Synthesis and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. An Overview
Abstract
:Introduction
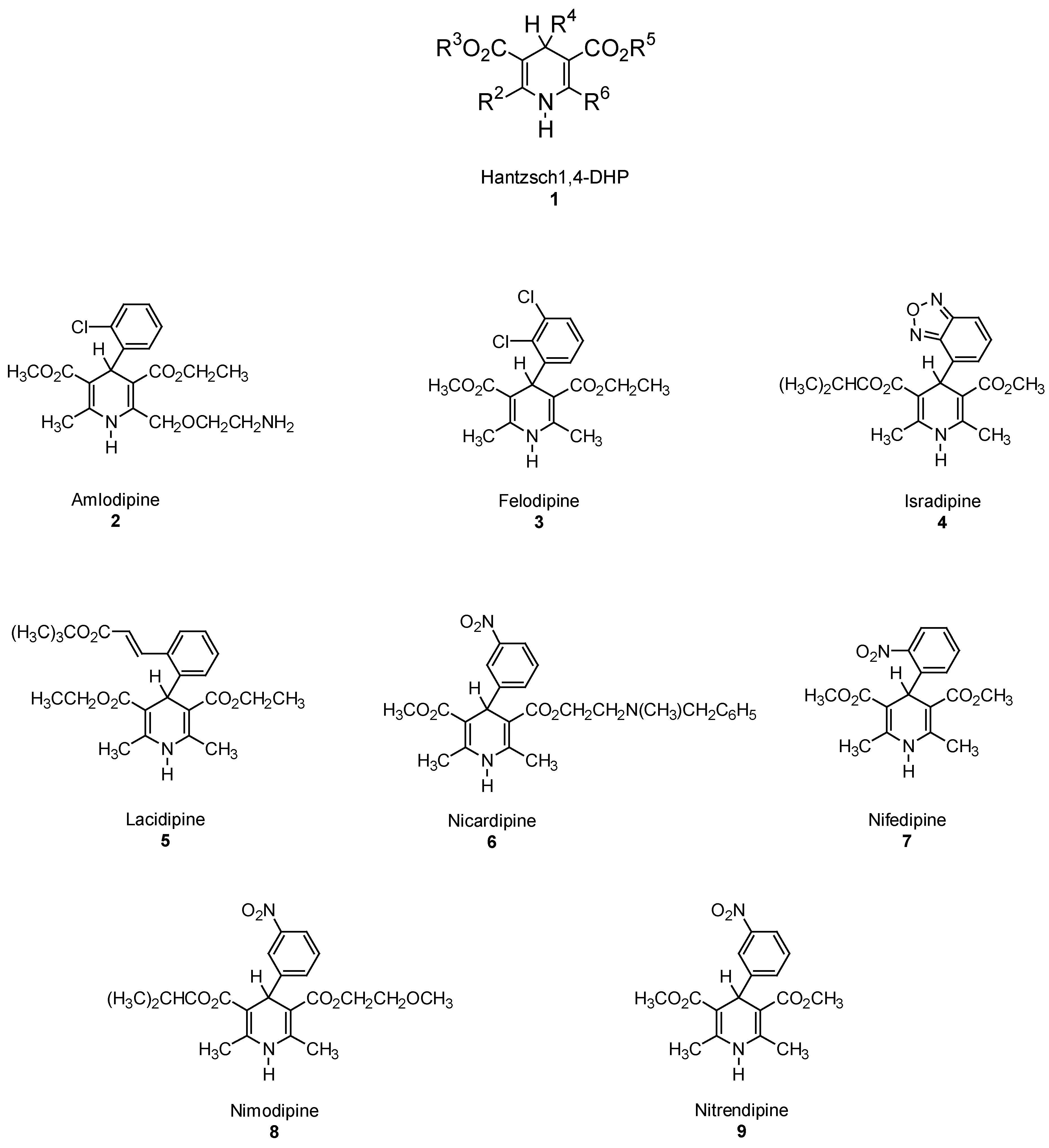
Hantzsch 1,4-DHP Synthesis
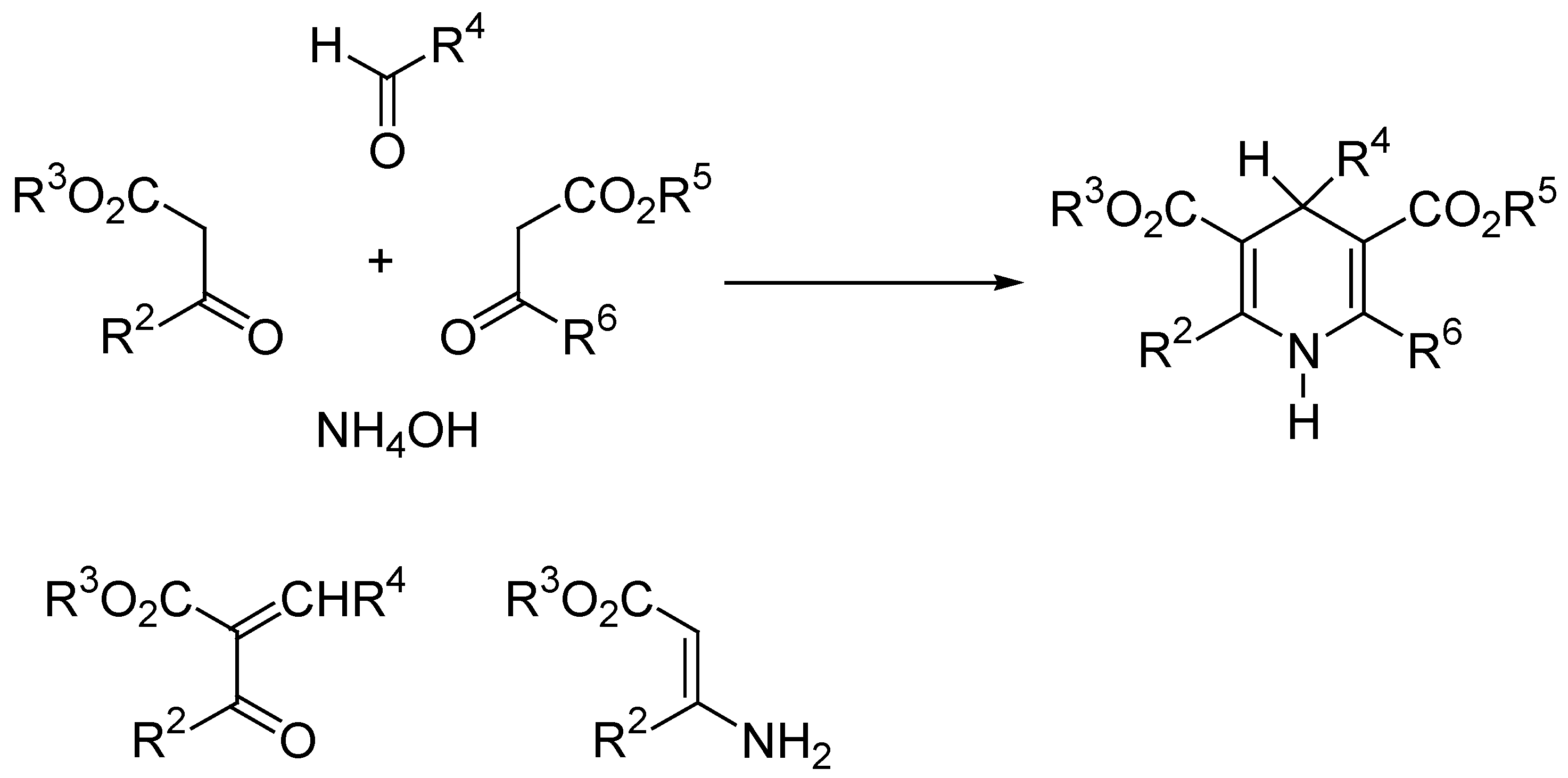
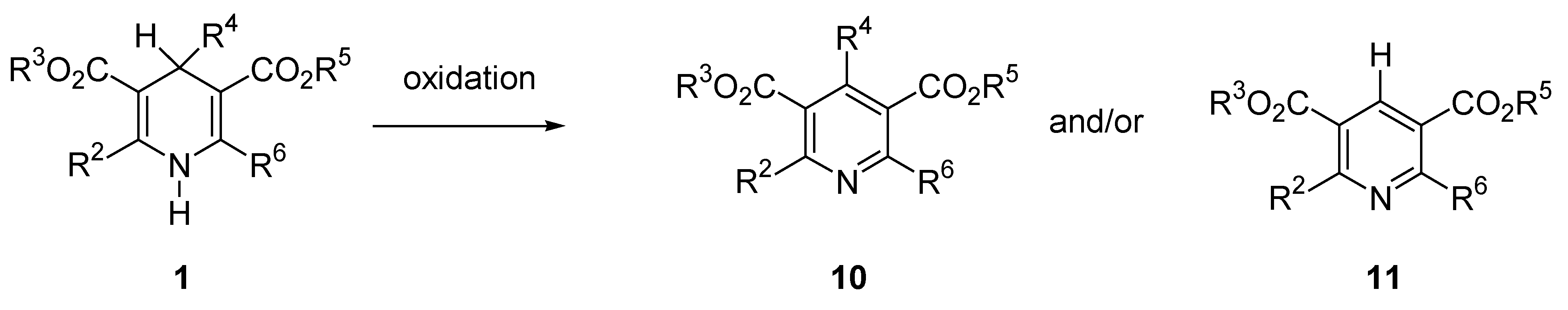
Aromatization of Hantzsch 1,4-DHP
- -
- Heterocycles bearing an aryl (or heteroaryl) group in position 4 always undergo a dehydrogenation process [18];
- -
- -

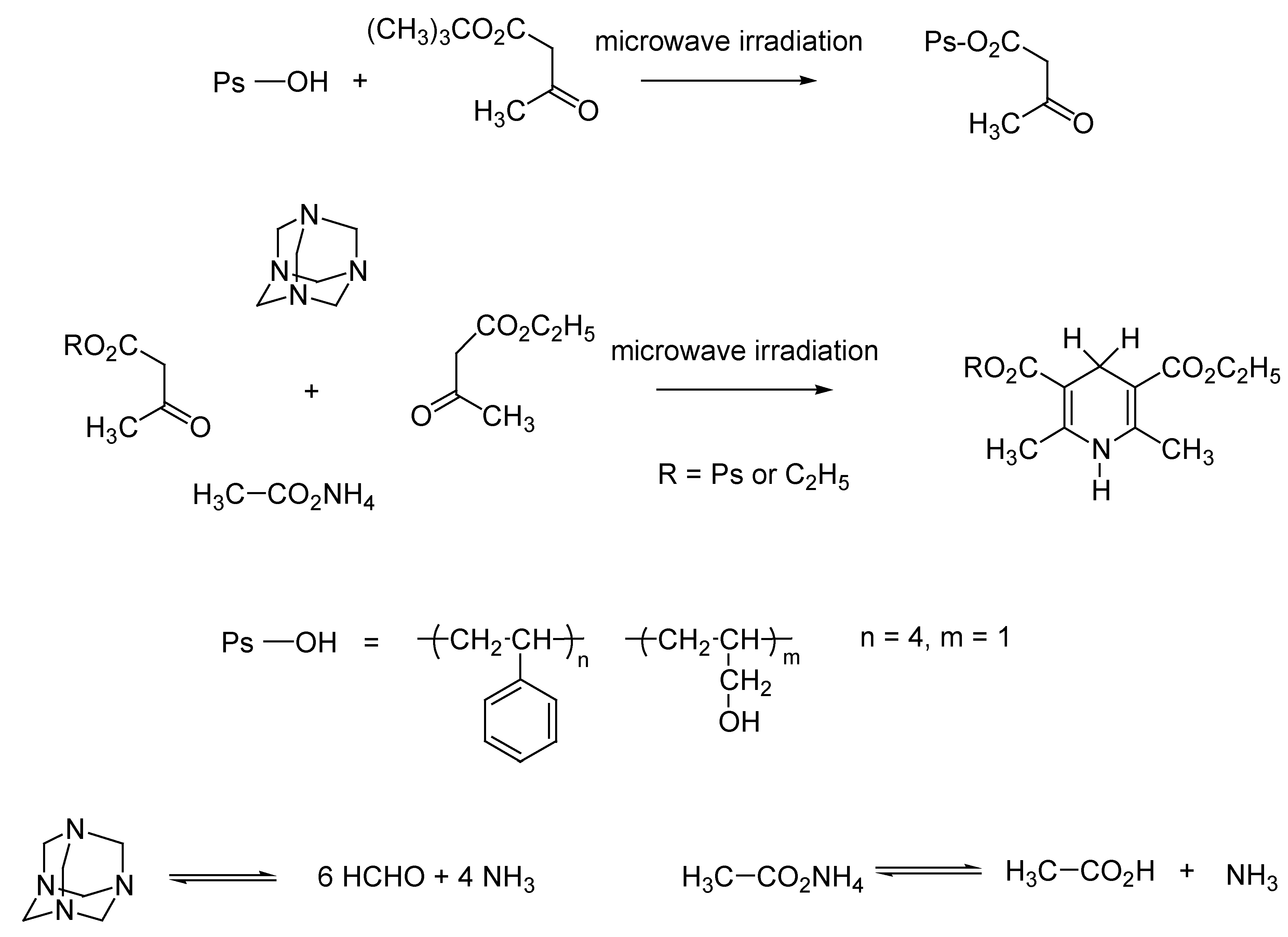
Domino Synthesis of Hantzsch Pyridines
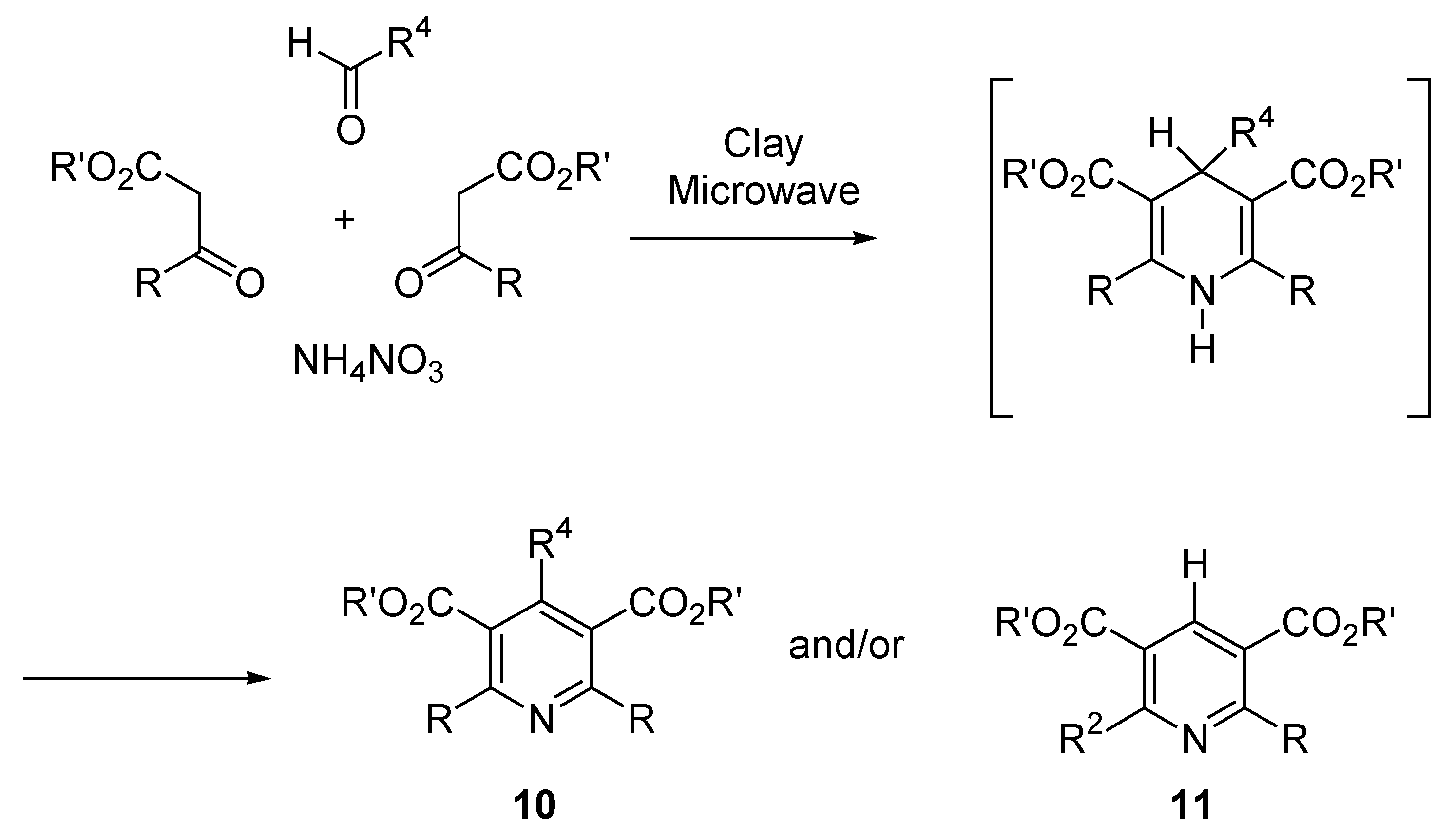
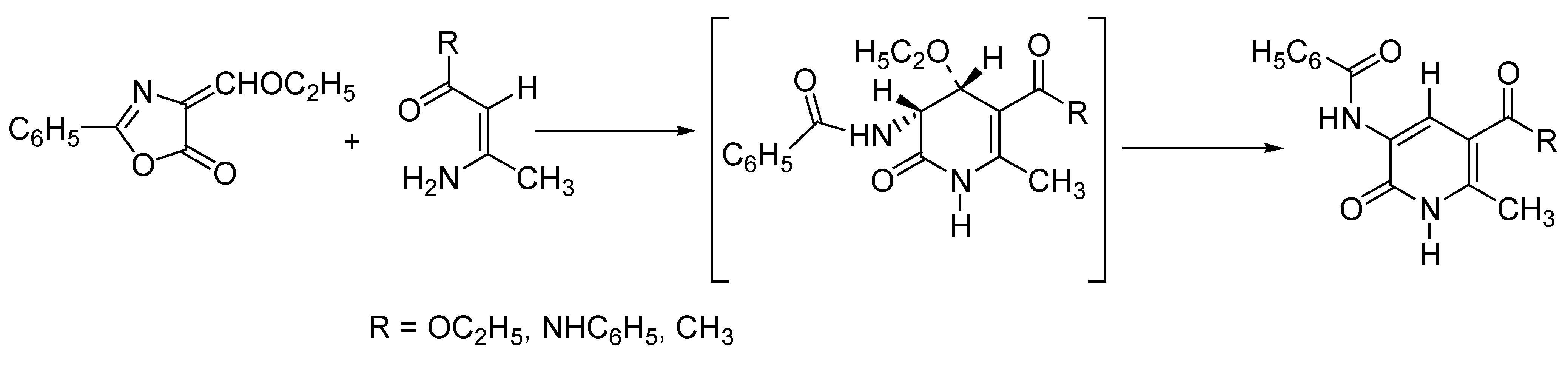
High-Throughput Synthesis
Conclusions
References and Notes
- Hantzsch, A. Condensationprodukte aus Aldehydammoniak und Ketoniartigen Verbindungen. Ber. 1881, 14, 1637–1638. [Google Scholar] [CrossRef]
- Love, B.; Goodman, M.; Snader, K.; Tedeschi, R.; Macko, E. “Hantzsch-type” dihydropyridine hypotensive agents. 3. J. Med. Chem. 1974, 17, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Bossert, F.; Meyer, H.; Wehinger, E. 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew. Chem. Int. Ed. Engl. 1981, 20, 762–769. [Google Scholar]
- Katzung, B.G. Basic & Clinical Pharmacology; Appleton & Lange: Lange, Stamford, CT (USA), 1998. [Google Scholar]
