Novel Cytotoxic Oxopyridoindolizines: iso-Propyl-7,8,9-trichloro-6,7,8,9-tetrahydro-5-oxopyrido[2,3-a]-indolizine-10-carboxylates (OPIC)
Abstract
:Introduction
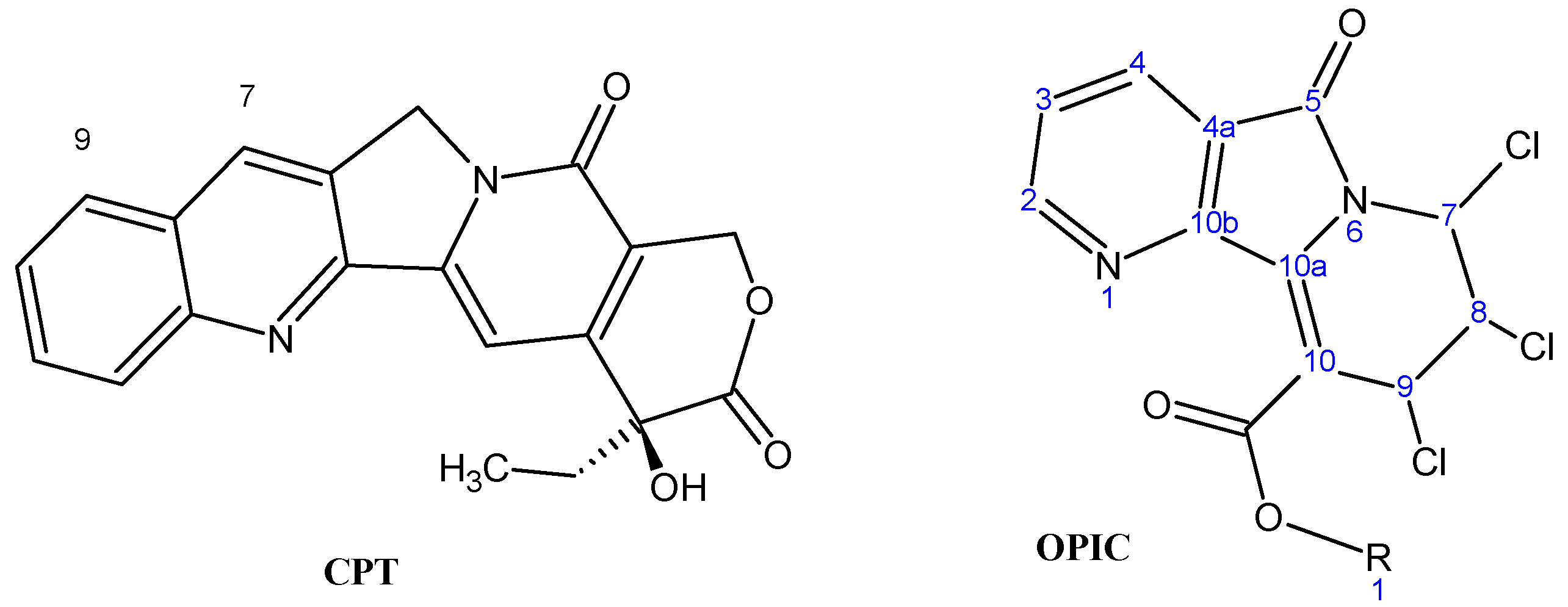
Results and Discussion
Chemistry
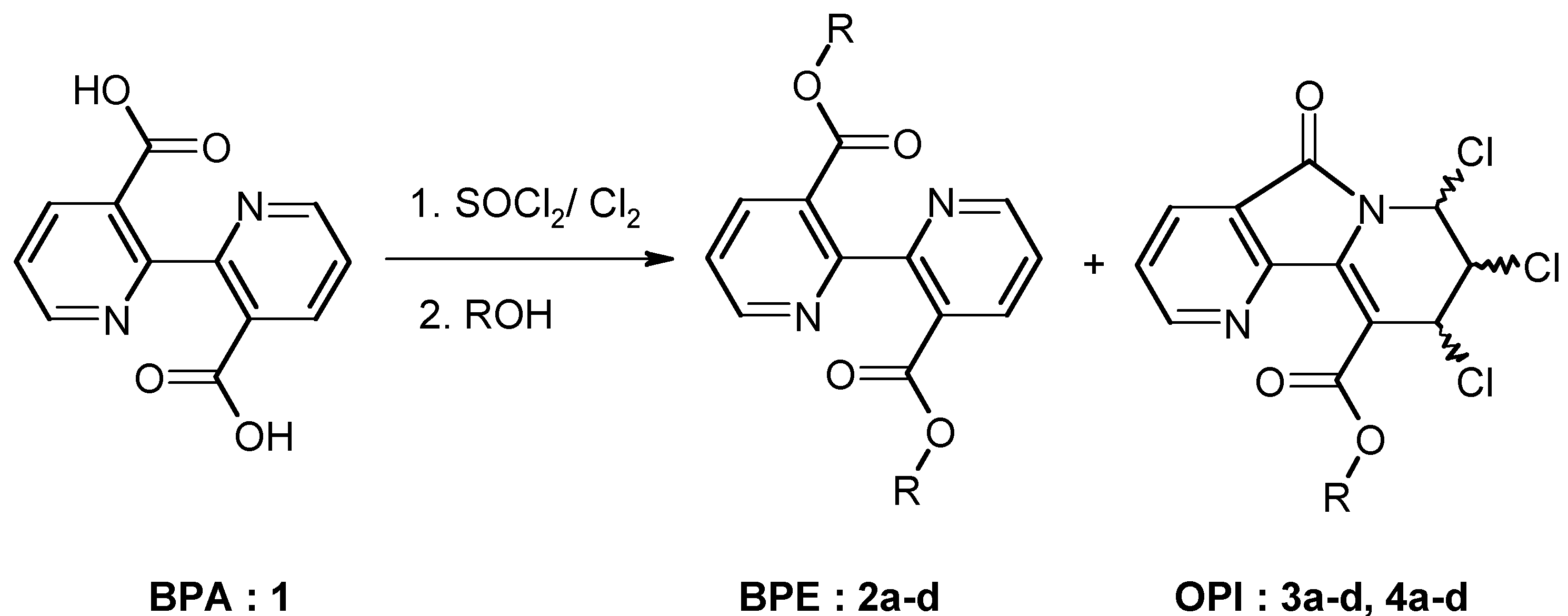
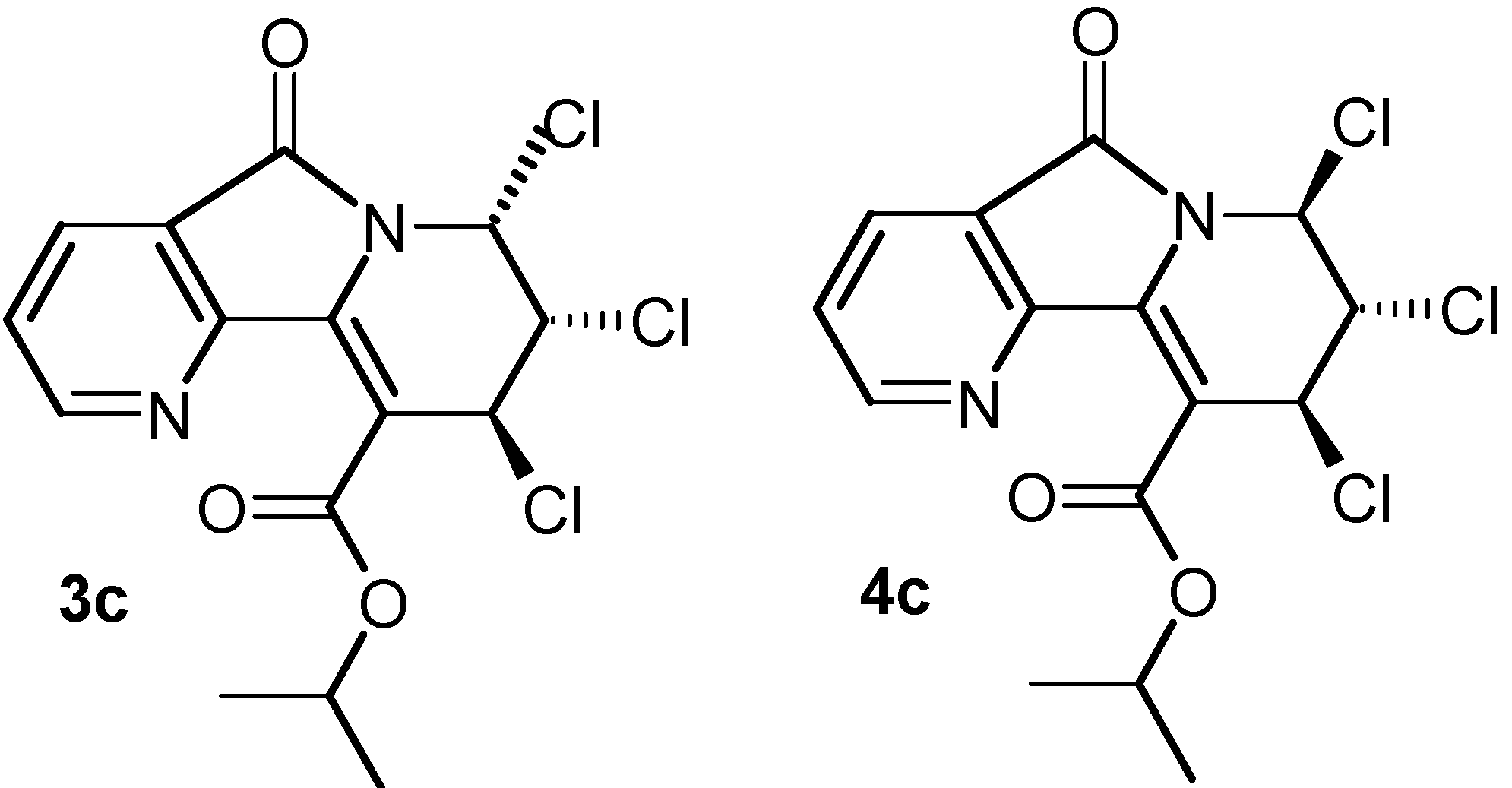
| 2b: (R = Et) 81% | 3b: (R = Et) 4% | 4b: (R = Et) 12% |
| 2c: (R =i-Pr) 73% | 3c: (R =i-Pr) 6% | 4c: (R =i-Pr) 12% |
Evaluation of in vitro Anti-tumour Activity
| Compound | R | Growth Percentages20 | Activity | ||
| (Lung) | (Breast) | (CNS) | |||
| NCI-H460 | MCF7 | SF-268 | |||
| 2b | Et | 97 | 89 | 90 | Inactive |
| 2c | i-Pr | 105 | 90 | 114 | Inactive |
| 2d | i-Bu | 81 | 82 | 74 | Inactive |
| 3c | i-Pr | 96 | 20 | 72 | Active |
| 4b | Et | 99 | 67 | 96 | Inactive |
| 4c | i-Pr | 105 | 19 | 62 | Active |
| 4d | i-Bu | 100 | 62 | 100 | Inactive |
| Panel | Cell line | GI50 | |
| 3c | 4c | ||
