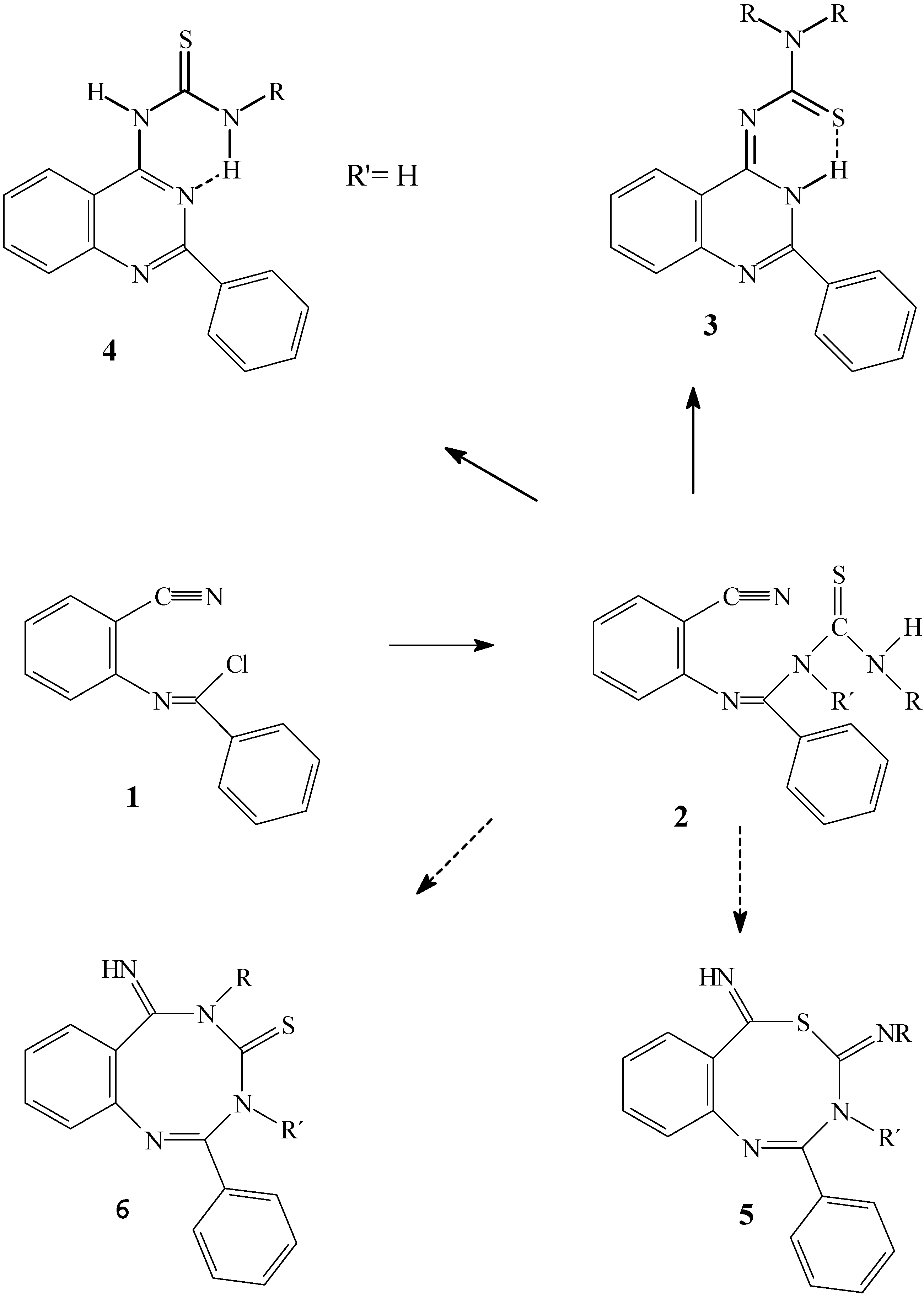
General procedure for reactions of N-(2-cyanophenyl)benzimidoyl chloride (1)
A mixture of
N-(2-cyanophenyl)benzamide [
10] (0.5 g, 2.25 mmol), and phosphorous pentachloride (0.5 g, 2.4 mmol) in dry toluene were refluxed for 8 h. The solvent was removed under reduced pressure to give a brownish colored oil of
N-(2-cyanophenyl)benzimidoyl chloride (
1) [
1,
2] which was not further purified. To the solution of crude oil in dry chloroform, appropriate equimolar amounts of the thioamide derivatives solution in dry chloroform were added. The reaction mixture was refluxed under inert atmosphere for 8h to give the precipitated solid, which was filtered, and crystallized from the appropriate solvent. The reaction were carried out under varied reaction conditions, either in the presence of triethylamine and reflux for 4 h, reflux in chloroform over night or by stirring of reaction mixture at room temperature for 4 days.
