane (25), 7α,13-dihydroxy-7,17-epoxy-14,15-dinor-labdane (26) and 7α,8β,13,17-tetrahydroxy-14,15-dinor-labdane (27).
Method A: To a solution of 24 (98.5 mg, 0.26 mmol) in methanol (4 mL) was added K2CO3 (120 mg). The reaction mixture was stirred at room temperature for 10 h, water was added and the mixture extracted with ether washed with 2N HCl and H2O, dried, filtered, evaporated and chromatographed yielding 59.1 mg of 25+26 (60%), and 29.6 mg of 27 (30%).
Method B: To a solution of 24 (110 mg, 0.29 mmol) was added a solution of 4% NaOH in methanol (6 mL). The reaction mixture was stirred at room temperature for 5 h, water was added and the mixture extracted with ether, washed with 2N HCl and H2O, dried, filtered, evaporated and chromatographed yielding 33 mg (30%) of 25, 16.5 mg (15%) of 26 and 36.3 mg (33%) of 27.
Compound 25: 1H-NMR δ: 3.83 (d, 1H, J= 12.2, H-17a), 3.70 (m, 1H, H-13), 3.65 (d, 1H, J=12.2 Hz, H-17b), 3.30 (m, 1H, H-7, α and β isomer), 1.90 – 0.90 (m, 14H), 1.42 (d, 3H, J=6.8, Me-16), 0.87 (s, 3H, Me-19) and 0.85 (s each, 2 x 3H, Me-18 and Me-20); 13C-NMR δ: 38.5 and 40.9 (C-1, isomers), 18.0 and 18.4 (C-2, isomers), 41.6 and 41.9 (C-3, isomers), 32.9 (C-4), 45.6 (C-5), 20.4 (C-6), 57.0 (C-7), 58.6 (C-8), 54.3 (C-9), 35.8 (C-10), 22.8 (C-11), 38.5 and 40.9 (C-12 isomer), 67.8 and 67.9 (C-13 isomers), 23.5 and 23.9 (C-16 isomers), 65.4 (C-17), 32.6 and 33.1 (C-18 isomers), 21.6 and 21.9 (C-19 isomers), 13.9 and 14.2 (C-20 isomers); IR cm-1: 3420, 2924, 1474, 1253, 1090, 1070.
Compound 26: 1H-NMR δ: 3.70 (m, 1H, H-13), 3.34 (t, 1H, J=2.9, H-7), 2.68 (d, 1H, J=4.3, H-17a), 2.44 (d, 1H, J=4.3, H-17b), 1.89 – 0.95 (m, 14H), 1.16 (d, 3H, J=6.7, Me-16), 0.87 (s, 3H, Me-19) and 0.85 (s each, 2X3H, Me-18 and Me-20); 13C-NMR δ: 38.5 and 40.9 (C-1 isomers), 18.0 and 18.4 (C-2 isomers), 41.6 and 41.9 (C-3 isomers), 32.9 (C-4), 46.8 (C-5), 27.7 (C-6), 73.5 (C-7), 61.2 (C-8), 46.9 (C-9), 35.8 (C-10), 22.8 (C-11), 38.5 and 40.9 (C-12 isomers), 67.8 and 67.9 (C-13 isomers), 23.5 and 23.9 (C-16 isomers), 48.6 (C-17), 32.6 and 33.1 (C-18 isomers), 21.6 and 21.9 (C-19 isomers),13.9 and 14.2 (C-20 isomers); IR cm-1: 3420, 2924, 1472, 1252, 1090, 1070.