- Gordeev, M.F.; Patel, D.V.; Gordon, E.M. Approaches to combinatorial synthesis of heterocycles: a solid-phase synthesis of 1,4-dihdropyridines. J. Org. Chem. 1996, 61, 924–928. [Google Scholar] [CrossRef]
- Breitenbucher, J.G.; Figliozzi, G. Solid-phase synthesis of 4-aryl-1,4-dihydropyridines via the Hantzsch three component condensation. Tetrahedron Lett. 2000, 41, 4311–4315. [Google Scholar] [CrossRef]
- Vanden Eynde, J.J.; Mayence, A. New methodologies for the preparation of drugs and their metabolites. Application to 1,4-dihydropyridines structurally related to calcium channel modulators of the Nifedipine-type. Intl. J. Med. Biol. Environ. 2000, 28, 25–31. [Google Scholar]
- Böcker, R.H.; Guengerich, F.P. Oxidation of 4-aryl- and 4-alkyl-substituted 2,6-dimethyl-3,5-bis(alkoxycarbonyl)-1,4-dihydropyridines by human liver microsomes and immunochemical evidence for the involvement of a form of cytochrome P-450. J. Med. Chem. 1986, 29, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Alajarin, R.; Vaquero, J.J.; Garcia Navio, J.L.; Alvarez-Builla, J. Synthesis of 1,4-dihydropyridines under microwave irradiation. Synlett 1992, 297–298. [Google Scholar] [CrossRef]
