Experimental
General
Melting points (uncorrected) were determined on a Kofler hot-stage microscope (Reichert).
1H- NMR spectra were recorded on a Bruker Avance DPX 200 (200 MHz) or on a Varian UnityPlus 300 (300 MHz) spectrometer. IR spectra were taken on a Perkin-Elmer 1605 FT-IR instrument. Mass spectra were obtained on a Shimadzu QP5050A DI 50 instrument, high-resolution mass spectra were recorded on a Finnigan MAT 8230 spectrometer at the Department of Organic Chemistry, University of Vienna. Column chromatography was carried out on Merck Kieselgel 60, 0.063–0.200 mm, thin layer chromatography was done on Merck aluminium sheets pre-coated with Kieselgel F
254. Microanalyses [
13] were performed at the Institute of Physical Chemistry (Microanalytical Laboratory), University of Vienna.
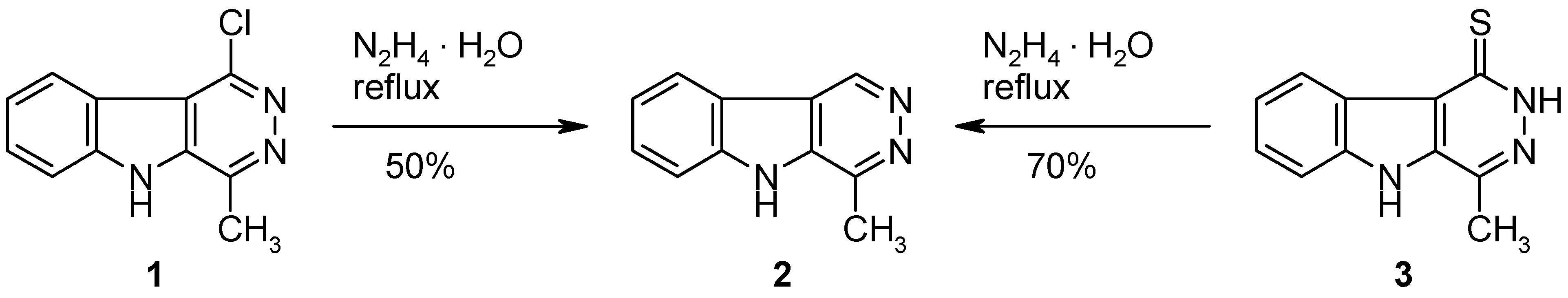
4-Methyl-5H-pyridazino[4,5-b]indole (2).
Method A: A mixture of the chloro compound 1 [1] (217 mg, 1 mmol) and hydrazine hydrate (5 mL, 0.1 mol) was refluxed for 48 h. The excess reagent was removed under reduced pressure and the residue was triturated with water (10 mL). The product was collected by filtration and recrystallized from EtOH to give 2 (92 mg, 50%) as colourless crystals, mp >320 °C (dec.; sublimation above 280 °C; lit. [1]: >320 °C dec.); identified by 1H-NMR and MS [1].
Method B: A mixture of the thione
3 [
1] (215 mg, 1 mmol) and hydrazine hydrate (2 mL, 0.04 mol) in EtOH (10 mL) was refluxed for 48 h. The volatile components were removed under reduced pressure and the residue was triturated with water (10 mL). The product was collected by filtration and recrystallized from EtOH to give
2 (129 mg, 70%) as colourless crystals (see above).
