Microwave-Assisted Synthesis of Some 3,5-Arylated 2-Pyrazolines
Abstract
:Introduction
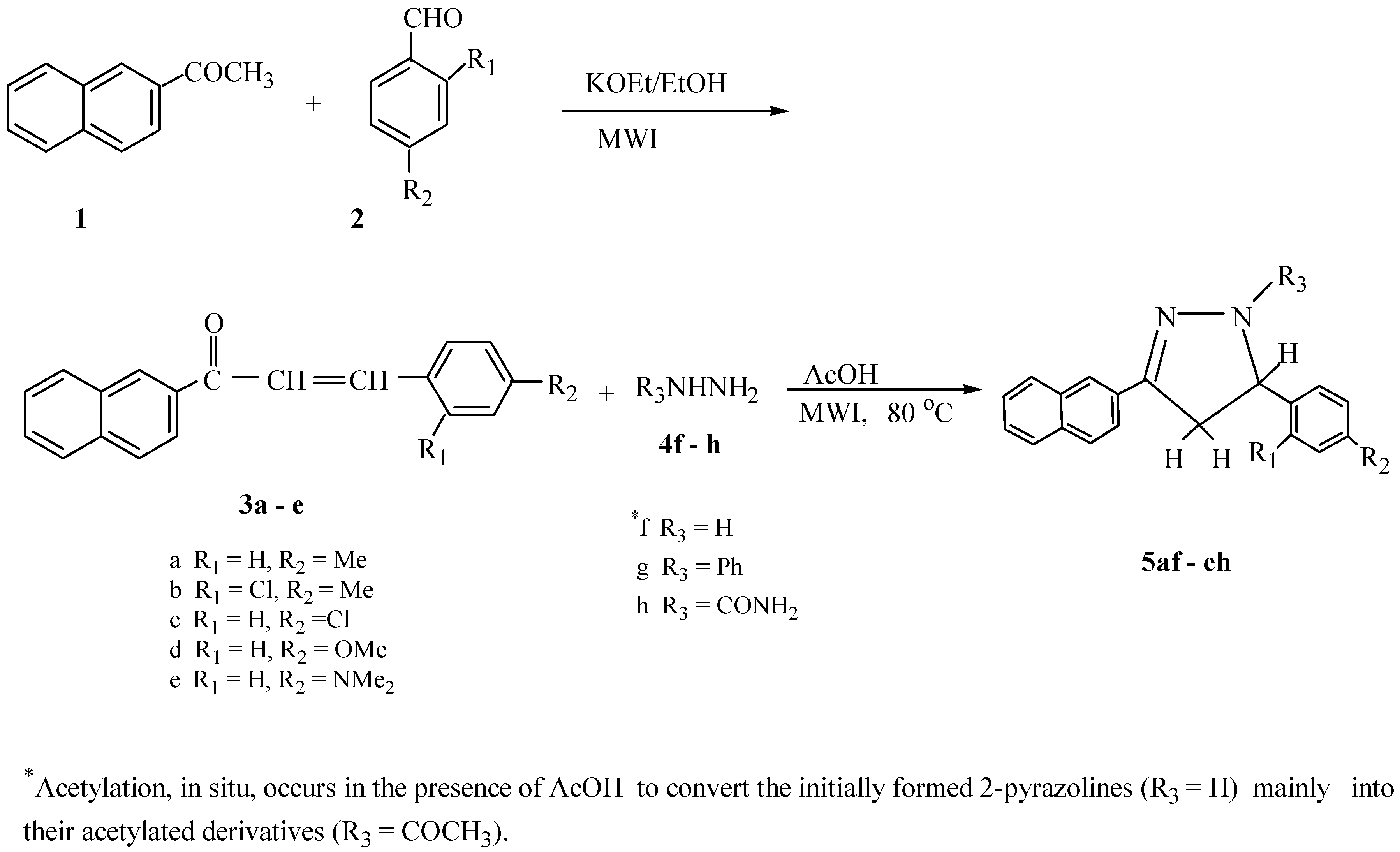
Results and Discussion
Conclusions
Experimental
General
General procedure for microwave-assisted preparation of 3,5-diaryl-2-pyrazoline derivatives (5af-eh)
| Compound | Molecular Formula | Irradiation Time (min) | Yielda (%) | M.P. (ºC) | Elemental Analysis Calcd (found) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 5af | C22H20ON2 | 7 | 85 | 175-176 | 80.49 (80.71) | 6.10 (6.29) | 8.54 (8.59) |
| 5ag | C26H22N2 | 1.4 | 98 | 167-168 | 86.19 (86.35) | 6.08 (6.04) | 7.73 (7.82) |
| 5ah | C21H19ON3 | 7 | 88 | 182-183 | 76.60 (76.74) | 5.77 (5.84) | 12.76 (12.72) |
| 5bf | C22H19ON2Cl | 2.3 | 96 | 172-173 | 72.83 (72.70) | 5.24 (5.29) | 7.72 (7.54) |
| 5bg | C26H21N2Cl | 1.7 | 98 | 131-132 | 78.69 (78.99) | 5.30 (5.43) | 7.06 (6.83) |
| 5bh | C21H18ON3 Cl | 6.4 | 82 | 162-164 | 69.32 (69.38) | 4.95 (4.82) | 11.55 (11.54) |
| 5cf | C21H17ON2Cl | 1.5 | 99 | 177-178 | 72.31 (72.34) | 4.88 (4.85) | 8.03 (8.16) |
| 5cg | C25H19N2Cl | 1.4 | 98 | 129-130 | 78.43 (78.58) | 4.97 (5.12) | 7.32 (7.52) |
| 5ch | C20H16ON3Cl | 11 | 85 | 180-182 | 68.67 (68.87) | 4.58 (4.67) | 12.02 (11.88) |
| 5df | C22H20O2N2 | 1.3 | 98 | 186-187 | 76.74 (76.93) | 5.81 (5.59) | 8.14 (8.54) |
| 5dg | C26H22ON2 | 1.2 | 98 | 135-136 | 82.54 (82.51) | 5.82 (5.72) | 7.41 (7.48) |
| 5dh | C21H19 O2N3 | 7.5 | 86 | 173-175 | 73.04 (73.12) | 5.51 (5.46) | 12.17 (12.21) |
| 5ef | C23H23ON3 | 4.5 | 85 | 183-184 | 77.31 (77.42) | 6.44 (6.48) | 11.76 (11.79) |
| 5eg | C27H25N3 | 2.2 | 99 | 185-186 | 82.86 (82.83) | 6.39 (6.41) | 10.74 (10.78) |
| 5eh | C22H22ON4 | 6.8 | 86 | 192-193 | 73.74(73.87) | 6.14(6.34) | 15.64(15.28) |
| Compound | IR (cm-1) | 1H-NMR (ppm) | MS (m/z) |
|---|---|---|---|