- Alajarin, R.; Jordan, P.; Vaquero, J.J.; Alvarez-Builla, J. Synthesis of unsymmetrically substituted 1,4-dihydropyridines and analogous calcium antagonists by microwave heating. Synthesis 1995, 389–391. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Shan, Z.-X.; Pan, B.; Lu, X.-H.; Chen, M.-H. Research on the synthesis of 1,4-dihydropyridines under microwave. Synth. Commun. 1995, 25, 857–862. [Google Scholar] [CrossRef]
- Khadilkar, B.M.; Chitnavis, A.A. Rate enhancement in the synthesis of some 4-aryl-1,4-dihydropyridines using methyl 3-aminocrotonate under microwave irradiation. Ind. J. Chem. 1995, 34B, 652–653. [Google Scholar]
- Khadilkar, B.M.; Gaikar, V.G.; Chitnavis, A.A. Aqueous hydrotrope solution as a safer medium for microwave enhanced Hantzsch dihydropyridine ester synthesis. Tetrahedron Lett. 1995, 36, 8083–8086. [Google Scholar] [CrossRef]
- Suarez, M.; Loupy, A.; Perez, E.; Moran, L.; Gerona, G.; Morales, A.; Autié, M. An efficient procedure to obtain hexahydroquinoleines and unsymmetrical 1,4-dihydropyridines using solid inorganic supports and microwave activation. Heterocycl. Commun. 1966, 2, 275–280. [Google Scholar]