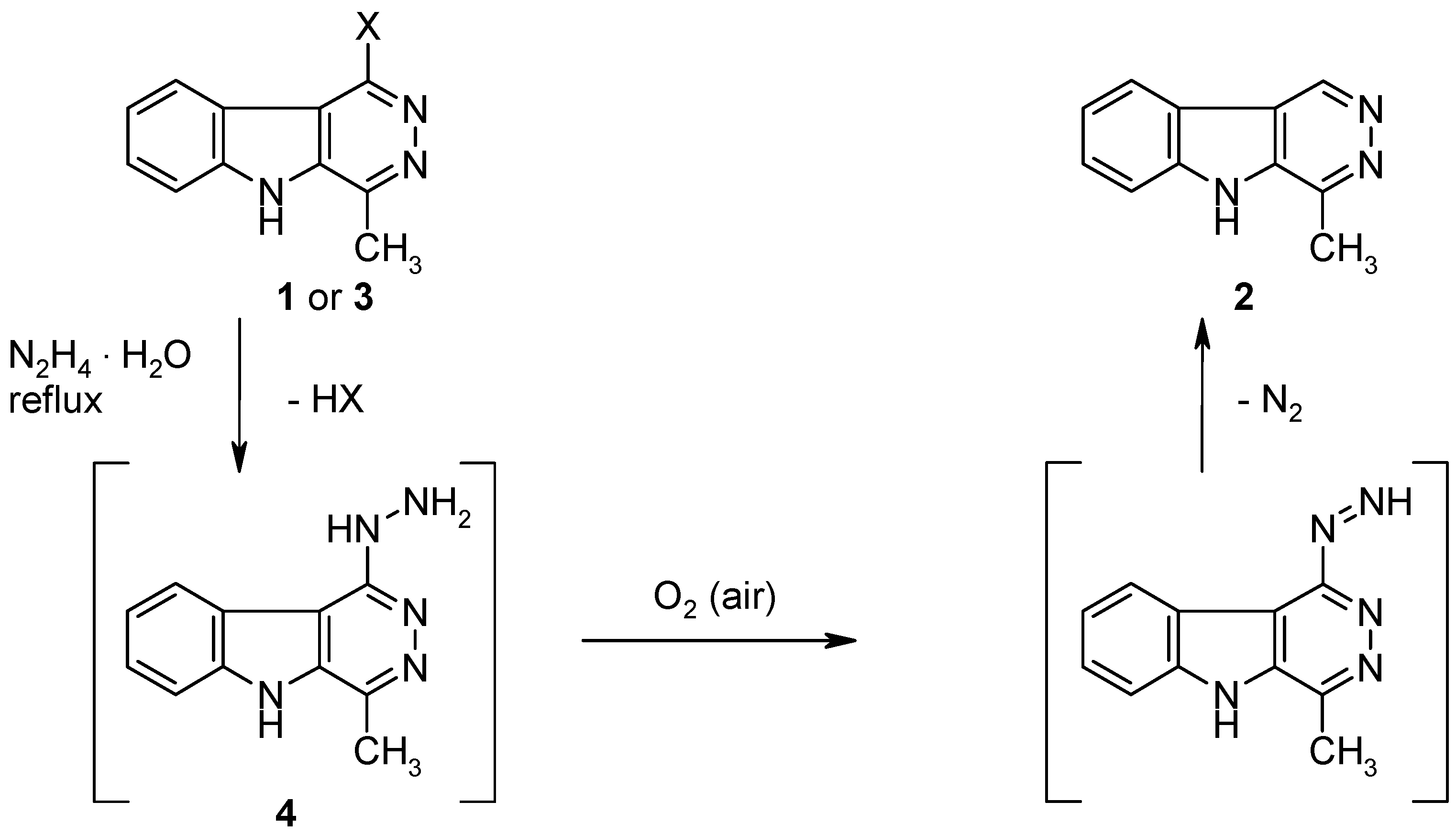
1-Hydrazino-4-methyl-5H-pyridazino[4,5-b]indole (4).
A mixture of the chloro compound 1 [1] (217 mg, 1 mmol) and hydrazine hydrate (5 mL, 0.1 mol) was flushed with argon, then it was refluxed under argon for 48 h. The excess reagent was removed under reduced pressure and the residue was triturated with water (10 mL). The product was collected by filtration and dried to give 4 (210 mg, 99%) as colourless crystals, mp >300 °C. This material is air-sensitive and was used for the following transformations without further purification. IR (KBr): 3321, 3298, 3155, 3141, 3078, 2981, 1620, 1573, 1423, 1328, 1216, 1111, 1017, 910, 744, 721 cm–1; MS (EI, 70 eV) m/z: 213 (M+, 100%), 183 (20), 168 (67), 156 (9), 142 (45), 128 (8), 115 (49), 101 (6), 88 (21), 70 (13), 63 (17); 1H-NMR (DMSO-d6) δ: 12.07 (s, 1H, 5-NH), 8.39 (d, J8,9 = 8.0 Hz, 1H, 9-H), 7.88 (s, 1H, NHNH2), 7.66 (d, J6,7 = 8.0 Hz, 1H, 6-H), 7.55–7.47 (m, 1H, 7-H), 7.34–7.27 (m, 1H, 8-H), 4.59 (s, 2H, NHNH2), 2.73 (s, 3H, CH3).