| 5af | 3068, 2975, 1660, 1620, 1580, 1484, 1337, 1638, 806 | 2.38 (s, 3H, Me), 2.46 (s, 3H, COMe), 3.16 (dd, J = 18.2, 5.1 Hz, 1H, CH2(Pyraz) ), 3.80 (dd, J = 18.2, 11.8 Hz, 1H, CH(Pyraz) ), 5.91 (dd, J = 11.8, 5.1 Hz, 1H, CH2(Pyraz) ), 7.12 (s, 4H, Ph ), 7.25-8.00 (m, 7H, Naph) | 57, 71, 77, 91, 127, 153, 255, 285, 286, 328, 329 |
| 5ag | 3178, 2938, 2813,1600, 1500, 1100, 870, 820, 750 | 2.40 (s, 3H, Me), 3.05 (dd, J = 16.9, 8.4 Hz, 1H, CH2(Pyraz) ), 3.82 (dd, J = 16.9, 12.0 Hz, 1H, CHPyraz), 5.29 (dd, J = 12.0, 8.4 Hz, 1H, CH2(Pyraz) ), 6.60 - 8.20 (m, 16H, Ar) | 42, 77, 91, 101, 118, 125, 127, 153, 167, 244, 271, 362 |
| 5ah | 3500, 3350, 3115, 2985, 1675, 1580, 1500, 1160, 855, 760 | 2.23 (s, 3H, Me), 3.26 (dd, J = 18.3, 5.8 Hz, 1H, CH2(Pyraz) ), 3.77 (dd, J = 18.3, 12.4 Hz, 1H, CHPyraz), 5.33 (bs, 2H, NH2), 5.50 (dd, J = 12.4, 5.8 Hz, 1H, CH2(Pyraz) ), 7.08 (s, 4H, Ph ), 7.20 - 8.00 (m, 7H, Naph ) | 42, 77, 91, 101, 127, 169, 153, 118, 195, 238, 285, 313, 329 |
| 5bf | 3065, 2915, 1670, 1590, 1470, 1450, 1330, 1150, 975, 865, 745, 630 | 2.42 (s, 3H, Me), 2.52 (s, 3H, COMe), 3.16 (dd, J = 18.0, 5.2 Hz, 1H, CH2(Pyraz) ), 3.83 (dd, J =18.0, 12.0 Hz, 1H, CHPyraz), 5.92 (dd, J = 12.0, 5.20 Hz, 1H, CH2(Pyraz) ), 7.10-8.10 (m, 10H, Ar) | 57, 71, 77, 112, 127, 138, 154, 271, 237, 277, 279, 294, 292, 305, 307, 308, 348, 350 |
| 5bg | 3058, 2883, 1594, 1500, 1460, 1325, 1120, 1050, 865, 745, 700 | 2.38 (s, 3H, Me), 3.14 (dd, J = 16.6, 8.1 Hz, 1H, CH2(Pyraz) ), 4.11 (dd, J = 16.6, 11.6 Hz, 1H, CHPyraz), 5.58 (dd, J = 11.6, 8.1 Hz, 1H, CH2(Pyraz) ), 6.50 - 8.20 (m, 15H, Ar) | 77, 91, 101, 113, 111, 127, 138, 140, 153, 167, 244, 271, 382, 384 |
| 5bh | 3450, 3250, 3085, 2965, 1680, 1580, 1478, 1240, 1075, 820, 750 | 2.28 (s, 3H, Me), 3.33 (dd, J = 16.6, 5.8 Hz, 1H, CH2(Pyraz) ), 3.89 (dd, J = 16.6, 12.2 Hz, 1H, CHPyraz), 5.38 (bs, 2H, NH2), 5.60 (dd, J = 12.2, 5.8 Hz, 1H, CH2(Pyraz) ), 7.26 (s, 3H, Ph ), 7.38 - 8.20 (m, 7H, Naph) | 42, 91, 115,127, 153, 169, 196, 195, 249, 305, 307, 349, 351 |