| HL-60(TB) | 2.58x10-5 | 3.82x10-5 | |
| K-563 | 3.34x10-5 | 3.06x10-5 | |
| Leukemia | MOLT-4 | 3.19x10-5 | 2.25x10-5 |
| RPMI-8226 | 2.98x10-5 | 2.18x10-5 | |
| SR | 4.96x10-5 | 2.65x10-5 | |
| A549/ATCC | > 10-4 | > 10-4 | |
| EKVX | > 10-4 | > 10-4 | |
| HOP-62 | > 10-4 | > 10-4 | |
| Non-small | HOP-92 | > 10-4 | 4.63x10-5 |
| cell lung | NCI-H226 | > 10-4 | > 10-4 |
| NCI-H23 | > 10-4 | 4.62x10-5 | |
| NCI-H322M | > 10-4 | > 10-4 | |
| NCI-H460 | > 10-4 | > 10-4 | |
| NCI-H522 | 3.57x10-5 | 2.62x10-5 | |
| Panel | Cell line | GI50 | |
| 3c | 4c | ||
| SF-268 | 8.21x10-5 | 4.79x10-5 | |
| SF-295 | > 10-4 | > 10-4 | |
| CNS cancer | SF-539 | > 10-4 | > 10-4 |
| SNB-19 | > 10-4 | > 10-4 | |
| U251 | > 10-4 | 5.68x10-5 | |
| LOX IMVI | > 10-4 | 6.88x10-5 | |
| MALME-3M | > 10-4 | 2.88x10-5 | |
| M14 | > 10-4 | > 10-4 | |
| SK-MEL-2 | > 10-4 | > 10-4 | |
| Melanoma | SK-MEL-28 | > 10-4 | > 10-4 |