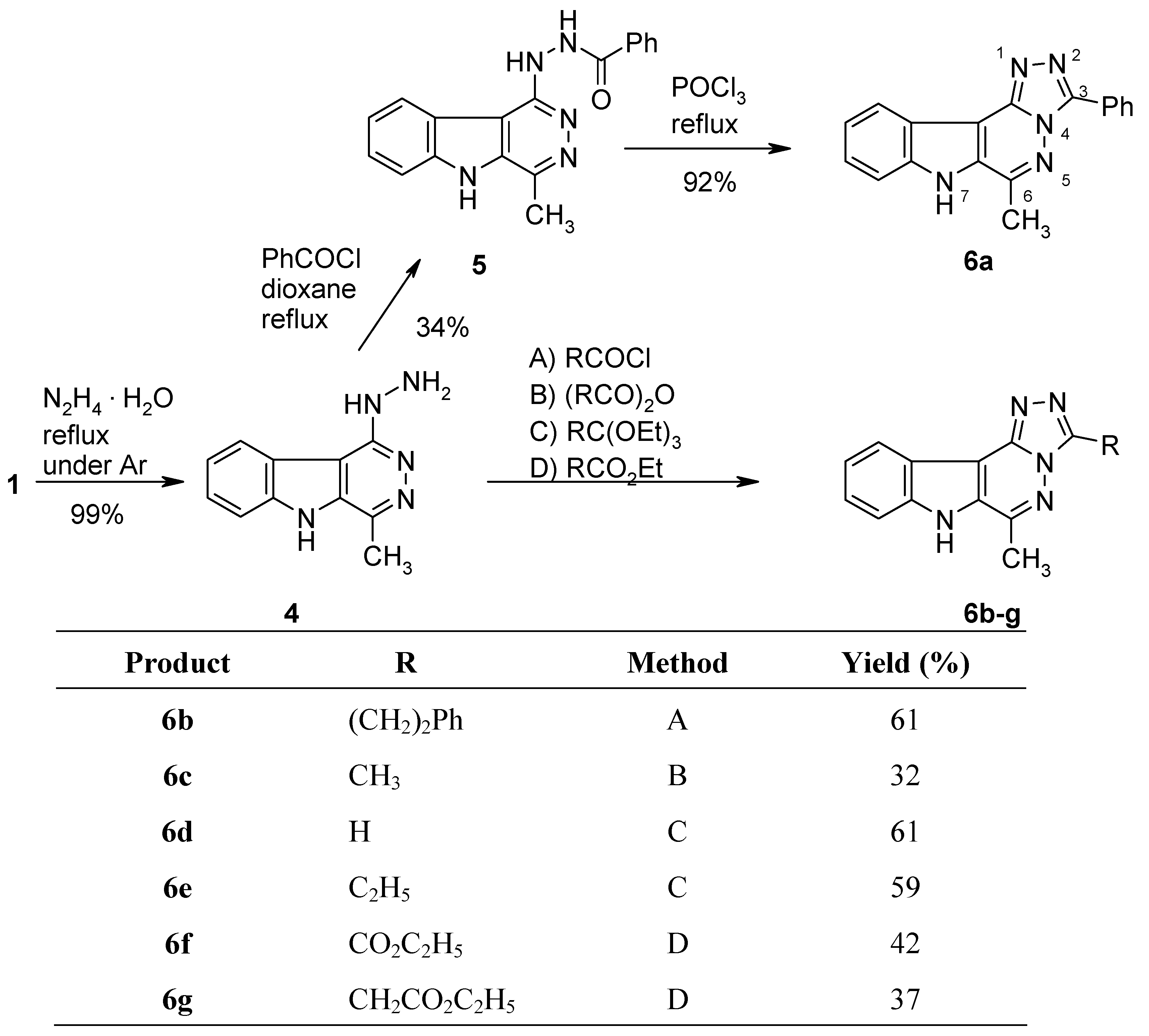
N'-(4-Methyl-5H-pyridazino[4,5-b]indol-1-yl)benzohydrazide (5).
Benzoyl chloride (140 mg, 1 mmol) was added dropwise to a solution of the hydrazino compound 4 (213 mg, 1 mmol) in dry dioxane (7 mL) and the mixture was refluxed under argon for 14 h. After cooling, the volatile components were removed under reduced pressure and the residue was recrystallized from EtOH to give the hydrochloride-monohydrate of 5 (126 mg, 34%) as colourless crystals, mp >350 °C. IR (KBr): 3422, 3154, 3062, 2778, 1668, 1616, 1581, 1555, 1513, 1460, 1399, 1311, 1291, 1262,, 1223 1155, 1046, 1021, 896, 775, 754, 710, 688, 640 cm–1; MS (EI, 70 eV) m/z: 318 (5%), 317 (M+, 20), 299 (100), 284 (31), 260 (22), 212 (36), 168 (21), 167 (19), 140 (24), 135 (9), 114 (12), 105 (37), 83 (15), 77 (41), 58 (40); 1H-NMR (DMSO-d6) δ: 13.87 (s, 1H, NH), 11.31 (s, 1H, NH), 8.74 (d, J8,9 = 8.1 Hz, 1H, 9-H), 8.08–8.06 (m, 2H, phenyl-H), 7.91 (d, J6,7 = 8.4 Hz, 1H, 6-H), 7.76–7.71 (m, 1H, 7-H), 7.68–7.53 (m, 4H, phenyl-H, 8-H), 2.82 (s, 3H, CH3). Anal. calcd for C18H15N5 O · 0.90 HCl · H2O: C, 58.72; H, 4.90; N, 19.02. Found: C, 58.74; H, 4.90; N, 18.76.