| 5cf | 3048, 2980, 1665, 1600, 1500, 1475, 1452, 1195, 986, 735, 640 | 2.42 (s, 3H, COMe), 3.12 (dd, J = 18.0, 6.20 Hz, 1H, CH2(Pyraz) ), 3.78 (dd, J = 18.0, 12.6 Hz, 1H, CHPyraz ), 5.95 (dd, J = 18.0, 6.2 Hz, 1H, CH2(Pyraz) ), 7.20 (s, 4H, Ph ), 7.32 - 8.30 (m, 7H, Naph) | 57, 77, 114, 127, 138, 153, 271, 237, 277, 276, 294, 292, 306, 307, 308, 348, 349, 350 |
| 5cg | 3072, 2918, 1600, 1500, 1360, 1135, 825, 730 | 3.18 (dd,
J = 16.6, 8.5 Hz, 1H, CH2(Pyraz) ), 3.83 (dd, J = 16.6, 11.4 Hz, 1H, CHPyraz ), 5.18 (dd, J = 11.4, 8.5 Hz, 1H, CH2(Pyraz) ), 6.60 - 8.20 (m, 16H, Ar) | 77, 91, 101, 113, 127, 139, 140, 153, 168, 244, 271, 382, 383, 384 |
| 5ch | 3420, 3300, 3120, 2900, 1675, 1582, 1487, 1500, 1220, 1085, 820, 745 | 3.25 (dd, J= 17.4, 6.4 Hz, 1H, CH2(Pyraz) ), 3.81 (dd, J = 17.4, 12.2 Hz, 1H, CHPyraz ), 5.35 (bs, 2H, NH2), 5.48 (dd, J = 12.2, 6.4 Hz, 1H, CH2(Pyraz) ), 7.18 (s, 4H, Ph ), 7.30 - 8.10 (m, 7H, Naph ) | 42, 77, 101, 115, 127, 153, 169, 196, 195, 228, 293, 305, 307, 349, 351 |
| 5df | 3068, 2887, 2838, 1670, 1600, 1495, 1448, 1120, 1095, 954, 740 | 2.45 (s, 3H, COMe), 3.25 (dd, J = 18.1, 5.8 Hz, 1H, CH2(Pyraz) ), 3.75 (s, 3H, OMe), 3.82 (dd, J = 18.1, 12.2 Hz, 1H, CHPyraz), 5.54 (dd, J = 12.2, 5.8 Hz, 1H, CH2(Pyraz)), 6.82 (d, J = 9.5 Hz, 2H, 3-HPh), 7.20 (d, J = 9.5 Hz, 2H, 2-HPh ), 7.50 - 8.10 (m, 7H, Naph ) | 57, 77, 101, 108, 127, 134, 153, 167, 274, 301, 329, 344, 345 |
| 5dg | 3150, 2880, 1600, 1495, 1410, 1350, 1240, 1115, 1035, 820, 740 | 3.29 (dd, J = 17.8, 6.0 Hz, 1H, CH2(Pyraz)), 3.82 (dd, J = 17.8, 12.0 Hz, 1H, CHPyraz), 3.70 (s, 3H, Me), 5.18 (dd, J = 12.0, 6.0 Hz, 1H, CH2(Pyraz) ), 6.60 - 8.20 (m, 16H, Ar) | 77, 107, 127, 134, 153, 154, 167, 244, 271, 378 |