Compound 27: 1H-NMR δ: 3.78 – 3.65 (m, 1H, H-13), 3.75 (d, 1H, J=12.0, H-17a), 3.53 (d, 1H, J=12.0, H-17b), 3.17 (sb, 1H, H-7), 1.90 – 1.00 (m, 14H), 1.15 (d, 3H, J=6.4, Me-16), 0.85 (s, 3H, Me-19), 0.83 (s, 3H, Me-19) and 0.80 (s, 3H, Me-20); 13C-NMR δ: 38.7 (C-1), 18.2 (C-2), 42.0 (C-3), 33.1 (C-4), 46.6 (C-5), 25.6 (C-6), 70.7 (C-7), 76.5 (C-8), 50.2 (C-9), 35.8 (C-10), 20.9 (C-11), 42.0 (C-12), 68.6 (C-13), 23.7 (C-16), 66.3 (C-17), 33.1 (C-18), 21.7 (C-19), 15.1 (C-20); IR cm-1: 3420, 1080.
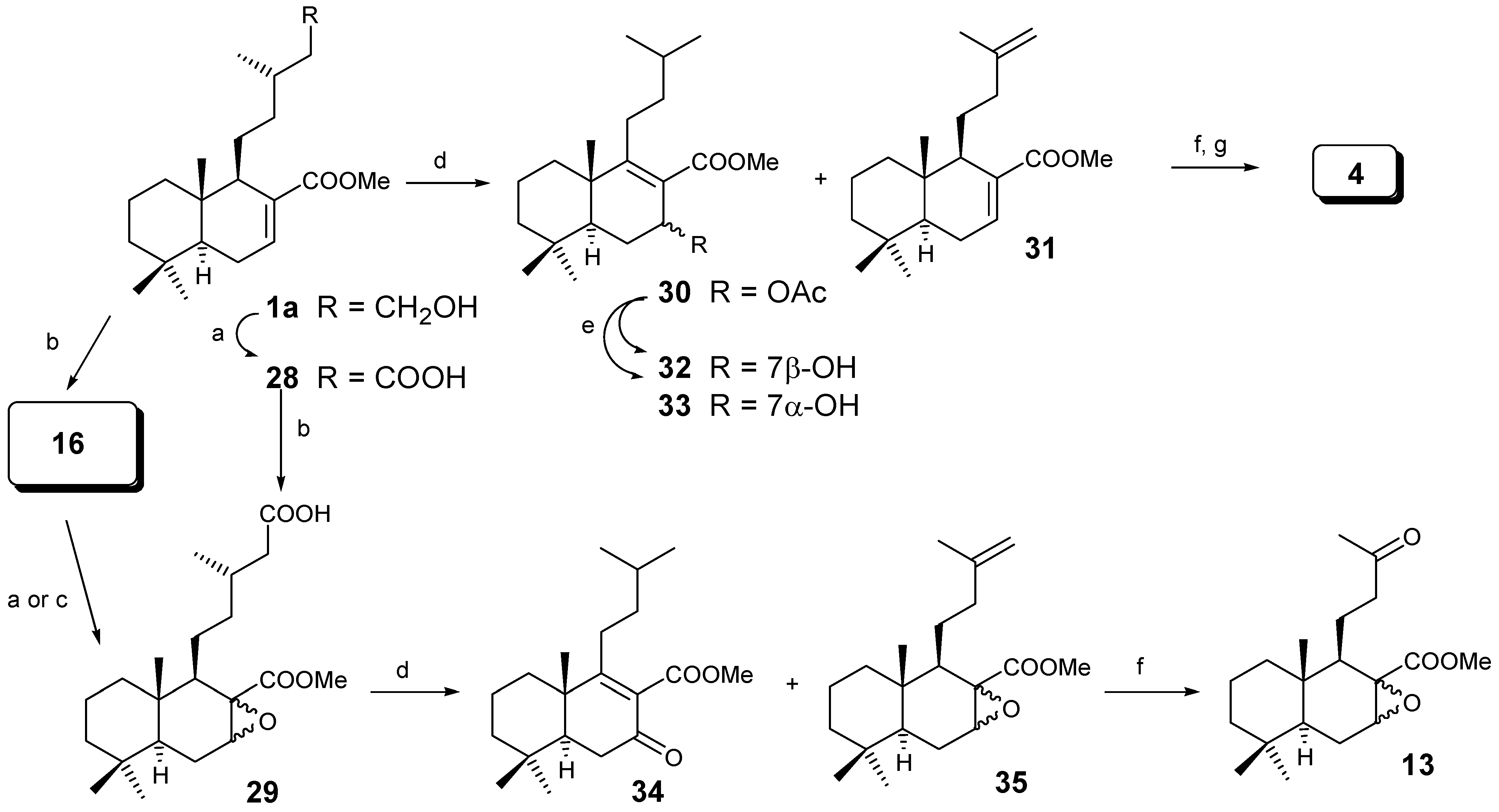
Oxidation of 1a: Synthesis of 7-labden-17-methoxycarbonyl-15-oic acid (28).