6-Methyl-3-phenyl-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indole (6a).
A mixture of 5 · HCl · H2O (35 mg, 0.095 mmol) and POCl3 (5 mL) was heated to 100 °C for 1 h. After cooling, the excess POCl3 was removed under reduced pressure and the residue was triturated with ice-water and made basic with concd. NH4OH. The precipitate was collected by filtration and recrystallized from EtOH to furnish 6a (26 mg, 92%) as colourless crystals, mp >350 °C. IR (KBr): 3405, 3066, 2954, 2923, 2882, 2770, 1635, 1616, 1539, 1503, 1470, 1324, 1272, 1239, 1181, 1143, 1070, 748, 669 cm–1; MS (EI, 70 eV) m/z: 300 (5%), 299 (M+, 100), 168 (11), 150 (6), 140 (15), 114 (8), 105 (37), 83 (15), 77 (41), 58 (40); 1H-NMR (DMSO-d6) δ: 12.74 (s, 1H, NH, shows positive NOE on irradiation at 2.88 ppm), 8.48–8.44 (m, 2H, phenyl 2’-H, 6’-H), 8.33 (d, J10,11 = 8.1 Hz, 1H, 11-H), 7.78 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.65–7.51 (m, 4H, 9-H, phenyl 3’-H, 4’-H, 5’-H), 7.49–7.43 (m, 1H, 10-H), 2.88 (s, 3H, CH3). Anal. calcd for C18H13N5 · 0.1 H2O: C, 71.79; H, 4.42; N, 23.26. Found: C, 71.75; H, 4.53; N, 23.19.
6-Methyl-3-(2-phenylethyl)-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indole (6b).
3-Phenyl propionyl chloride (168 mg, 1 mmol) was added dropwise over 5 min, to a solution of the hydrazino compound 4 (213 mg, 1 mmol) in dry dioxane (7 mL), then the mixture was refluxed under argon for 16 h. After cooling, the volatile components were removed under reduced pressure and the residue was recrystallized from EtOH to afford the hydrochloride of 6b (223 mg, 61%) as pale yellow crystals, mp 325–327 °C. IR (KBr): 3404, 3058, 3023, 2921, 2742, 2574, 1652, 1617, 1537, 1493, 1453, 1416, 1383, 1252, 1197, 700, 638 cm–1; MS (EI, 70 eV) m/z: 327 (M+, 44%), 326 (49), 299 (12), 237 (29), 236 (100), 200 (12), 172 (17), 156 (14), 140 (24), 127 (10), 114 (15), 91 (61), 73 (15), 67 (64); 1H-NMR (DMSO-d6) δ: 13.63 (s, 1H, NH, shows positive NOE on irradiation at 2.95 ppm), 8.66 (d, J10,11 = 7.8 Hz, 1H, 11-H), 7.87 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.73–7.68 (m, 1H, 9-H), 7.55–7.50 (m, 1H, 10-H), 7.32–7.19 (m, 5H, phenyl-H, shows positive NOE on irradiation at 3.24 ppm), 3.52 (t, J = 7.7 Hz, 2H, PhCH2CH2), 3.24 (t, J = 7.7 Hz, 2H, PhCH2CH2), 2.95 (s, 3H, CH3). Anal. calcd for C20H17N5 · HCl · 0.1 H2O: C, 65.70; H, 5.02; N, 19.15. Found: C, 65.40; H, 5.03; N, 18.94.
3,6-Dimethyl-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indole (6c).