| 5dh | 3480, 3375, 3150, 2900, 1685, 1580, 1520, 1490, 1250, 1070, 865, 750 | 3.27 (dd, J = 18.1, 5.0 Hz, 1H, CH2(Pyraz)), 3.69 (s, 3H, Me), 3.78 (dd, J = 18.1, 11.2 Hz, 1H, CHPyraz), 5.40 (bs, 2H, NH2), 5.52 (dd, J = 11.2, 5.0 Hz, 1H, CH2(Pyraz) ), 6.77 (d, J = 9.8 Hz, 2H, 3-HPh ), 7.15 (d, J = 9.8 Hz, 2H, 2-HPh ), 7.30 - 8.00 (m, 7H, Naph ) | 77, 91, 121,134, 149,153, 169, 191, 195, 303, 302, 345, 346 |
| 5ef | 3087, 2983, 2810, 1657, 1648, 1615, 1595, 1340, 1075, 815 | 2.43 (s, 3H, COMe), 2.83 (s, 6H, NMe2), 3.12 (dd, J = 15.9, 7.9 Hz, 1H, CH2(Pyraz) ), 3.75 (dd, J = 15.9, 11.8 Hz, 1H, CHPyraz ), 5.85 (dd, J = 11.8, 7.9 Hz, 1H, CH2(Pyraz) ), 7.35 (d, J = 9.6 Hz, 2H, 3-HPh ), 7.63 (d, J = 9.6 Hz, 2H, 2-HPh ), 7.80 - 8.10 (m, 7H, Naph) | 44, 77, 101, 110, 127, 137, 153, 167, 195, 237, 313, 314, 342, 357 |
| 5eg | 3134, 3006, 2871, 1616, 1594, 1523, 1453, 1117, 865, 826, 747 | 2.79 (s, 6H, NMe2), 3.15 (dd, J = 15.5, 8.1 Hz, 1H, CH2(Pyraz) ), 3.71 (dd, J = 15.5, 11.2 Hz, 1H, CHPyraz ), 5.09 (dd, J = 11.2, 8.1 Hz, 1H, CH2(Pyraz) ), 6.30 - 8.20 (m, 16H, Ar) | 48, 64, 77, 91, 120, 121, 134, 147, 153, 171, 244, 271, 389, 391, 392, 393 |
| 5eh | 3120, 2818, 1660, 1635, 1600, 1589, 1348, 1150, 845, 650 | 2.81 (s, 6H, NMe2), 3.26 (dd, J = 15.0, 8.2 Hz, 1H, CH2(Pyraz) ), 3.82 (dd, J = 15.0, 12.2 Hz, 1H, CHPyraz), 5.36 (bs, 2H, NH2), 5.68 (dd, J = 12.2, 8.2 Hz, 1H, CH2(Pyraz) ), 7.21 (d, J = 10.3 Hz, 2H, 3-HPh ), 7.58 (d, J = 10.3 Hz, 2H, 2-HPh ), 7.70 - 8.10 (m, 7H, Naph ) | 42, 77, 120, 127, 147, 153, 154, 169, 191, 195, 314, 358 |
Acknowledgements
References
- Taylor, E. C.; Patel, H.; Kumar, H. Tetrahedron 1992, 48, 8089.
- Roelfvan, S. G.; Arnold, C.; Wellnga, K. J. Agric. Food Chem. 1979, 84, 406.
- Keats, G. H. Brit. Pat. 1,209,631 1970.
- Kedar, R. M.; Vidhale, N. N.; Chincholkar, M. M. Orient. J. Chem. 1997, 13, 143.
- Singh, A.; Rathod, S.; Berad, B. N.; Patil, S. D.; Dosh, A. G. Orient. J. Chem. 2000, 16, 315.