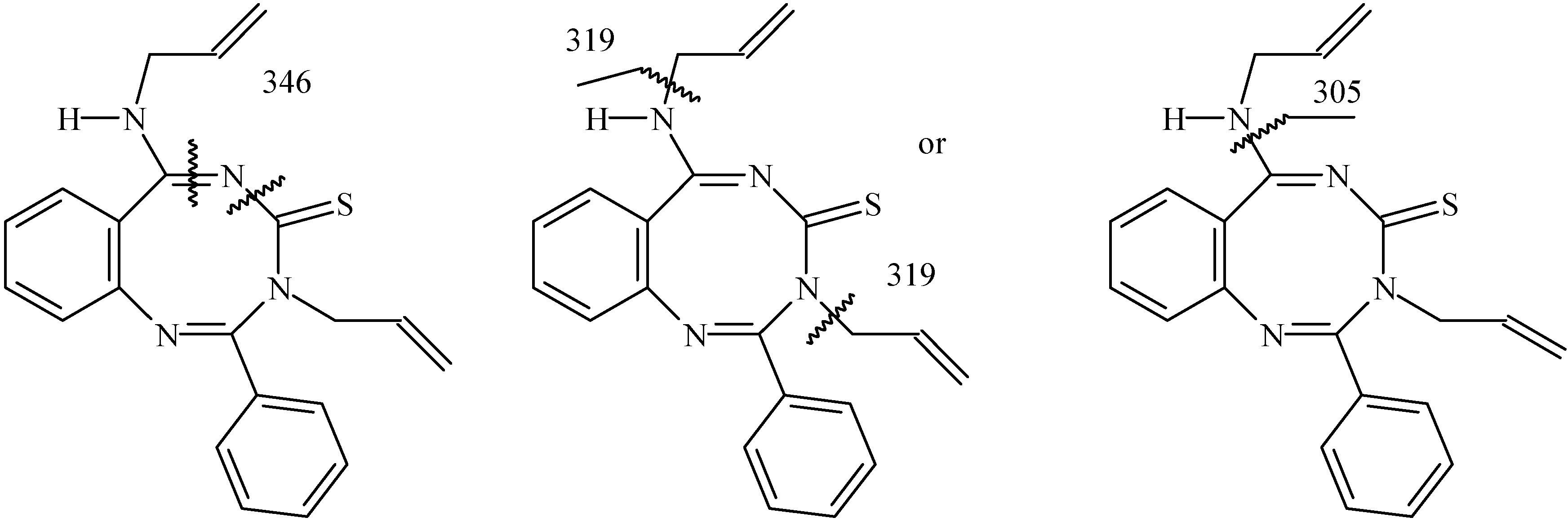
3-Allyl-6-(allylamino)-2-phenyl-3,4-dihydro-1,3,5-benzotriazocin-4-thione (7): 0.18g (38%); M.p. 153-154 oC; For C21H20N4S (360.48) calculated: 69.97% C, 5.59% H, 15.54% N, 8.89% S; found: 69.78% C, 5.34% H, 15.48% N, 8.76% S; FTIR, ṽ/cm-1: 3107 (NH), 2903 (CH), 1620 (C=N); 1H-NMR (DMSO) δ/ ppm: 8.91 (1H, t, NHCH2, J= 7.2 Hz), 8.54– 7.58 (9H, m, ArH), 6.14-6.01 (1H, m, NCH2CH), 5.79-5.66 (1H, m, NHCH2CH), 5.23 (1H, d, CH=CH2, J = 9.52 Hz), 5.14 (2H, d, CH=CH2, J= 8.4 Hz), 5.01 (2H, d, NCH2CH, J= 3.9 Hz), 4.93 (1H, d, CH=CH2, J= 8.4 Hz), 4.12-4.09 (2H, m, NHCH2CH); 13C-NMR (DMSO) δ/ ppm: 183.92 (C=S), 162.79 (Cq), 159.03 (Cq), 151.32 (Cq), 136.64 (Cq), 134.36 (CHAr), 133.44 (CHAr), 131.11 (CHAr), 128.73 (CHAr), 128.14 (CHAr), 127.28 (CHAr), 127.43 (CHAr), 124.86 (CHAr), 118.77 (Cq), 117.79 (CH=CH2), 116.57 (CH=CH2), 55.03 (NCH2), 47.85 (NHCH2); Mass spectrum, m/z (Ir/%): 361 (27), 360 (49), 345 (16), 320 (19), 319 (100), 302 (22), 260 (95), 246 (30), 205 (53), 99 (32), 77 (22), 72 (14), 41 (58).