CrO3 (620 mg, 6.2 mmol) was added to 90% acetic acid (10.0 mL). After stirring for 15 min, 1a (1.4 g, 4.17 mmol) in CH2Cl2 (10 mL) were added and stirring was continued at 40º C for 40 h. MeOH was added and stirred for 30 min, the solvent removed in vacuo. The reaction product was extracted with ether, the ether solution was washed with a solution of 4% NaOH and the pH of the aqueous solution was adjusted to a value of 2 with HCl and the acidic products were recovered by extraction with ether. The solution of the acidic products was dried with anhydrous Na2SO4. Solvent removal and chromatography on silica-gel gave 860 mg (60%) of 28 in the 6:4 hexane/EtOAc fractions. 1H-NMR δ: 9.70 (sb, 1H, COOH), 6.62 (m, 1H, H-7), 3.62 (s, 3H, COOMe), 2.42 – 1.70 (m, 4H), 1.68 – 0.90 (m, 13H), 0.94 (d, 3H, J=6.9, Me-16), 0.87 (s, 3H, Me-19), 0.84 (s, 3H, Me-18) and 0.79 (s, 3H, Me-20); 13C-NMR δ: 39.5 (C-1), 18.5 (C-2), 42.0 (C-3), 32.7 (C-4), 49.4 (C-5), 23.9 (C-6), 137.2 (C-7), 135.3 (C-8), 50.9 (C-9), 36.9 (C-10), 25.6 (C-11), 38.1 (C-12), 31.0 (C-13), 41.3 (C-14), 179.7 (C-15), 19.7 (C-16), 170.7 (C-17), 33.1 (C-18), 21.9 (C-19), 14.3 (C-20), 51.3 (COOMe); IR cm-1: 3500 – 2500, 2929, 1710, 1645, 1254.
Epoxidation of 28: Synthesis of 29.
To a solution of 28 (846 mg, 2.41 mmol) in dry CH2Cl2 (5 mL), m-CPBA (800 mg, 4.6 mmol) was added and the mixture stirred at 40º C. After 30 h, the solvent was removed and ether was added, the organic phase was washed with 40% Na2S2O3, 10% Na2CO3 and water to neutrality, dried over Na2SO4, filtered and evaporated to give 29 (508 mg, 60%).
Oxidation of 16: Synthesis of 7,8(α+β)epoxylabdan-17-methoxycarbonyl-15-oic acid (29).
Method A: CrO3 (235 mg, 2.3 mmol) was added to 90% acetic acid (5.0 mL). After stirring for 15 min, 16 (330 mg, 0.9 mmol) in CH2Cl2 (5 mL) and glacial acetic acid (2 mL) were added and stirring was continued at room temperature for 8 h. MeOH was added and the solvent removed in vacuo. Work-up afforded 320 mg of crude product that after chromatography on silica-gel gave 181.5 mg (55%) of 29 in the EtOAc fractions.
Method B: To a stirred solution of PDC (1.2 mg, 3.3 mmol) in DMF (12.0 mL) was added 16 (340 mg, 1.0 mmol). After 40 h at room temperature the solvent is evaporated, extracted with ether, washed with 7-10 vol of water and dried over Na2SO4. The residue was chromatographed on silica-gel affording 889 mg (74%) of 29 in the EtOAc fractions.