A mixture of the hydrazino compound 4 (70 mg, 0.33 mmol) and acetic anhydride (5 mL) was heated under argon to 100 °C for 1 h. After cooling, the excess reagent was removed under reduced pressure and the residue was triturated with water (10 mL), filtered off and recrystallized from EtOH to give 6c (24 mg, 31%) as colourless crystals, mp >350 °C. IR (KBr): 3132, 3074, 2921, 2746, 1635, 1614, 1533, 1506, 1409, 1323, 1246, 1098, 825, 759 cm–1; MS (EI, 70 eV) m/z: 238 (16%), 237 (M+, 100), 168 (30), 167 (16), 142 (10), 141 (15), 140 (39), 115 (16), 114 (24), 100 (7), 84 (11), 76 (7), 63 (11), 57 (10), 51 (9); 1H-NMR (DMSO-d6) δ: 12.62 (bs, 1H, NH), 8.27–8.24 (m, 1H, 11-H), 7.77–7.75 (m, 1H, 8-H), 7.62–7.57 (m, 1H, 9-H), 7.54–7.40 (m, 1H, 10-H), 2.82 (s, 3H, 6-CH3), 2.70 (s, 3H, 3-CH3). HRMS (EI, 70 eV) m/z calcd for C13H11N5 (M+): 237.1014. Found: 237.1008.
6-Methyl-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indole (6d).
A suspension of the hydrazino compound 4 (100 mg, 0.47 mmol) in triethyl orthoformate (5 mL) was refluxed under argon for 6 h. After cooling, the excess reagent was removed by Kugelrohr distillation and the residue was purified by recrystallization from EtOH to give 6d (65 mg, 61%) as almost colourless crystals, mp >350 °C. IR (KBr): 3140, 3060, 2924, 2842, 2763, 1637, 1613, 1510, 1394, 1322, 1211, 1176, 1029, 916, 793, 754, 663, 625 cm–1; MS (EI, 70 eV) m/z: 224 (15%), 223 (M+, 100), 195 (6), 168 (13), 167 (11), 141 (11), 140 (33), 127 (7), 115 (20), 114 (27), 113 (14), 100 (10), 88 (10), 84 (7), 70 (18), 51 (8); 1H-NMR (DMSO-d6) δ: 12.68 (s, 1H, NH), 9.46 (s, 1H, 3-H), 8.29–8.26 (m, 1H, 11-H), 7.78–7.75 (m, 1H, 8-H), 7.63–7.58 (m, 1H, 9-H), 7.47–7.41 (m, 1H, 10-H), 2.81 (s, 3H, CH3). Anal. calcd for C12H9N5 · 0.1 C2H5OH: C, 64.31; H, 4.25; N, 30.74. Found: C, 64.26; H, 4.24; N, 30.69.
3-Ethyl-6-methyl-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indole (6e).
A suspension of the hydrazino compound 4 (213 mg, 1 mmol) in triethyl orthopropionate (8 mL) was refluxed under argon for 8 h. After cooling, the excess reagent was removed by Kugelrohr distillation, and the residue was purified by column chromatography (CH2Cl2/MeOH, 19:1) followed by recrystallization from EtOH to afford 6e (148 mg, 59%) as almost colourless crystals, mp 338–340 °C. IR (KBr): 3137, 3070, 2983, 2938, 2880, 2771, 1635, 1614, 1531, 1505, 1465, 1324, 1246, 1032, 810, 750 cm–1; MS (EI, 70 eV) m/z: 252 (16%), 251 (M+, 100), 250 (44), 237 (20), 236 (68), 167 (9), 152 (7), 141 (7), 140 (21), 115 (9), 114 (13), 113 (6), 88 (9), 76 (5), 71 (10), 63 (6); 1H-NMR (DMSO-d6) δ: 12.76 (s, 1H, NH, shows positive NOE on irradiation at 2.83 ppm), 8.28 (d, J10,11 = 8.1 Hz, 1H, 11-H), 7.76 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.64–7.58 (m, 1H, 9-H), 7.47–7.41 (m, 1H, 10-H), 3.13 (q, J = 7.5 Hz, 2H, CH2CH3), 2.83 (s, 3H, CH3), 1.41 (t, J = 7.5 Hz, 3H, CH2CH3). Anal. calcd for C14H13N5: C, 66.92; H, 5.21; N, 27.87. Found: C, 66.55; H, 5.11; N, 27.56.
Ethyl 6-methyl-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indole-3-carboxylate (6f).