2-Phenyl-4H-3,1-benzothiazin-4-imine (8): 0.25 g (78%); M.p. 216-217 oC; For C14H10N2S (238.31) calculated: 70.56% C, 4.23% H, 11.76% N, 13.45% S; found: 70.35% C, 4.12% H, 11.57% N, 13.37% S; FTIR, ṽ/cm-1: 3278.09 (NH), 3046.74 (CH), 1663.12 (C=N); 1H-NMR (DMSO) δ/ ppm: 13.84 (1H, s, NH), 8.62– 7.54 (9H, m, ArH); 13C-NMR (DMSO) δ/ ppm: 151.48 (Cq), 144.25 (Cq), 135.38 (CHAr), 132.08 (Cq), 131.45 (CHAr), 129.24 (CHAr), 128.43 (CHAr), 127.95 (CHAr), 127.55 (Cq); Mass spectrum, m/z (Ir/%): 238 (23), 238 (100), 222 (8), 206 (29), 205 (92), 178 (8), 151 (9), 134 (7), 119 (21), 108 (9), 102 (34), 77 (53), 76 (22), 51 (14).
2-Phenyl-3,4-dihydroquinazolin-4-thione (9): 0.17g (53%); M.p.162-163 oC; For C14H10N2S (238.31) calculated: 70.56% C, 4.23% H, 11.76% N, 13.45% S; found: 70.41% C, 4.08% H, 11.49% N, 13.29% S; FTIR, ṽ/cm-1: 3149 (NH), 3078, 3047 (CH), 1663 (C=N); 1H-NMR (CDCl3) δ/ ppm: 10.89 (1H, s, NH), 8.76– 7.52 (9H, m, ArH); 13C-NMR (CDCl3) δ/ ppm: 187.83 (C=S), 162.00 (Cq), 150.15 (Cq), 145.17 (Cq), 135.86 (CHAr), 132.26 (CHAr), 132.22 (Cq), 130.35 (CHAr), 129.61 (CHAr), 128.90 (CHAr), 128.43 (CHAr), 128.14 (Cq), 127.16 (CHAr).