Compound 29: 1H-NMR δ: 3.70 (s, 3H, COOMe), 3.28 (d, 1H, J=6.3, H-7 β), 3.26 (sb, 1H, H-7α), 2.40 – 2.00 (m, 3H), 1.95 – 1.00 (m, 14H), 0.96 (d, 3H, J=6.7, Me-16), 0.88 (s each, 2X3H, Me-18 and Me-19) and 0.85 (s, 3H, Me-20); 13C-NMR δ: 38.6 (C-1), 18.6 (C-2), 42.0 (C-3), 33.0 (C-4), 46.0 (C-5), 21.6 (C-6), 57.8 (C-7), 59.0 (C-8), 54.5 (C-9), 35.1 (C-10), 21.9 (C-11), 36.0 (C-12), 30.6 (C-13), 41.2 (C-14), 178.4 (C-15), 19.8 (C-16), 170.8 (C-17), 32.6 (C-18), 21.8 (C-19), 14.5 (C-20), 52.1 (COOMe); IR cm-1: 3550 – 2500, 1730, 1700, 1089, 758; HRMS (CI) for C21H34O5 (MH+): Calcd 366.4917; Found 366.4928.
Reaction of 28: Synthesis of methyl 15-nor-7(α+β)-acetoxy-8-labden-17-oate (30) and methyl 15-nor-7,13-labdadien-17-oate (31).
To a solution of 28 (343 mg, 0.98 mmol) in dry C6H6 (10 mL) was added dry pyridine (0.1 mL) under a N2 atmosphere and the mixture was stirred at room temperature. After 15 min, dry (AcO)2Cu (60 mg, 0.33 mmol) was added and the reaction mixture was heated at 80º C. (AcO)4Pb (1.280 g, 2.9 mmol) was added in six portions over the next 6 h. The solvent was removed and extracted with ether. The ethereal solution of neutral products was washed with water and dried over Na2SO4, filtered and evaporated to give 273 mg of crude mixture that by CC afforded 103.2 mg of 30 and 21.8 mg of 31.
Compound 30: 1H-NMR δ: 5.69 (t, 1H, J=8.7, H-7β), 5.68 (m, 1H, H-7α), 3.66 (s, 2 x 3H, 2 x COOMe), 2.60 – 2.00 (m, 4H), 1.98 (s, 2 x 3H, 2 x OOCMe), 1.97 – 1.10 (m, 10H), 1.09 (s, 3H, Me-20, β), 0.97 (s, 3H, Me-20, α), 0.87 (d, 2 x 3H, J=6.9, Me-16), 0.84 (d, 2 x 3H, J=6.9, Me-14) and 0.83 (s, 4 x 3H, Me-18 and Me-19); IR cm-1: 2956, 1733, 1651, 1238.
Compound 31: 1H-NMR δ: 6.61 (m, 1H, H-7), 4.63 (s, 2H, H-14), 3.69 (s, 3H, COOMe), 2.40 – 1.71 (m, 6H), 1.68 (s, 3H, Me-16), 1.62 – 0.98 (m, 8H), 0.88 (s, 3H, Me-19), 0.85 (s, 3H, Me-18) and 0.81 (s, 3H, Me-20); 13C-NMR (CDCl3) δ: 39.3 (C-1), 18.5 (C-2), 42.0 (C-3), 32.8 (C-4), 49.4 (C-5), 23.8 (C-6), 136.9 (C-7), 135.4 (C-8), 50.7 (C-9), 36.9 (C-10), 26.8 (C-11), 39.4 (C-12), 146.8 (C-13), 109.3 (C-14), 22.5 (C-16), 169.8 (C-17), 33.1 (C-18), 21.9 (C-19), 14.3 (C-20), 51.3 (COOMe); [α]D20 - 19.0 (c 0.2 in CHCl3); IR cm-1: 3079, 2960, 1720, 1652, 798.
Reaction of 31 with O3 and Norrish type II reaction: Synthesis of 5.