| SK-MEL-5 | > 10-4 | 8.30x10-5 | |
| UACC-257 | > 10-4 | 3.41x10-5 | |
| UACC-62 | > 10-4 | > 10-4 | |
| IGROV1 | 9.63x10-5 | 3.11x10-5 | |
| OVCAR-3 | > 10-4 | > 10-4 | |
| Ovarian cancer | OVCAR-4 | > 10-4 | > 10-4 |
| OVCAR-5 | > 10-4 | > 10-4 | |
| OVCAR-8 | 3.98x10-5 | 3.17x10-5 | |
| 786-0 | 7.39x10-5 | 5.52x10-5 | |
| A498 | > 10-4 | > 10-4 | |
| ACHIN | > 10-4 | > 10-4 | |
| Renal cancer | CAKI-1 | > 10-4 | > 10-4 |
| RXF 393 | > 10-4 | > 10-4 | |
| SN12C | 2.12x10-5 | 2.18x10-5 | |
| TK-10 | > 10-4 | > 10-4 | |
| UO-31 | > 10-4 | > 10-4 | |
| Panel | Cell line | GI50 | |
| 3c | 4c | ||
| PC-3 | > 10-4 | 5.66x10-5 | |
| Prostate Cancer | DU-145 | > 10-4 | > 10-4 |
| MCF7 | 8.44x10-5 | 3.91x10-5 | |
| NCI/ADR-RES | > 10-4 | > 10-4 | |
| Breast Cancer | MDA-MB-231/ATCC | > 10-4 | > 10-4 |
| HS 578T | 8.15x10-5 | > 10-4 | |
| MDA-MB-435 | > 10-4 | 8.10x10-5 | |
| MDA-N | 4.41x10-5 | 5.10x10-5 | |
| BT-549 | > 10-4 | 3.69x10-5 | |