2,3-Diphenyl-3,4-dihydroquinazolin-4-imine (10a): 0.26g (62%); M.p. 214-215 oC; For C20H14N2S (314.41) calculated: 76.40% C, 4.49% H, 8.91% N, 10.20% S; found: 76.32% C, 4.41% H, 8.88% N, 10.06% S; FTIR, ṽ/cm-1: 3250 (NH), 3039 (CH), 1634.04 (C=N); 1H-NMR (DMSO) δ/ ppm: 11.73 (1H, s, NH), 9.02– 7.35 (13H, m, ArH); 13C-NMR (DMSO) δ/ ppm: 158.94 (Cq), 157.13 (Cq), 136.88 (Cq), 135.70 (CHAr), 133.13 (CHAr), 129.17 (CHAr), 128.86 (CHAr), 128.58 (CHAr), 127.95 (CHAr), 126.32 (CHAr), 124.62 (CHAr), 124.48 (CHAr), 120.65 (CHAr), 112.66 (Cq); Mass spectrum, m/z (Ir/%): 314 (<1), 298 (29), 297 (62), 296 (100), 205 (19), 192 (6), 149 (18), 102 (11), 93 (7), 83 (14), 77 (42), 76 (6), 51 (5), 36 (26).
3-(4-Methylphenyl)-2-phenyl-3,4-dihydroquinazolin-4-imine (10b): 0.21g (48%); M.p.207-208 oC; For C21H16N2S (328.43) calculated: 76.80% C, 4.91% H, 8.53% N, 9.76% S; found: 76.69% C, 4.85% H, 8.29% N, 9.54% S; FTIR, ṽ/cm-1: 3274 (NH), 3069, 2926 (CH), 1634 (C=N); 1H NMR (DMSO) δ/ ppm: 11.52 (1H, s, NH), 8.93– 7.33 (13H, m, ArH), 2.38 (3H, s, CH3); 13C NMR (DMSO) δ/ ppm: 158.77 (Cq), 157.23 (Cq), 135.66 (CHAr), 134.32 (Cq), 133.08 (CHAr), 129.083 (CHAr), 128.92 (CHAr), 127.91 (CHAr), 124.42 (CHAr), 124.22 (CHAr), 122.34 (CHAr), 120.65 (CHAr), 112.67 (Cq).
3-(4-Chlorophenyl)-2-phenyl-3,4-dihydroquinazolin-4-imine (10c): 0.27g (57%); M.p. 183-184 oC; For C20H13ClN2S (348.85) calculated: 68.86% C, 3.76% H, 10.16% Cl, 8.03% N, 9.16% S; found: 68.74% C, 3.63% H, 10.14% Cl, 7.96% N, 9.03% S; FTIR, ṽ/cm-1: 3273 (NH), 3065, 2926 (CH), 1633 (C=N); 1H-NMR (DMSO) δ/ ppm: 11.56 (1H, s, NH), 8.91– 7.58 (13H, m, ArH); 13C-NMR (DMSO) δ/ ppm: 158.84 (Cq), 156.95 (Cq), 136.78 (Cq), 135.86 (CHAr), 132.83 (CHAr), 132.03 (CHAr), 129.17 (CHAr), 128.77 (CHAr), 128.59 (CHAr), 126.31 (CHAr), 126.32 (CHAr), 124.58 (CHAr), 124.35 (CHAr), 119.49 (CHAr), 112.36 (Cq).
3-Benzyl-6-imino-2-phenyl-3,4,5,6-tetrahydro-1,3,5-benzotriazocin-4-thione (11): 0.19g (38%);M.p. 170-171 oC; For C22H18N4S (370.47) calculated: 71.33% C, 4.90% H, 15.12% N, 8.65% S; found: 71.25% C, 4.89% H, 15.03% N, 8.57% S; FTIR, ṽ/cm-1: 3256 (NH), 3035 ,2978 (CH), 1645 (C=N); 1H-NMR (DMSO) δ/ ppm: 10.6 (1H, s, NH), 7,49– 7.15 (14H, m, ArH), 4.24 (2H, s, NCH2); 13C-NMR (DMSO) δ/ ppm: 177.01 (C=S), 159.24 (Cq), 152.29 (Cq), 151.18 (Cq), 138.19 (Cq), 133.08 (Cq), 128.67 (CHAr), 128.15 (CHAr), 128.04 (CHAr), 127.05 (CHAr), 126.96 (CHAr), 126.65 (CHAr), 43.07 (CH2).