A solution of 31 (100 mg, 0.3 mmol), in CH2Cl2 (4 mL) was cooled to –78º C with acetone/Dry Ice. Ozone (about 5.2 g of O3/h) was bubbled through this solution for 1.5 min. To the cooled reaction mixture Ph3P (164.5 mg, 0.6 mmol) in CH2Cl2 (3 mL) was added and then it was gradually allowed to reach room temperature. The solvent was removed under reduced pressure and the residue chromatographed on silica-gel to give 75 mg (75%) of product. A solution of the reaction product (75 mg, 0.24 mmol) in dry hexane (250 mL) was placed in a quartz flask and a stream of dry N2 was bubbled through it. The solution was irradiated with UV light (Hanau TQ-150, high pressure) for 2 h. Removal of solvent afforded a yellow oil which was purified by chromatography on silica-gel eluting with 95:5 hexane/EtOAc to yield 37.5 mg (50%) of 5 and 34.2 mg (45%) of the starting material.
Hydrolysis of 30: Synthesis of methyl 15-nor-7β-hydroxy-8-labden-17-oate (32) and methyl 15-nor-7α-hydroxy-8-labden-17-oate (33).
To a solution of 30 (41.4 mg, 0.12 mmol) in methanol (4 mL) was added K2CO3 (60 mg). The reaction mixture was stirred at room temperature for 10 h, water was added and the mixture extracted with ether, washed with 2N HCl and H2O, dried, filtered, evaporated and chromatographed yielding 15.5 mg of 32 and 10.4 mg of 33.
Compound 32: 1H-NMR δ: 4.68 (t, 1H, J=8.7, H-7), 3.76 (s, 3H, COOMe), 2.62 (m, 1H), 2.20 – 1.10 (m, 14H), 1.10 (s, 3H, Me-20), 0.90 (s, 3H, Me-19), 0.89 (d, 3H, J=6.8, Me-16), 0.88 (d, 3H, J=6.8, Me-14) and 0.86 (s, 3H, Me-18); 13C-NMR δ: 40.1 (C-1), 18.7 (C-2), 41.4 (C-3), 33.0 (C-4), 49.5 (C-5), 28.4 (C-6), 69.7 (C-7), 128.6 (C-8), 158.5 (C-9), 40.6 (C-10), 26.6 (C-11), 35.9 (C-12), 29.1 (C-13), 22.3 (C-14), 22.3 (C-16), 170.4 (C-17), 33.0 (C-18), 21.7 (C-19), 20.1 (C-20), 51.3 (COOMe); [α]D20 + 38.4 (c 0.2 in CHCl3); IR cm-1: 3400, 2940, 1720, 1630, 1460, 1250, 1060; MS m/z (EI+) 322 (M+, 3%), 304 (17), 290 (4), 273 (4), 263 (17), 248 (19), 230 (7), 219 (20), 201 (19), 191 (10), 177 (14), 173 (13), 166 (30), 163 (63), 159 (12), 151 (19), 149 (13), 147 (11), 145 (16), 142 (32), 139 (15), 133 (19), 131 (17), 129 (11), 123 (26), 119 (37), 117 (17), 109 (60), 105 (40), 91 (40), 83 (21), 81 (27), 79 (27), 77 (22), 59 (20), 55 (70), 43 (79), 41 (100); HRMS (CI) for C20H34O3 (MH+): Calcd 322.2508; Found 322.2520.