- Ohberg, L.; Westman, J. An efficient and fast procedure for the Hantzsch dihydropyridine synthesis under microwave conditions. Synlett 2001, 1296–1298. [Google Scholar] [CrossRef]
- Vanden Eynde, J.J.; Rutot, D. Microwave-mediated derivatization of poly(styrene-co-allyl alcohol), a key step for the soluble polymer-assisted synthesis of heterocycles. Tetrahedron 1999, 55, 2687–2694. [Google Scholar] [CrossRef]
- Torchy, S.; Cordonnier, G.; Barbry, D.; Vanden Eynde, J.J. Hydrogen transfer from Hantzsch 1,4-dihydropyridines to carbon-carbon double bonds under microwave irradiation. Molecules 2002, 7, 528–533. [Google Scholar] [CrossRef]
- Love, B.; Snader, K. The Hantzsch reaction. I. Oxidation dealkylation of certain dihydropyridines. J. Org. Chem. 1965, 30, 1914–1916. [Google Scholar] [CrossRef]
- de Matteis, F.; Hollands, C.; Gibbs, A.H.; de Sa, N.; Rizzardini, M. Inactivation of cytochrome P-450 and production of N-alkylated porphyrins caused in isolated hepatocytes by substituted dihydropyridines. Structural requirements for loss of haem and alkylation of the pyrrole nitrogen atom. FEBS Lett. 1982, 145, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Augusto, O.; Beilan, H.S.; Ortiz de Montellano, P.R. The catalytic mechanism of cytochrome P-450. Spin-trapping evidence for one-electron substrate oxidation. J. Biol. Chem. 1982, 257, 11288–11295. [Google Scholar] [PubMed]
- Ayling, E.E. The influences of alkyl groups in carbonyl compounds. J. Chem. Soc. 1938, 1014–1023. [Google Scholar] [CrossRef]
- Vanden Eynde, J.J.; Delfosse, F.; Mayence, A.; Van Haverbeke, Y. Old reagents, new results: aromatization of Hantzsch 1,4-dihydropyridines with manganese dioxide and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Tetrahedron 1995, 51, 6511–6516. [Google Scholar] [CrossRef]
- Alvarez, C.; Delgado, F.; Garcia, O.; Medina, S.; Marquez, C. MnO2/bentonite: a new reactive for the oxidation of Hantzsch’s dihydropyridines using microwave irradiation in the absence of solvent (I). Synth. Commun. 1991, 21, 619–624. [Google Scholar] [CrossRef]
- Delgado, F.; Alvarez, C.; Garcia, O.; Penieres, G.; Marquez, C. Unusual oxidative dealkylation of certain 4-alkyl-1,4-dihydropyridines with MnO2/bentonite using microwave irradiation in the absence of solvent (II). Synth. Commun. 1991, 21, 2137–2141. [Google Scholar] [CrossRef]