- Katri, H. Z.; Vunii, S. A. J. Ind. Chem. Soc. 1981, 58, 168.
- Das, N. B.; Mittra, A. S. Ind. J. Chem. 1978, 16B, 638.
- Azarifar, D.; Shaebanzadeh, M. Molecules 2002, 7, 885.
- Holla, B. Shivarama; Akberali, P. M.; Shivanada, M. K. Farmaco 2000, 55, 256.
- Palaska, E.; Aytemir, M.; Tayfun, I.; Erol, K.; Dilek, E. Eur. J. Med. Chem. Chim. Ther. 2001, 36, 539.
- Garge, H. G. Chandraprakash. J. Pharm. Sc. 1971, 14, 649. [Google Scholar]
- Regaila, H. A.; El-Bayonk, A. K.; Hammad, M. Egypt. J. Chem. 1979, 20, 197.
- Krishna, R.; Pande, B. R.; Bharthwal, S. P.; Parmar, S. S. Eur. J. Med. Chem. 1980, 15, 567.
- Husain, M. I.; Shukla, S. Ind. J. Chem. 1986, 25B, 983.
- Tomilovi, Yu. V.; Okonnishnikova, G. P.; Shulishov, E. V.; Nefedov, O. M. Russ. Chem. Bt. 1995, 44, 2114.
- Klimova, E. I.; Marcos, M.; Klimova, T. B.; Cecilio, A. T.; Ruben, A. T.; Lena, R. R. J. Organomet. Chem. 1999, 585, 106.
- Bhaskarreddy, D.; Padmaja, A.; Ramanareddy, P. V.; Seenaiah, B. Sulfur Lett. 1993, 16, 227.
- Padmavathi, V.; Sumathi, R. P.; Chandrasekhar, B. N.; Bhaskarreddy, D. J. Chem. Research 1999, 610.
- Bhaskarreddy, D.; Chandrasekhar, B. N.; Padmavathi, V.; Sumathi, R. P. Synthesis 1998, 491.
- Knorr, L. Ber. Dt. Chem. Ges. 1893, 26, 100.
- Thakare, V. G.; Wadodkar, K. N. Ind. J. Chem. Sect. B 1986, 25, 610.
- Ankhiwala, M. D.; Hathi, M. V. J. Ind. Chem. Soc. 1994, 71, 587.
- Vounauwers, K.; Muller, A. Ber. Dt. Chem. Ges. 1908, 41, 4230.
- Baker, A.; Butt, V. S. J. Chem. Soc. 1949, 2142.
- Kadu, V. B.; Dashi, A. G. Orient. J. Chem. 1997, 13, 285.
- Abdelhamid, A. O.; Zohdi, H. F.; Sallam, M. M. M.; Ahmed, N. A. Molecules 2000, 5, 967.
- Rurack, K.; Bricks, J. L.; Schultz, B.; Maus, M.; Reck, G.; Resch-Genger, U. J. Phys. Chem. A. 2000, 104, 6171.
- Guerra, F. M.; Mish, M. R.; Carreira, E. M. Org. Lett. 2000, 2, 4265.
- Molteni, G.; Garanti, L. Heterocycles 2001, 55, 1573.
- Yadav, J. S.; Reddy, B. V. S.; Greetha, V. Syn. Lett. 2002, 3, 513.
- Nakamichi, N.; Kawashita, Y.; Hayashi, M. Org. Lett. 2002, 4, 3955.
- Wang, S.; Shi, B.; Li, Y.; Wang, Q.; Huang, R. Synth. Commun. 2003, 33, 1449.
- Chen, Y.; Lam, Y.; Lai, Y. H. Org. Lett. 2003, 5, 1067.
- Sample Availability: Available from the authors.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Azarifar, D.; Ghasemnejad, H. Microwave-Assisted Synthesis of Some 3,5-Arylated 2-Pyrazolines. Molecules 2003, 8, 642-648. https://doi.org/10.3390/80800642
Azarifar D, Ghasemnejad H. Microwave-Assisted Synthesis of Some 3,5-Arylated 2-Pyrazolines. Molecules. 2003; 8(8):642-648. https://doi.org/10.3390/80800642
Chicago/Turabian StyleAzarifar, Davood, and Hassan Ghasemnejad. 2003. "Microwave-Assisted Synthesis of Some 3,5-Arylated 2-Pyrazolines" Molecules 8, no. 8: 642-648. https://doi.org/10.3390/80800642




