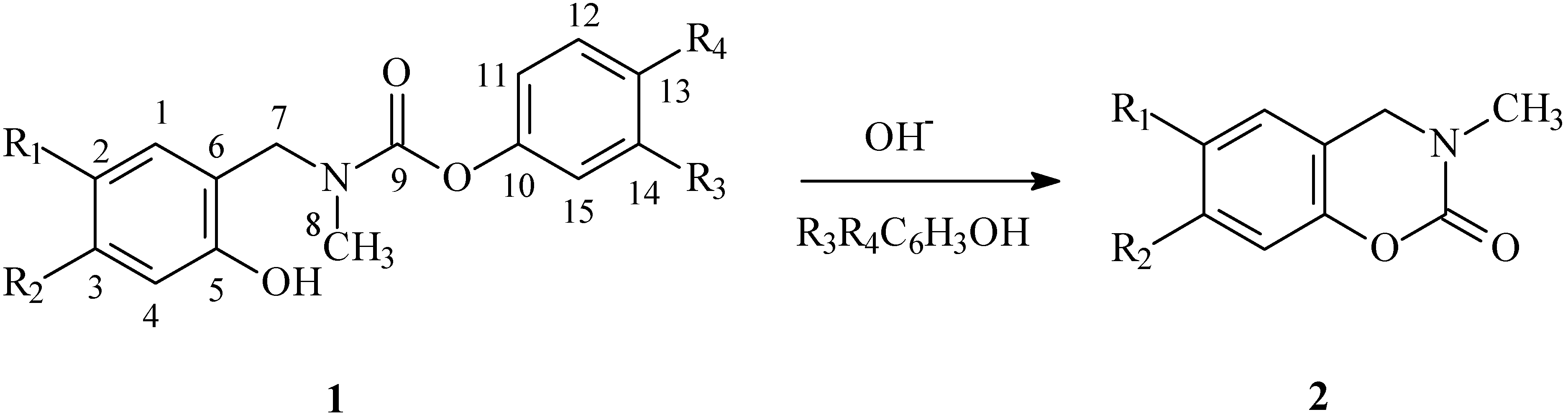
Yields and physicochemical properties:
Phenyl N-(4-chloro-2-hydroxybenzyl)carbamate (1a). Yield 61%; m.p. 110.5-112.0oC; Calculated for C15H14ClNO3 (291.2): 61.76 % C, 4.81% H, 4.80% N; found 61.73% C, 4.81% H, 4.95% N; 1H-NMR: 8.93 (br, 1H, OH), 7.35 (m, 2H, H-12), 7.21 (m, 1H, H-13), 7.08 (m, 2H, H-11), 7.05 (d, J=8.1, 1H, H-1), 6.95 (s, 1H, H-4), 6.82 (dd, J=2.1, J=8.0, 1H, H-2), 4.37 (s, 2H, H-7), 3.11 (s, 3H, H-8); 13C-NMR: 157.1 (C-5), 156.7 (C-9), 150.8 (C-10), 135.4 (C-3), 131.8 (C-1), 129.3 (C-12), 125.8 (C-13), 121.4 (C-11), 120.3 (C-6), 119.6 (C-4), 118.0 (C-2), 49.5 (C-7), 34.2 (C-8).
3-Chlorophenyl N-(4-chloro-2-hydroxybenzyl)carbamate (1b). Yield 55%; m.p. 101-103o C; Calculated for C15H13ClNO3 (325.3): 55.24 % C, 4.02% H, 4.29% N; found 55.19% C, 4.10% H, 4.47% N; 1H-NMR: 8.75 (br, 1H, OH), 7.29 (m, 1H, H-14), 7.21 (m, 1H, H-15), 7.14 (m, 1H, H-11), 7.06 (d, J=8.1, 1H, H-1), 7.01 (m, 1H, H-13), 6.96 (s, 1H, H-4), 6.84 (dd, J=1.8, J=8.0, 1H, H-2), 4.38 (s, 2H, H-7), 3.11 (s, 3H, H-8); 13C-NMR: 156.7 (C-5), 156.6 (C-9), 151.2 (C-10), 135.6 (C-12), 134.6 (C-3), 131.9 (C-1), 130.0 (C-14), 126.1 (C-13), 122.1 (C-11), 120.0 (C-6), 119.8 (C-15), 119.8 (C-4), 118.1 (C-2), 49.5 (C-7), 34.3 (C-8).