- Garcia, O.; Delgado, F.; Cano, A.C.; Alvarez, C. Oxydation d’esters de Hantzsch, par le nouveau système HNO3/bentonite, et irradiation aux micro-ondes. Tetrahedron Lett. 1993, 34, 623–625. [Google Scholar] [CrossRef]
- Varma, R.S.; Kumar, D. Solid state oxidation of 1,4-dihydropyridines to pyridines using phenyliodine (III) bis(trifluoroacetate) or elemental sulfur. J. Chem. Soc., Perkin Trans. 1 1999, 1755–1757. [Google Scholar]
- Vanden Eynde, J.J.; Mayence, A.; Maquestiau, A. A novel application of the oxidizing properties of pyridinium chlorochromate: aromatization of Hantzsch 1,4-dihydropyridines. Tetrahedron 1992, 48, 463–468. [Google Scholar] [CrossRef]
- Vanden Eynde, J.J. Unpublished results.
- Penieres, G.; Garcia, O.; Franco, K.; Hernandez, O.; Alvarez, C. A modification to the Hantzsch method to obtain pyridines in a one-pot reaction: use of a bentonitic clay in a dry medium. Heterocycl. Commun. 1996, 2, 359–360. [Google Scholar]
- Cotterill, I.C.; Usyatinsky, A.Y.; Arnold, J.M.; Clark, D.S.; Dordick, J.S.; Michels, P.C.; Khmelnitsky, Y.L. Microwave assisted combinatorial chemistry, synthesis of substituted pyridines. Tetrahedron Lett. 1998, 39, 1117–1120. [Google Scholar] [CrossRef]
- Vanden Eynde, J.J.; Labuche, N.; Van Haverbeke, Y. Microwave-mediated domino reaction in dry medium. Preparation of dihydropyridinones and pyridinones structurally related to Hantzsch esters. Synth. Commun. 1997, 27, 3683–3690. [Google Scholar] [CrossRef]
- Sample Availability: Available from MDPI.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Vanden Eynde, J.J.; Mayence, A. Synthesis and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. An Overview. Molecules 2003, 8, 381-391. https://doi.org/10.3390/80400381
Vanden Eynde JJ, Mayence A. Synthesis and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. An Overview. Molecules. 2003; 8(4):381-391. https://doi.org/10.3390/80400381
Chicago/Turabian StyleVanden Eynde, Jean Jacques, and Annie Mayence. 2003. "Synthesis and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. An Overview" Molecules 8, no. 4: 381-391. https://doi.org/10.3390/80400381
APA StyleVanden Eynde, J. J., & Mayence, A. (2003). Synthesis and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. An Overview. Molecules, 8(4), 381-391. https://doi.org/10.3390/80400381