| COLO 205 | > 10-4 | 4.39x10-5 | |
| HCC-2998 | > 10-4 | > 10-4 | |
| HCT-116 | > 10-4 | 5.06x10-5 | |
| Colon Cancer | HCT-15 | > 10-4 | 7.03x10-5 |
| HT29 | > 10-4 | > 10-4 | |
| KM12 | > 10-4 | > 10-4 | |
| SW-620 | 3.49x10-5 | 3.46x10-5 | |
Conclusions
Experimental
General
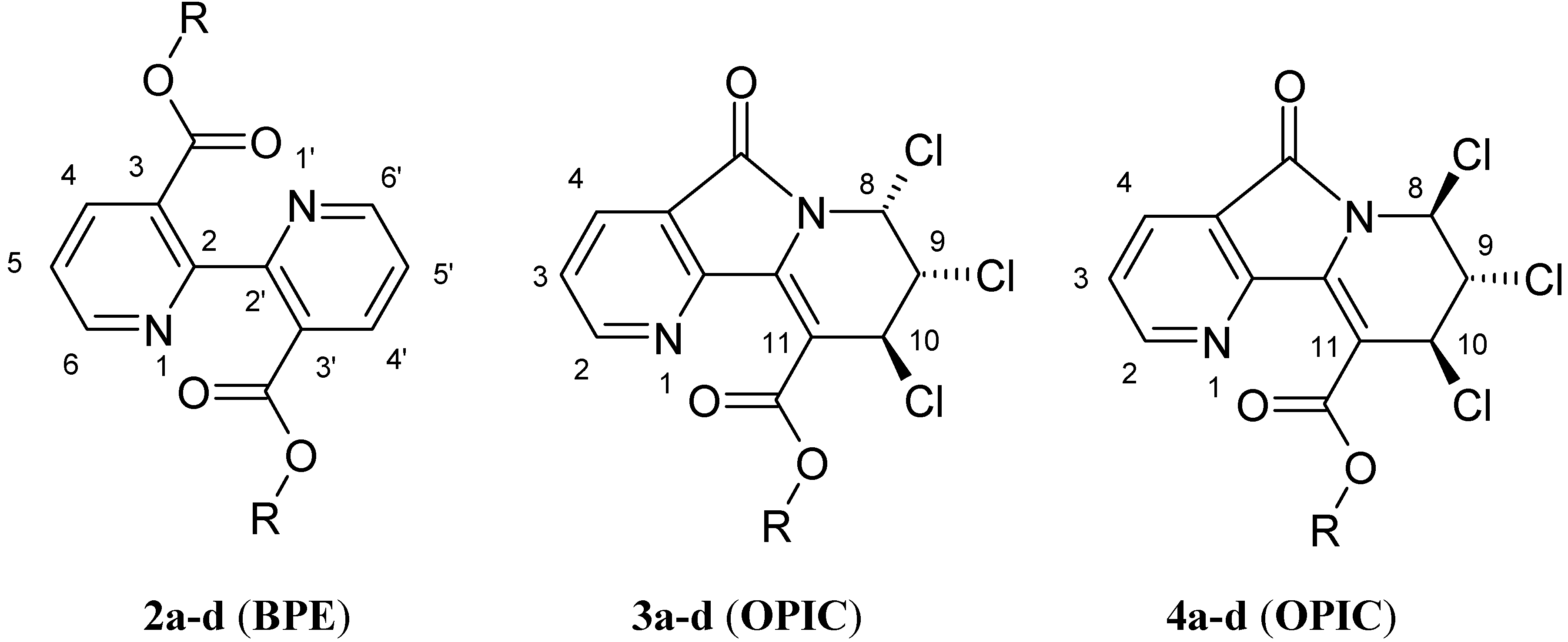
General procedure for the synthesis of 3,3'-di-alkyloxycarbonyl-2,2'-bipyridines (BPE: 2) and alkyl-7,8,9-trichloro-6,7,8,9-tetrahydro-5-oxopyrido [2,3-a]indolizine-10-carboxylates (OPIC: 3 and 4).
Acknowledgements
References and Notes
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. J. Amer. Chem. Soc. 1966, 88, 3888.
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. J. Bioi. Chem. 1985, 260, 14873.
- Wang, J.C. Annu. Rev. Pharmacol. Toxicol. 1996, 94, 194.
- Pommier, Y. Sem. Oncol. 1996, 23, 1.
- Camptothecins: New Anticancer Agents; Potmesil, H.; Pinedo, H. (Eds.) CRC: Boca Raton, 1995.