3-Nitrophenyl N-(4-chloro-2-hydroxybenzyl)carbamate (1c). Yield 81%; m.p. 123-126o C; Calculated for C15H13ClN2O5 (336.3): 53.50 % C, 3.89% H, 8.32% N; found 53.64% C, 3.92% H, 8.31% N; 1H-NMR: 8.53 (br, 1H, OH), 8.08 (d, J = 8.0, 1H, H-13), 8.00 (m, 1H, H-11), 7.54 (m, 1H, H-14), 7.47 (d, J=8.2, 1H, H-15), 7.08 (d, J=8.1, 1H, H-1), 6.91 (d, J=1.4, 1H, H-4), 6.84 (dd, J=2.0, J=8.0, 1H, H-2), 4.41 (s, 2H, H-7), 3.14 (s, 3H, H-8); 13C-NMR: 156.4 (C-5), 156.0 (C-9), 151.0 (C-10), 148.5 (C-12), 135.6 (C-3), 131.9 (C-1), 129.9 (C-14), 127.9 (C-15), 120.6 (C-13 + C-6), 119.9 (C-4), 117.9 (C-2), 117.1 (C-11), 49.5 (C-7), 34.3 (C-8).
Phenyl N-(5-bromo-2-hydroxybenzyl)carbamate (1d). Yield 72%; m.p. 113-115o C; Calculated for C15H14BrNO3 (336.4): 53.61 % C, 4.17% H, 4.17% N; found 53.47% C, 4.20% H, 4.29% N; 1H-NMR: 8.76 (br, 1H, OH), 7.31 (m, 3H, H-3 + H-12 + H-14), 7.24 (s, 1H, H-1), 7.18 (m, 1H, H-13), 7.07 (m, 2H, H-11 + H-15), 6.78 (d, J=8.5, 1H, H-4), 4.33 (s, 2H, H-7), 3.08 (s, 3H, H-8); 13C-NMR (CDCl3): 157.0 (C-5), 155.0 (C-9), 150.8 (C-10), 133.3 (C-3), 132.8 (C-1), 129.3 (C-12 + C-14), 125.8 (C-13), 123.9 (C-6), 121.4 (C-11 + C-15), 119.5 (C-4), 111.2 (C-2), 49.4 (C-2), 29.6 (C-8).
4-Chlorophenyl N-(5-bromo-2-hydroxybenzyl)carbamate (1e). Yield 79%; m.p. 124 - 126o C; Calculated for C15H13BrClNO3 (370.4): 48.62% C, 3.51% H, 3.78% N; found 48.66% C, 3.52% H, 3.77% N; 1H-NMR: 8.65 (br, 1H, OH), 7.32 (m, 3H, H-3 + H-12+ H-14), 7.26 (d, J=2.4, 1H, H-1), 7.04 (m, 2H, H-11 + H-15), 6.83 (d, J=8.6, 1H, H-4), 4.37 (s, 2H, H-7), 3.13 (s, 3H, H-8); 13C-NMR: 156.6 (C-5), 154.9 (C-9), 149.2 (C-10), 133.3 (C-3), 132.9 (C-1), 131.1 (C-13), 129.2 (C-12 + C-14), 123.6 (C-6), 122.7 (C-11 + C-15), 119.5 (C-4), 111.2 (C-2), 49.5 (C-7), 34.4 (C-8).