Compound 33: 1H-NMR δ: 4.44 (m, 1H, H-7), 3.76 (s, 3H, COOMe), 2.61 – 2.41 (m, 2H), 1.99 – 1.10 (m, 11H), 0.98 (s, 3H, Me-20), 0.96 (d, 3H, J=6.8, Me-16), 0.88 (d, 3H, J=6.8, Me-14) and 0.86 (s, 2X3H, Me-18 and Me-19); 13C-NMR δ: 40.1 (C-1), 18.9 (C-2), 41.4 (C-3), 33.0 (C-4), 44.9 (C-5), 27.1 (C-6), 65.8 (C-7), 126.3 (C-8), 161.7 (C-9), 41.3 (C-10), 27.2 (C-11), 35.6 (C-12), 29.2 (C-13), 22.3 (C-14), 22.4 (C-16), 170.6 (C-17), 33.1 (C-18), 21.8 (C-19), 18.5 (C-20), 51.4 (COOMe); [α]D20 + 41.4 (c 0.3 in CHCl3); IR cm-1: 3450, 2950, 1715, 1620, 1460, 1230, 1100; MS m/z (EI+) 322 (M+, 1%), 304 (17), 263 (17), 248 (16), 234 (17), 233 (100), 219 (25), 201 (28), 191 (15), 177 (16), 167 (10), 164 (18), 163 (98), 159 (15), 149 (11), 147 (11), 145 (18), 133 (20), 121 (19), 119 (48), 117 (17), 105 (40), 95 (23), 91 (46), 83 (24), 81 (22), 79 (25), 77 (23), 69 (50), 67 (22), 55 (55), 43 (73), 41 (92).
Reaction of 29: Synthesis of methyl 7-oxo-15-nor-8-labden-17-oate (34) and methyl 7β,8β-epoxy-15-nor-13-labden-17-oate (35β).
To a solution of 29 (250 mg, 0.68 mmol) in dry C6H6 (10 mL) was added dry pyridine (0.1 mL) under a N2 atmosphere and the mixture was stirred at room temperature. After 15 min, dry (AcO)2Cu (50 mg, 0.27 mmol) was added and the reaction mixture is heated to 80º C. Over the next 6 h (AcO)4Pb (774 mg, 1.74 mmol) was added in six portions. The solvent was removed and the residue extracted with ether. The ethereal solution was washed with a solution of 4% NaOH for extraction of the unreacted acid 29 (44.2 mg). The solution of neutral products was washed with water and dried over Na2SO4, filtered and evaporated to give 195 mg of a mixture that by CC afforded 155 mg (62%) of 34 and 30 mg (12%) of 35.
Compound 34: 1H-NMR δ: 3.76 (s, 3H, COOMe), 2.60 – 2.00 (m, 4H), 1.90 – 1.12 (m, 13H), 1.12 (s, 3H, Me-20), 0.87 (d, 3H, J=7.2, Me-16), 0.86 (s each, 2X3H, Me-18 and Me-19) and 0.85 (d, 3H, J=7.2, Me-14); 13C-NMR δ: 38.9 (C-1), 18.3 (C-2), 41.0 (C-3), 33.2 (C-4), 49.8 (C-5), 34.9 (C-6), 196.4 (C-7), 132.1 (C-8), 167.9 (C-9), 40.5 (C-10), 28.1 (C-11), 34.9 (C-12), 29.0 (C-13), 22.0 (C-14), 22.1 (C-16), 172.7 (C-17), 32.4 (C-18), 21.3 (C-19), 18.3 (C-20), 52.1 (COOMe); [α]D20 + 22.3 (c 0.4 in CHCl3); IR cm-1: 2956, 1750, 1667, 1583, 1462, 1348, 1242, 1144, 1095, 1022, 801; MS m/z (EI+) 320 (M+, 0.8%), 288 (25), 273 (14), 245 (100), 217 (41), 189 (27), 175 (29), 161 (25), 149 (32), 135 (23), 121 (22), 109 (39), 91 (31), 79 (22), 77 (17); HRMS (CI) for C20H32O3 (MH+): Calcd 320.2351; Found 320.2363.