- Pantaziz, P.; Giovannella, B.C. (Eds.) The Camptothecins: From discovery to the patient. Ann. N. Y.: Acad. Sci. 1996, 803.
- Lerchen, H.G. Drugs 1999, 2, 896.
- Robinson, C.; Robinson, K.; Castaner, J. Drugs of Future 1996, 21, 881.
- Eckhardt, S.G.; Baker, S.D.; Eckhardt, J.R.; Burke, T.G.; Warner, D.L.; Kuhn, J.G.; Rodriguez, G.; Fields, S.; Thurman, A.; Smith, L.; Rothenberg, M.L.; White, L.; Wissel, P.; Kunka, R.; Depee, S.; Littlefield, D.; Burris, H.A.; Von Hoff, D.D.; Rowinsky, E.K. Clinic Cancer Res. 1998, 4, 595.
- Kumazawa, E.; Jimbo, T.; Ochi, Y; Tohgo, A. Cancer Chemother. Pharmacol. 1998, 42, 210.
- Kawato, Y.; Terasawa, H. Progress in Medicinal Chemistry; Ellis, G.P., Luscombe, D.K., Eds.; Elsevier: London, 1997. [Google Scholar]
- Dallava11e, S.; Ferrari, A.; Merlini, L; Penco, S.; Carenini, N.; De Cesare, M.; Perego, P.; Pratesi, G.; Zunino, F. Bioorg. Med. Chem. Lett. 2001, 1.