3-chlorophenyl N-(5-bromo-2-hydroxybenzyl)carbamate (1f). Yield 54%; m.p. 130 -134o C; Calulated for C15H13BrClNO3 (370.4): 48.62% C, 3.51% H, 3.78% N; found 48.38% C, 3.51% H, 3.97% N; 1H-NMR: 8.62 (br, 1H, OH), 7.33 (dd, J=2.3, J=8.7, 1H, H-3), 7.27 (m, 2H, H-14 + H-1), 7.21 (m, 1H, H-15), 7.14 (t, J = 2.00, 1H, H-11), 7.01 (m, 1H, H-13), 6.84 (d, J=8.6, 1H, H-4), 4.37 (s, 2H, H-7), 3.13 (s, 3H, H-8); 13C-NMR: 156.5 (C-5), 155.0 (C-9), 151.2 (C-10), 134.6 (C-12), 133.4 (C-3), 133.1 (C-1), 130.0 (C-14), 126.1 (C-13), 123.5 (C-11), 119.8 (C-15), 119.7 (C-4), 111.2 (C-2), 49.6 (C-7), 34.5 (C-8).
3-Nitrophenyl N-(5-bromo-2-hydroxybenzyl)carbamate (1g). Yield 74%; m.p. 147oC (dec); Calculated for C15H13BrClN2O5 (416.5): 47.27% C, 3.41% H, 7.35% N; found 47.44% C, 3.50% H, 7.26% N; 1H-NMR: 8.41 (br, 1H, OH), 8.11 (m, 1H, H-13), 8.02 (t, J=2.2, 1H, H-11), 7.56 (t, J=8.1, 1H, H-14), 7.49 (m, 1H, H-15), 7.35 (dd, J=2.3, J=8.6, 1H, H-3), 7.29 (d, J=2.4, 1H, H-1), 6.85 (d, J=8.6, 1H, H-4), 4.41 (s, 2H, H-7), 3.18 (s, 3H, H-8); 13C-NMR: 155.0 (C-9), 154.9 (C-5), 150.9 (C-10), 148.5 (C-12), 133.4 (C-3), 133.2 (C-1), 129.9 (C-14), 127.9 (C-15), 123.3 (C-6), 120.7 (C-13), 119.7 (C-4), 117.2 (C-11), 111.4 (C-2), 49.7 (C-7), 34.6 (C-8).
Phenyl N-(4-nitro-2-hydroxybenzyl)carbamate (1h). Yield 49%; m.p. 147.5 – 150 °C; Calulated for C15H14N2O5 (302.3): 59.62% C, 4.63% H, 9.27% N; found 59.44% C, 4.63% H, 9.38% N; 1H-NMR: 9.25 (br, 1H, OH), 7.75 (s, 1H, H-4), 7.70 (d, J=8.2, 1H, H-1), 7.37 (m, 2H, H-12 + H-14), 7.30 (d, J=8.1, 1H, H-2), 7.23 (m, 1H, H-13), 7.10 (m, 2H, H-11 + H-15), 4.96 (s, 2H, H-7), 3.16 (s, 3H, H-8); 13C-NMR: 157.2 (C-5), 156.7 (C-9), 150.8 (C-10), 149.3 (C-3), 131.4 (C-1), 129.4 (C-12 + C-14), 128.6 (C-6), 125.9 (C-13), 121.4 (C-11 + C-15), 114.3 (C-2), 113.0 (C-4), 49.5 (C-7), 34.7 (C-8).
3-Nitrophenyl N-(4-nitro-2-hydroxybenzyl)carbamate (1i). Yield 84%; m.p. 137oC (dec.); Calculated for C15H13N3O7 (347.4): 51.89% C, 3.74% H, 12.11% N; found 51.96% C, 3.76% H, 12.16% N;1H-NMR: 8.95 (br, 1H, OH), 8.13 (d, J=8.1, 1H, H-13), 8.02 (m, 1H, H-11), 7.78 (d, J=1.90, 1H, H-4), 7.73 (dd, J=2.0, J=8.3, 1H, H-2), 7.59 (m, 1H, H-14), 7.49 (dd, J=1.0, J=8.7, 1H, H-15), 7.32 (d, J=8.3, 1H, H-1), 4.53 (s, 2H, H-7), 3.20 (s, 3H, H-8).