Compound 35β: 1H-NMR δ: 4.65 (s, 1H, H-14a), 4.63 (s, 1H, H-14b), 3.70 (s, 3H, COOMe), 3.28 (d, 1H, J=6.4, H-7), 2.22 – 0.99 (m, 14H), 1.67 (s, 3H, Me-16), 0.86 (s, 3H, Me-19), 0.83 (s, 3H, Me-19) and 0.81 (s, 3H, Me-20); 13C-NMR δ: 40.5 (C-1), 18.1 (C-2), 42.0 (C-3), 32.9 (C-4), 47.9 (C-5), 25.8 (C-6), 59.7 (C-7), 60.6 (C-8), 49.4 (C-9), 35.9 (C-10), 21.0 (C-11), 37.3 (C-12), 146.0 (C-13), 109.8 (C-14), 22.5 (C-16), 172.5 (C-17), 33.2 (C-18), 22.0 (C-19), 15.3 (C-20), 52.3 (COOMe); IR cm-1: 3070, 2931, 1740, 1650, 1454, 1381, 1283, 1161, 1054, 891, 769; MS m/z (EI+) 320 (M+, 0.8%), 281 (8), 243 (7), 234 (9), 219 (49), 207 (23), 193 (20), 135 (26), 123 (44), 109 (100), 95 (65), 93 (43), 81 (83), 79 (45); HRMS (CI) for C20H32O3 (MH+): Calcd 320.2351; Found 320.2362.
Reaction of 35 with O3: Synthesis of 13.
A solution of 35 (220 mg, 0.69 mmol) in CH2Cl2 (6 mL), was cooled to –78º C with acetone/dry ice. Ozone (about 5.2 g of O3/h) was bubbled through this solution for 8 min. To the cooled reaction mixture Ph3P (230 mg, 0.90 mmol) in CH2Cl2 (6 mL) was added and the mixture was gradually allowed to reach room temperature. The solvent was then removed under reduced pressure and the residue chromatographed on silica-gel affording 16.7 mg (7.6%) of 35 and 158.4 mg (72%) of 13.
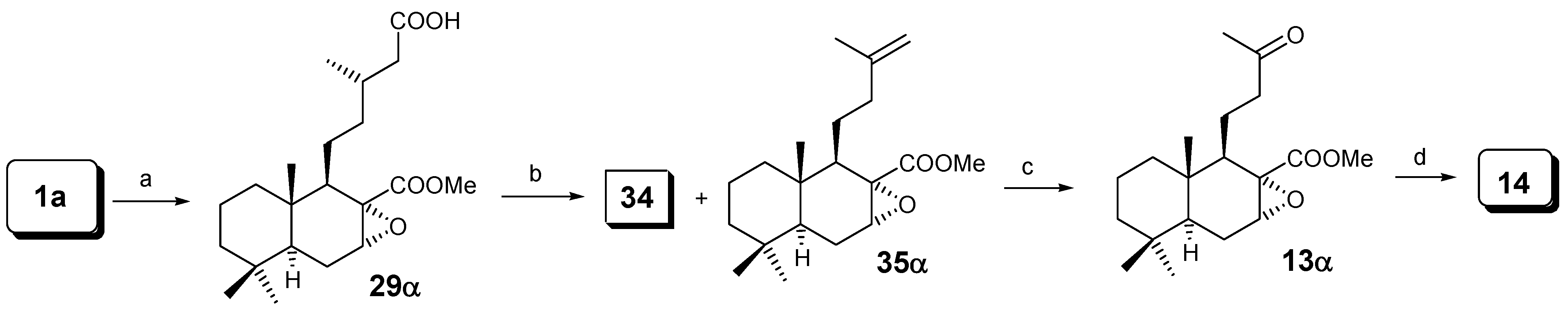
Reaction of 1a with dimethyldioxirane: Synthesis of 7α,8α-epoxylabdan-17-methoxycarbonyl-15-oic acid (29α).