A mixture of the hydrazino compound 4 (100 mg, 0.47 mmol) and diethyl oxalate (7 mL) was refluxed under argon for 4 h. After cooling, the excess reagent was removed by Kugelrohr distillation and the residue was purified by column chromatography (CH2Cl2/MeOH, 19:1) and subsequent recrystallization from EtOH to give 6f (60 mg, 42%) as colourless crystals, mp 280–282 °C. IR (KBr): 3327, 3064, 2990, 1716, 1618, 1536, 1483, 1367, 1348, 1294, 1270, 1238, 1195, 1064, 835, 769, 750, 663 cm–1; MS (EI, 70 eV) m/z: 295 (M+, 62%), 250 (15), 239 (9), 224 (11), 223 (69), 194 (9), 182 (9), 169 (14), 168 (100), 167 (19), 140 (25), 125 (6), 114 (20), 100 (7), 88 (11), 70 (11); 1H-NMR (DMSO-d6) δ: 12.89 (s, 1H, NH), 8.33 (d, J10,11 = 8.1 Hz, 1H, 11-H), 7.79 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.67–7.61 (m, 1H, 9-H), 7.49–7.44 (m, 1H, 10-H), 4.48 (q, J = 7.1 Hz, 2H, CH2CH3), 2.87 (s, 3H, CH3), 1.40 (t, J = 7.1 Hz, 3H, CH2CH3). Anal. calcd for C15H13N5O2 · 0.2 C2H5OH: C, 60.74; H, 4.70; N, 23.00. Found: C, 60.72; H, 4.39; N, 22.82.
Ethyl (6-methyl-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indol-3-yl)acetate (6g).
A mixture of the hydrazino compound 4 (150 mg, 0.7 mmol) and diethyl malonate (5 mL) was refluxed under argon for 6 h. After cooling, the excess reagent was removed by Kugelrohr distillation and the residue was purified by recrystallization from EtOH to give 6g (80 mg, 37%) as almost colourless needles, mp 283–285 °C. IR (KBr): 3422, 3072, 2984, 2771, 1740, 1635, 1615, 1534, 1419, 1368, 1200, 1185, 751, 505 cm–1; MS (EI, 70 eV) m/z: 310 (14%), 309 (M+, 64), 238 (6), 237 (44), 236 (100), 178 (6), 168 (6), 167 (10), 152 (13), 140 (19), 127 (6), 115 (10), 113 (8), 104 (4), 100 (4), 88 (7); 1H-NMR (DMSO-d6) δ: 12.71 (s, 1H, NH), 8.28 (d, J10,11 = 8.1 Hz, 1H, 11-H), 7.77 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.64–7.59 (m, 1H, 9-H), 7.47–7.42 (m, 1H, 10-H), 4.31 (s, 2H, CH2), 4.14 (q, J = 7.1 Hz, 2H, CH2CH3), 2.81 (s, 3H, CH3), 1.84 (t, J = 7.1 Hz, 3H, CH2CH3). Anal. calcd for C16H15N5O2: C, 62.13; H, 4.89; N, 22.64. Found: C, 61.95; H, 4.83; N, 22.39.
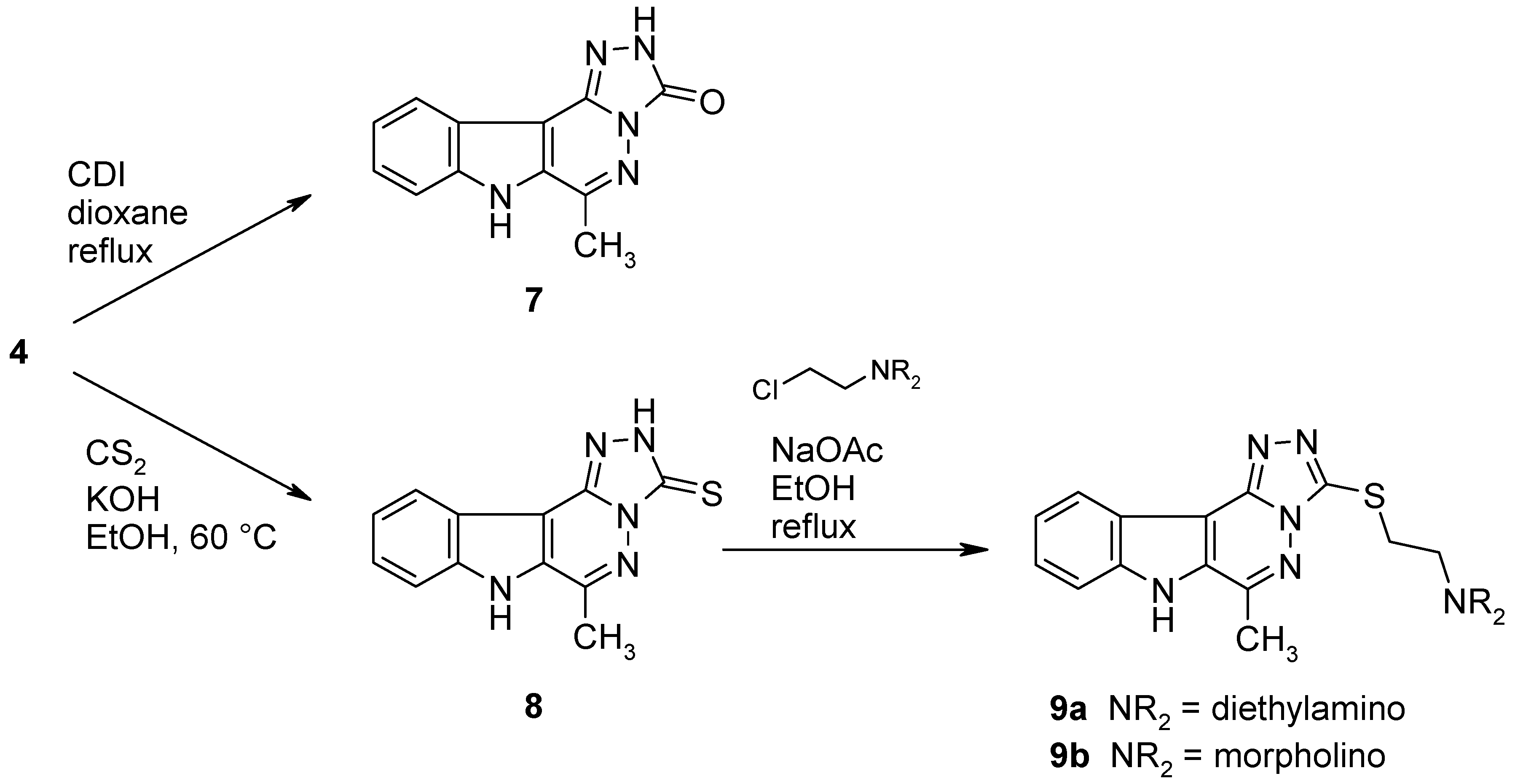