- Redinbo, R.R.; Stewart, L.; Kuhn, P.; Champoux, J.J.; Hol, W.G.J. Science 1998, 279, 1504.
- Fan, J.; Weinstein, J.N.; Kohn, K.W.; Shi, L.M.; Pommier, Y.J. Med. Chem. 1998, 41, 2216.
- Pantazis, P. Leuk. Res. 1995, 19, 775.
- Cai, Q.; Lindsey, J.R.; Zhang, R. Int. J. 0ncol. 1997, 10, 953.
- Ben-Hadda, T.; Sam, N.; Le Bozec, H.; Dixneuf, P.H. Inorg. Chem. Comm. 1999, 2, 460.
- Lewis; Wedekind. Proc. Chem. 1909, 25, 59.
- Pascal, P. Nouveau Traitǔ de Chimie Minǔrale; Tome, XVI, Ed.; Masson et Cie: Paris, 1960; pp. 153–156. [Google Scholar]
- Sam, N.; Elkadiri, S.; Le Bozec, H.; Toupet, L.; Daoudi, M.; Bitit, N.; Ben Hadda, T.; Dixneuf, P.H. J. Chem. Soc., Perkin Trans. 1 2002, 1688–1692.
- In the current protocol, each cell line is inoculated and preincubated on a microtiter plate. Test agents are then added at a single concentration and the culture incubated for 48 hours. End-point determinations are made with Alamar blue (Biotechniques 1996, 21, 780). Results for each test agent are reported as the percent growth of the treated cells compared to the untreated controls. Compounds which reduce the growth of any one of the cell lines to approximately 32% or less (negative numbers indicate cell kill) are passed on for evaluation in the full panel of 60 cell lines over a 5-log dose range. All compounds were tested at a final concentration of 10-4 M.
- Le Bozec, H.; Toupet, L.; Daoudi, M.; Ben Larbi, N.; Sam, N.; Mimouni, M.; Ben Hadda, T. Orgchem/0206001. 2002. Available online: http://preprint.chemweb.com/Orgchem/0206001.
- Sample Availability: Not available.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zunino, F.; Kotchevar, A.T.; Waring, M.; Daoudi, M.; Larbi, N.B.; Mimouni, M.; Sam, N.; Zahidi, A.; Ben-Hadda, T. Novel Cytotoxic Oxopyridoindolizines: iso-Propyl-7,8,9-trichloro-6,7,8,9-tetrahydro-5-oxopyrido[2,3-a]-indolizine-10-carboxylates (OPIC). Molecules 2002, 7, 628-640. https://doi.org/10.3390/70800628
Zunino F, Kotchevar AT, Waring M, Daoudi M, Larbi NB, Mimouni M, Sam N, Zahidi A, Ben-Hadda T. Novel Cytotoxic Oxopyridoindolizines: iso-Propyl-7,8,9-trichloro-6,7,8,9-tetrahydro-5-oxopyrido[2,3-a]-indolizine-10-carboxylates (OPIC). Molecules. 2002; 7(8):628-640. https://doi.org/10.3390/70800628
Chicago/Turabian StyleZunino, Franco, Ann T. Kotchevar, Michael Waring, Maria Daoudi, Najib Ben Larbi, Mostafa Mimouni, Najat Sam, Assou Zahidi, and Taibi Ben-Hadda. 2002. "Novel Cytotoxic Oxopyridoindolizines: iso-Propyl-7,8,9-trichloro-6,7,8,9-tetrahydro-5-oxopyrido[2,3-a]-indolizine-10-carboxylates (OPIC)" Molecules 7, no. 8: 628-640. https://doi.org/10.3390/70800628
APA StyleZunino, F., Kotchevar, A. T., Waring, M., Daoudi, M., Larbi, N. B., Mimouni, M., Sam, N., Zahidi, A., & Ben-Hadda, T. (2002). Novel Cytotoxic Oxopyridoindolizines: iso-Propyl-7,8,9-trichloro-6,7,8,9-tetrahydro-5-oxopyrido[2,3-a]-indolizine-10-carboxylates (OPIC). Molecules, 7(8), 628-640. https://doi.org/10.3390/70800628