Phenyl N-(5-nitro-2-hydroxybenzyl)carbamate (1j). Yield 73%; m.p. 146 (dec.); Calculated for C15H14N2O5 (302.3): 59.62% C, 4.63% H, 9.27% N; found 59.73% C, 4.65% H, 9.43% N; 1H-NMR:9.50 (br, 1H, OH), 8.11 (m, 2H, H-1 + H-3), 7.37 (m, 2H, H-12 + H-14), 7.21 (t, J=7.4, 1H, H-13), 7.10 (m, 2H, H-11 + H-15), 6.97 (d, J=8.8, 1H, H-4), 4.47 (s, 2H, H-7), 3.17 (s, 3H, H-8); 13C-NMR: 162.2 (C-5), 157.5 (C-9), 150.7 (C-10), 140.2 (C-2), 129.4 (C-12 + C-14), 127.2 (C-3), 126.4 (C-1), 126.0 (C-13), 122.0 (C-6), 121.3 (C-11 + C-15), 118.2 (C-4), 49.7 (C-7), 34.5 (C-8).
4-Chlorophenyl N-(5-nitro-2-hydroxybenzyl)carbamate (1k). Yield 88%; m.p. 144.5-147.0o C; Calculated for C15H13ClN2O5 (336.3): 53.50 % C, 3.89% H, 8.32% N; found 53.20% C, 3.86% H, 8.58% N; 1H-NMR: 9.47 (br, 1H, OH), 8.16 (dd, J=2.7, J=9.0, 1H, H-3), 8.11 (d, J=2.7, 1H, H-1), 7.34 (m, 2H, H-12 + H.14), 7.06 (m, 2H, H-11 + H-15), 7.00 (d, J=8.9, 1H, H-4), 4.47 (s, 2H, H-7), 3.18 (s, 3H, H-8).
3-Chlorophenyl N-(5-nitro-2-hydroxybenzyl)carbamate (1l). Yield 71%; m.p. 117-119oC; Calculated for C15H13ClN2O5 (336.3): 53.50 % C, 3.89% H, 8.32% N; found 53.44% C, 3.89% H, 8.44% N; 1H-NMR: 9.54 (br, 1H, OH), 8.09 (m, 2H, H-1 + H-3), 7.28 (t, J=8.1, 1H, H-14), 7.19 (d, J=8.1, 1H, H-13), 7.14 (s, 1H, H-11), 7.02 (d, J=8.0, 1H, H-15), 6.91 (d, J=9.1, 1H, H-4), 4.54 (s, 2H, H-7), 3.15 (s, 3H, H-8); 13C-NMR: 161.9 (C-5), 156.8 (C-9), 150.9 (C-10), 140.1 (C-2), 134.5 (C-12), 130.0 (C-14), 1287.0 (C-13), 127.0 (C-1), 126.4 (C-3), 126.2 (C-11), 121.9 (C-15), 121.7 (C-6), 119.6 (C-4), 49.6 (C-7), 34.5 C-8).
3-Nitrophenyl N-(5-nitro-2-hydroxybenzyl)carbamate (1m). Yield 90%; m.p. 151-154oC; Calculated for C15H13N3O7 (347.4): 51.89% C, 3.74% H, 12.11% N; found 52.18% C, 3.83% H, 12.22% N; 1H-NMR: 9.52 (br, 1H, OH), 8.14 (m, 3H, H-3 + H-1 + H-13), 8.03 (t, J=2.2, 1H, H-11), 7.58 (t, J=8.1, 1H, H-14), 7.50 (m, 1H, H-15), 7.01 (d, J=8.9, 1H, H-4), 4.51 (s, 2H, H-7), 3.23 (s, 3H, H-8); 13C-NMR: 161.9 (C-5), 156.5 (C-9), 150.9 (C-10), 148.6 (C-12), 140.4 (C-2), 130.0 (C-14), 127.8 (C-15), 127.2 (C-1), 126.6 (C-3), 121.6 (C-6), 120.9 (C-13), 118.3 (C-4), 117.2 (C-11), 49.9 (C-7), 34.7 (C-8).