To a solution of 1a (196 mg, 0.58 mmol) in acetone (5 mL) was added dimethyldioxirane (8 mL, 0.1 M). The reaction is carried out at room temperature. After 20 min, the solvent was removed and the residue chromatographed on silica-gel (elution with 7:3 hexane/EtOAc) affording 88.2 mg (45%) of 29α. 1H-NMR δ: 3.72 (s, 3H, COOMe), 3.22 (sb, 1H, H-7), 2.40 – 1.00 (m, 17H), 0.96 (d, 3H, J=6.7, Me-16), 0.89 (s, 3H, Me-19), 0.88 (s, 3H, Me-18) and 0.85 (s, 3H, Me-20); 13C-NMR δ: 38.6 (C-1), 18.6 (C-2), 42.0 (C-3), 33.0 (C-4), 46.0 (C-5), 21.6 (C-6), 57.8 (C-7), 59.0 (C-8), 54.5 (C-9), 35.1 (C-10), 21.9 (C-11), 36.0 (C-12), 30.6 (C-13), 41.2 (C-14), 178.4 (C-15), 19.8 (C-16), 170.8 (C-17), 32.6 (C-18), 21.8 (C-19), 14.5 (C-20), 52.1 (COOMe); IR cm-1: 3380 – 2600, 1736, 1712, 1437, 1279, 1161, 1054, 755.
Reaction of 29α: Synthesis of 34 and methyl 7α,8α-epoxy-15-nor-13-labden-17-oate (35α).
To a solution of 29α (250 mg, 0.68 mmol) in dry C6H6 (10 mL) was added dry pyridine (0.1 mL) under a N2 atmosphere and the mixture was stirred at room temperature. After 15 min, dry (AcO)2Cu (42 mg, 0.23 mmol) was added and the reaction mixture was heated to 80º C. Over 6 h additional (AcO)4Pb (882.8 mg, 2.0 mmol) was added in six portions. The solvent was removed and the residue extracted with ether. The ethereal solution was washed with a solution of 4% NaOH to extract the unreacted acid 29α (25 mg). The solution of neutral products was washed with water and dried over Na2SO4, filtered and evaporated to give 220 mg of a mixture that after CC afforded 149 mg (60%) of 34 and 30.1 mg (12%) of 35α. 1H-NMR δ: 4.71 (s, 1H, H-14a), 4.68 (s, 1H, H-14b), 3.73 (s, 3H, COOMe), 3.23 (sb, 1H, H-7), 2.20 – 1.00 (m, 14H), 1.63 (s, 3H, Me-16), 0.89 (s, 3H, Me-19), 0.87 (s, 3H, Me-18) and 0.85 (s, 3H, Me-20); 13C-NMR δ: 38.5 (C-1), 18.6 (C-2), 42.0 (C-3), 33.0 (C-4), 46.0 (C-5), 22.0 (C-6), 57.8 (C-7), 59.0 (C-8), 53.3 (C-9), 35.3 (C-10), 22.3 (C-11), 36.9 (C-12), 145.2 (C-13), 110.7 (C-14), 22.2 (C-16), 171.0 (C-17), 32.6 (C-18), 21.9 (C-19), 14.6 (C-20), 52.2 (COOMe); IR cm-1: 3073, 2927, 1733, 1649, 1437, 1277, 1053, 886, 768.
Reaction of 35α with O3: Synthesis of 13α.
A solution of 35α (100 mg, 0.31 mmol) in CH2Cl2 (5 mL), was cooled to –78º C. Ozone (about 5.2 g of O3/h) was bubbled through this solution for 8 min. To the cooled reaction mixture Ph3P (158.4 mg, 0.62 mmol) in CH2Cl2 (6 mL) was added and the mixture was gradually allowed to reach room temperature. The solvent was then removed under reduced pressure and the residue chromatographed on silica-gel affording 6.0 mg (6%) of 35α and 72.0 mg (72%) of 13α.
Norrish type II reaction of 13α: Synthesis of 14.
A solution of 13α (100 mg, 0.31 mmol) in dry hexane (250 mL) was placed in a quartz flask and a stream of dry N2 was bubbled through. The solution was irradiated with UV light (Hanau TQ-150, high pressure) for 90 min. Removal of the solvent afforded a yellow oil which was purified by chromatography on silica-gel eluting with 98:2 hexane/EtOAc, to yield 28.5 mg (28%) of 14 and 40.0 mg (40%) of the starting material 13α.