6-Methyl-2,7-dihydro-3H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indol-3-one (7).
A mixture of the hydrazino compound 4 (107 mg, 0.5 mmol) and 1,1’-carbonyldiimidazole (162 mg, 1 mmol) in dry dioxane (10 mL) was refluxed under argon for 14 h. After cooling, the solvent was removed under reduced pressure and the residue was triturated with water (20 mL). The product was collected by filtration, dried and recrystallized from EtOH to afford 7 (80 mg, 64%) as colourless crystals, mp >350 °C. IR (KBr): 3419, 3133, 3083, 2891, 1708, 1652, 1614, 1532, 1400, 1243, 1077, 746 cm–1; MS (EI, 70 eV) m/z: 240 (13%), 239 (M+, 100%), 168 (37), 167 (15), 156 (8), 142 (43), 115 (20), 114 (27), 91 (12), 88 (13), 71 (21), 63 (8), 57 (10); 1H-NMR (DMSO-d6) δ: 12.24 (bs, 2H, NH), 8.01 (d, J10,11 = 7.8 Hz, 1H, 11-H), 7.71 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.56–7.51 (m, 1H, 9-H), 7.39–7.35 (m, 1H, 10-H), 2.69 (s, 3H, CH3). Anal. calcd for C12H9N5O · 0.65 H2O: C, 57.44; H, 4.14; N, 27.91. Found: C, 57.40; H, 3.97; N, 27.62.
6-Methyl-2,7-dihydro-3H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indole-3-thione (8).
A mixture of the hydrazino compound 4 (213 mg, 1 mmol), CS2 (3 mL) and KOH (500 mg, 9 mmol) in EtOH (10 mL) was heated under argon to 60 °C until the starting material was consumed (TLC monitoring). The volatile components were removed under reduced pressure and the residue was taken up in water (100 mL). The resulting mixture was acidified with 2N HCl, the precipitate was collected by filtration and recrystallized from EtOH to give 8 (185 mg, 72%) as yellow crystals, mp >300 °C. IR (KBr): 3419, 3145, 2998, 2929, 2776, 1646, 1616, 1533, 1507, 1443, 1399, 1333, 1267, 1242, 1206, 1039, 958, 863, 836, 801, 746, 668, 562. cm–1; MS (EI, 70 eV) m/z: 256 (15%), 255 (M+, 100), 168 (10), 156 (5), 140 (11), 128 (8), 115 (49), 114 (10), 88 (4), 58 (25); 1H-NMR (DMSO-d6) δ: 14.33 (s, 1H, 2-NH), 12.80 (s, 1H, 7-NH, shows positive NOE on irradiation at 2.81 ppm), 8.08 (d, J10,11 = 7.8 Hz, 1H, 11-H), 7.75 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.61–7.56 (m, 1H, 9-H), 7.44–7.39 (m, 1H, 10-H), 2.81 (s, 3H, CH3). HRMS (EI, 70 eV) m/z calcd for C12H9N5S (M+): 255.0579. Found: 255.0584.
N,N-Diethyl-N-{2-[(6-methyl-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indol-3-yl)sulfanyl]-ethyl}amine (9a).
2-Diethylaminoethyl chloride hydrochloride (125 mg, 0.73 mmol) was added to a mixture of the thione 8 (185 mg, 0.72 mmol) and sodium acetate (400 mg, 4.9 mmol) in EtOH (10 mL) and the mixture was refluxed for 16 h. The solvent was removed under reduced pressure, the residue was dissolved in water (50 mL) and the product was extracted with CH2Cl2 (3 × 50 mL). The combined extracts were washed with water and dried over Na2SO4. The solvent was removed under reduced pressure and the product was purified by column chromatography (CH2Cl2/MeOH, 9:1). Evaporation of the main fraction, followed by recrystallization from EtOH/EtOAc gave 9a (125 mg, 49%) as colourless needles, mp 228–230 °C. IR (KBr): 3418, 3141, 2967, 2934, 2801, 1635, 1613, 1539, 1399, 1244, 1216, 1076, 803, 747, 664 cm–1; MS (EI, 70 eV) m/z: 354 (M+, 1%), 255 (23), 140 (5), 114 (6), 100 (100), 99 (60), 86 (90), 71 (24), 56 (22); 1H-NMR (CDCl3) δ: 10.70 (s, 1H, NH), 8.39 (d, J10,11 = 8.1 Hz, 1H, 11-H), 7.67 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.53–7.48 (m, 1H, 9-H), 7.37–7.32 (m, 1H, 10-H), 3.53 (t, J = 7.4 Hz, 2H, SCH2), 3.01 (t, J = 7.4 Hz, 2H, SCH2CH2N), 2.89 (s, 3H, CH3), 2.66 (q, J = 7.2 Hz, 4H, SCH2CH2N(CH2CH3)2), 1.06 (t, J = 7.2 Hz, 6H, SCH2CH2N(CH2CH3)2). Anal. calcd for C18H22N6S: C, 60.99; H, 6.26; N, 23.71. Found: C, 60.69; H, 6.20; N, 23.59.
4-{2-[(6-Methyl-7H-[1,2,4]triazolo[4’,3’:1,6]pyridazino[4,5-b]indol-3-yl)sulfanyl]ethyl}morpholine (9b).
4-(2-Chloroethyl)morpholine hydrochloride (146 mg, 0.78 mmol) was added to a mixture of the thione 8 (200 mg, 0.78 mmol) and sodium acetate (400 mg, 4.9 mmol) in EtOH (10 mL) and the mixture was refluxed for 16 h. The solvent was removed under reduced pressure, the residue was dissolved in water (50 mL) and the product was extracted with CH2Cl2 (3 × 50 mL). The extract was washed with water and dried over Na2SO4. The solvent was removed under reduced pressure and the product was purified by column chromatography (CH2Cl2/MeOH, 9:1). Evaporation of the main fraction, followed by recrystallization from EtOH/EtOAc gave 9b (240 mg, 83%) as colourless crystals, mp 242–244 °C. IR (KBr): 3410, 3143, 3078, 2959, 2812, 2737, 1635, 1613, 1533, 1503, 1399, 1302, 1243, 1117, 1004, 916, 867, 803, 770, 749, 663 cm–1; MS (EI, 70 eV) m/z: 368 (M+, 2%), 268 (5), 255 (62), 250 (16), 114 (56), 100 (100), 85 (19), 70 (13), 56 (40); 1H-NMR (CDCl3) δ: 10.92 (s, 1H, NH, shows positive NOE on irradiation at 2.89 ppm or at 7.62 ppm), 8.36 (d, J10,11 = 7.8 Hz, 1H, 11-H), 7.62 (d, J8,9 = 8.4 Hz, 1H, 8-H), 7.49–7.44 (m, 1H, 9-H), 7.35–7.30 (m, 1H, 10-H), 3.69–3.65 (m, 4H, OCH2), 3.56 (t, J = 7.1 Hz, 2H, SCH2), 2.89 (s, 3H, CH3), 2.87 (t, J = 6.8 Hz, 2H, SCH2CH2N), 2.56–2.51 (m, 4H, NCH2, shows positive NOE on irradiation at 2.87 ppm). Anal. calcd for C18H20N6OS: C, 58.68; H, 5.47; N, 22.81. Found: C, 58.49; H, 5.46; N, 22.69.